Abstract
Using solid-phase microextraction and gas chromatography–mass spectrometry procedure, volatile compounds were studied during ripening of Reggianito cheeses stored under different temperature–time combinations. Volatile profile was characterised for cheeses ripened under standard conditions at 0, 61, 124 and 180 days of ripening, and changes occurring between 61 and 180 days were evaluated for cheeses in which an elevated temperature of 20 °C was applied within the first month of maturation. A total of 41 volatile compounds including acids, ketones, aldehydes, esters, alcohols and hydrocarbons were identified. Despite the presence of other compounds of potential relevance for cheese flavour, carboxylic acids clearly dominated the volatile profile of Reggianito cheeses. The more notable variations in the volatile fraction of Reggianito cheeses occurred during the first 2 months of ripening and were mostly explained by changes observed for the predominant acid fraction. Significant changes in the quantities for some volatile compounds were observed for the different temperature–time combinations applied, though the balance between the different groups of compounds was not considerably affected and small changes were observed for total chromatographic area values. In this work, a first description of changes occurring during ripening for the different volatile compounds present in traditional Reggianito cheese was provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence, content and composition of volatile substances in food are determinant for odour and aroma characteristics, which in turn have a substantial influence on quality. Each product has a characteristic and unique composition of volatile components [1]. In this context, each cheese variety has a particular volatile profile. Volatile compounds in different cheeses are often the same, although their ratio can vary considerably. The study of volatile compounds in each cheese variety is useful for both characterisation and quality definition purposes, linking it with the area and methodology of production [2]. There are many works in which cheese volatile fraction characterisation and studies of its evolution during ripening have been performed in both internationally recognised and rather local cheese varieties [1, 3–7]. The study of volatile fraction in cheese has been also useful for evaluating the effect of different changes made along cheese manufacture [8–11].
Reggianito is the major hard cheese variety produced in Argentina, in the vast central zone known as Pampa region. Its manufacture process is similar in many aspects to that of Parmigiano Reggiano and Grana Padano hard Italian cheeses, and includes the use of pasteurised bovine milk, natural whey starter and bovine chymosin, while a curd-cooking step up to 52 °C and brine salting are applied. Cheeses (6–7 kg blocks of cylindrical geometry) are usually ripened at 11–13 °C for 6 months [12]. Several research works have been carried out to elucidate the effects of different technological changes applied during Reggianito cheese manufacturing [13–16]. In order to reduce the storage period, the use of elevated temperatures has showed to be an effective strategy. Sihufe et al. [17] carried out a study to assess the impact of an increased storage temperature of 18 °C and suggested an optimal ripening period between 2 and 3 months. Later, another study also showed encouraging results when elevated temperatures of 20 °C were applied during the first month of the ripening period, showing an important reduction in storage time of 2 months [18] with a milder development of the final sensory characteristics [19].
Regarding volatile compounds found in Reggianito cheese, Wolf et al. [20] made a volatile profile characterisation of fully ripened commercial Reggianito cheeses. The study was developed using 18 cheese samples from six different leader brands, purchased in local markets. A total of 53 volatile compounds were identified (including acids, alcohols, ketones, esters and aldehydes), despite only 40 of them were detected in at least 50 % of the samples analysed. The volatile profile was dominated by acidic volatile compounds (principally acetic and butyric acids), alcohols (principally ethanol) and ketones. Although many previous studies, those referring to the volatile profile composition for Reggianito cheese are scarce and no data have been published following the evolution of individual volatile compounds for this traditional cheese type during ripening. Therefore, this study was conducted with the aim of contributing to already existing characterisation of the volatile fraction of Reggianito cheese, describing its evolution over the ripening period, and assessing the impact of the different temperature–time combinations applied on this area of cheese chemistry.
Materials and methods
Cheese sampling and ripening conditions
Ripening conditions and sampling were previously described by Ceruti et al. [21]. Briefly, 20 cheeses (7.8 ± 0.1 kg weight, 23.7 ± 0.2 cm diameter, 15.3 ± 0.2 cm height) were manufactured at a local factory, using pasteurised milk from the same cheese vat and following a standard cheese manufacture procedure. Cheeses were brined during 7 days and brought to our laboratory immediately after. Two cheeses were used for initial composition determination, and 18 cheeses were kept under three different temperature–time combinations for 6 months. Six cheeses were ripened at 12 °C and 85 % relative humidity (cheeses C), whereas the other 12 cheeses were stored at two different temperature–time combinations: six cheeses at 20 °C for 2 weeks followed by 12 °C up to 6 months (cheeses E 1) and six cheeses at 20 °C for 4 weeks followed by 12 °C up to 6 months (cheeses E 2), both at 85 % relative humidity. Cheeses in duplicate were sampled at 61, 124 and 180 days of ripening.
Analysis of volatile compounds by SPME–GC–MS
Sample preparation
Before chromatographic analysis, volatile compounds were both concentrated and isolated from a cheese matrix by a solid-phase microextraction (SPME) procedure. Five grams of grated cheese was placed in a 30-mL glass vial and sealed with 20-mm-diameter PTFE-silicone septa (Supelco, Inc., Bellefonte, PA, USA) and an aluminium crimp seal. For each cheese, two samples were prepared and subsequently analysed by GC–MS.
Headspace-SPME
A 1-cm-long DVB/CAR/PDMS 50/30 μm SPME fibre (Supelco, Inc., Bellefonte, PA, USA) housed in its manual holder (Supelco, Inc., Bellefonte, PA, USA) was used to concentrate volatile compounds on the vial headspace. The vial was kept at 40 °C for 10 min, and then the fibre was exposed to headspace during 30 min at 40 °C.
Gas chromatography–mass spectrometry
Compounds loaded onto the fibre were then analysed with a Clarus 600 gas chromatograph coupled to a Clarus 600T mass spectrometer (Perkin-Elmer, Shelton, CT, USA), equipped with a split/splitless injector and an Elite-5 (30 m × 0.25 mm ID × 0.25 μm film thickness) column (Perkin-Elmer, Shelton, CT, USA). Volatile compounds were desorbed from the fibre at the injection port of the chromatograph, splitless mode, during 5 min at 250 °C. The carrier gas was He at 1 mL min−1 flow rate, oven temperature programme being 10 min at 40 °C, then 6 °C min−1 to 150 °C, 10 °C min−1 to 230 °C and 9 min at 230 °C. Transfer line temperature was set at 200 °C and source temperature at 180 °C. Peak detection was made by mass spectrometry on the total ion current obtained by electron impact at 70 eV, and data were acquired at a rate of 2 scans s−1 over an m/z scan range of 40–500 amu. A C7–C40 n-alkanes mixture (Sigma-Aldrich, Inc., Saint Louis, MO, USA) was injected and run under the same chromatographic conditions, in order to obtain Kováts indices (KI) of detected compounds. KI were calculated according to Bianchi et al. [22]. Compounds were tentatively identified by computer matching of mass spectra with those of NIST2008 Mass Spectral Library (version 2.2). These identities were also confirmed with bibliographical data and by comparison of their KI with those reported in the literature, collected by online databases [23–26]. Area counts of volatiles were provided by integration using TurboMass software version 5.4.2 (Perkin-Elmer, Shelton, CT, USA).
Statistical analysis
One-way ANOVA was performed with area values for control cheeses, considering ripening time as the analysis factor. When differences between treatment effects were significant (P < 0.05), the least significant differences (LSD) test was used for multiple comparison of means. Two-way ANOVA was also performed, considering ripening temperature and ripening time as main factors, for area values corresponding to C, E 1 and E 2 cheeses at 61, 124 and 180 days of ripening. Both statistical tests were performed using Statgraphics (Statgraphics Inc., Rockville, MD, USA). Principal component analysis (PCA) was performed taking into account information from the 41 volatile compounds identified for each cheese at 61, 124 and 180 days of ripening. PCA was applied to a correlation matrix, using Minitab (Minitab Inc., State College, PA, USA).
Results and discussion
Volatile profile composition
A total number of 41 volatile compounds were identified from SPME–GC–MS analysis of Reggianito cheese samples (Table 1). Peaks detected were grouped according to their structure in carboxylic acids (11 compounds), ketones (8), aldehydes (10), esters (1), alcohols (5) and hydrocarbons (6). All compounds found in this study have been previously described in other cheese varieties, and most of them (36) in Reggianito [20] or in related hard Italian cheese varieties [27–29].
Groups of volatile compounds observed in this study were the same found by Wolf et al. [20], and their contribution to total chromatographic area value was quite similar, with the exception of the alcohols fraction for which Wolf et al. [20] found a considerably higher proportion corresponding to alcohols, mostly explained by the high content of ethanol. Ethanol may have been present in our cheese samples but could not be detected in this work, probably due to its very high volatility. Nevertheless, particularly high odour threshold values have been reported for ethanol [28, 30], thus being improbable an important direct contribution to cheese flavour for this compound. Although acids were the predominant group in both studies, we found in this work a relatively higher contribution of this group to the volatile fraction of Reggianito cheese.
For control cheeses at 180 days of ripening (cheeses ripened under standard conditions), the volatile profile was dominated by acids, accounting for around 80 % of total area value. Among this group, seven even-chain FFA (C2–C14) were detected, as well as two uneven-chain FFA (heptanoic and nonanoic acids) and two branched-chain acids (isovaleric and 2-methylbutanoic acids) (Table 1). Acetic, butyric and caproic acids clearly predominated among volatile acids. In a second, yet important area value level was caprylic and capric acids, as well as isovaleric and 2-methylbutanoic acids, while minor quantitative contributions were observed for heptanoic, nonanoic, lauric and myristic acids. A similar composition of acidic fraction was observed in grana-type Trentingrana cheese, for which up to 90 % of total area value was due to acids [9]. All of these compounds have been reported to be odour-active compounds in cheese, and this fraction has been described as important or even predominant for flavour in many cheese types, including hard Italian cheeses [31]. Furthermore, acids are not only aroma compounds by themselves, but also serve as precursors of other volatile compounds as methyl ketones, alcohols, lactones and esters [32].
Butyric acid may play a major role in Reggianito cheese flavour, considering its higher concentration than caproic acid and relatively lower threshold value in relation to acetic acid [28–31]. Acetic, caproic, caprylic, capric, isovaleric and 2-methylbutanoic acids may also have influence on Reggianito cheese flavour characteristics due to the combination of the presence at relatively high proportions and the low odour thresholds values reported for these compounds [28–31]. Previous works in Italian grana cheeses reported a role as key odorants for butyric and caproic acids, whereas acetic, caprylic, capric, isovaleric and 2-methylbutanoic acids also were considered contributors to global flavour [5, 33, 34].
Ketones were the second group of importance, accounting for 8 % of total area value. As Table 1 shows, the most abundant compounds within this group were acetoin (3-hydroxy-butanone) and methyl ketones 2-heptanone and 2-nonanone, for which low odour thresholds have been reported [31]. Other ketones (2-pentanone, 2-undecanone, 2-hydroxy-3-pentanone, 6-methyl-5-hepten-2-one and 3,5-octadien-2-one) were present at considerably lower quantities. Methyl ketones, particularly 2-heptanone and 2-nonanone, are often described as the most abundant ketones found in cheese. These compounds are normally dominant in mould-ripened cheeses, but may also play a role in the aroma of grana-type cheeses, contributing to fruity and blue cheese-like aromas [28].
Aldehydes represented around 4 % of total area value, and higher amounts were observed for hexanal, heptanal and benzaldehyde, followed by nonanal, 3-methylbutanal, benzeneacetaldehyde, 3-hydroxy-butanal, octanal, decanal and dodecanal (Table 1). Almost all of these compounds, except for 3-hydroxy-butanal, were described to be present in grana-type cheese and to have low threshold values. Straight-chain aldehydes are associated with green, grass-like odours. Those with shorter chain are also described as pungent and malty, whereas those with longer chain as presenting fatty and citric notes [28]. Branched-chain aldehyde 3-methylbutanal is usually described with strong malty, green and cocoa-like aromas, while almond-like aroma is usually described for benzaldehyde and a strong, rosy and green aroma for benzeneacetaldehyde [28].
Ethyl butanoate was the only ester detected, but it represented around 2.5 % of total area value. For this compound, a particularly low odour threshold has been described [31], and due to the relatively high proportion in which it is present, it probably makes a contribution to Reggianito cheese aroma. Wolf et al. [20] found that ethyl butanoate accounted for higher mean area values and it was the only ester present in all Reggianito samples analysed. Ethyl esters are usually regarded as main contributors to the fruity character in cheese. Further, these compounds can contribute to the aroma of cheese by minimising the sharpness imparted by fatty acids. Ethyl butanoate has been identified as one of the most potent odorants in several cheese varieties, including Grana Padano, Pecorino [31] and Parmigiano Reggiano cheeses [35].
Minor area contributions were explained by the groups of alcohols and hydrocarbons, each one responsible of around 2 % of total area value. Within alcohols found, 3-methyl-1-butanol and 2-heptanol predominated, being also found in almost all samples by Wolf et al. [20], and 1-butanol, 3-metil-3-buten-1-ol and 2-ethyl-1-hexanol were detected in a lower proportion (Table 1). All of these compounds have been previously found in hard Italian cheeses [27], and particularly, branched-chain alcohol 3-methyl-1-butanol has been described as the alcohol probably making the highest contribution to Parmigiano Reggiano cheese flavour [36]. Hydrocarbons found were toluene, octane, ethylbenzene, decane, limonene and decahydronaftalene having all of them, except for limonene, relatively high thresholds values [30], thus making improbable an important contribution to cheese flavour by these compounds.
Volatile profile evolution during Reggianito cheese ripening
Except for heptanoic acid and 2-heptanol at day 0, detected peaks in this study were present in all samples from 0 to 180 days of ripening. Heptanoic acid and 2-heptanol appeared during the first 61 days, and no further peaks appeared or disappeared afterwards, and thus changes during ripening in the volatile profile of Reggianito cheese were caused by variations in individual quantities. Total area value at day 0 was 130.8 ± 20.7 × 106, while values towards 2 months of ripening were around 200 × 106. A great increase in total area was then observed for cheeses in the first 2 months of ripening, while no significant differences were observed for this value in the following 4 months.
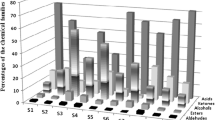
In Fig. 1, changes in the volatile fraction composition during ripening for control cheeses are shown. The increase in total area value during the first 2 months of ripening can be explained by the increase in the area values for acids during this period, while no significant changes were observed for this group in the following months. Because of this increase, the proportion of the remaining groups of compounds decreased notably between 0 and 61 days, regardless the change each had during this period. A tendency to increase for volatile acids during ripening has already been reported for various cheese varieties. Constant increases were observed for Nanos cheese [37] and Brie cheese [8]. For other cheese varieties, this change has been observed to occur during the first months of ripening, this quantity remaining constant thereafter, as reported for Ibores [38] and Montasio cheeses [6]. An important part of the increase observed in this work for volatile acids during the first 2 months of ripening was explained by the increment of FFA of 4 or more carbon atoms. The presence of these compounds in cheese is largely attributed to lipolysis [31]. In Reggianito cheese, lipolytic activity is explained by the presence of lipases and esterases from starter and NSLAB microflora, starter being composed by thermophilic lactobacilli L. helveticus (66 %) and L. delbrueckii subsp. lactis (33 %) [16]. Lipases and esterases from L. helveticus and L. delbrueckii subsp. lactis strains are intracellular, and thus, cell lysis is important for lipolytic activity [39]. It was already described that thermophilic lactobacilli in Reggianito cheese, being at counts of 106–107 cfu g−1 at the beginning of ripening, virtually disappeared within the first 2 months of ripening [19]. It can be stated therefore that liberation of intracellular lipolytic enzymes due to cell lysis may have been partially responsible of the increase in total area values for volatile acids.
Evolution during ripening of the main chemical groups of volatile compounds isolated in Reggianito cheese ripened under traditional conditions (12 °C for 180 days). Mean values with different letters within the same group of compounds mean significant differences (P < 0.05) at LSD test. Error bars indicate standard deviations between replicates
Ketones and aldehydes decreased between 61 and 180 days of ripening, mainly due to decrease in areas for 2-pentanone, acetoin, 2-hydroxy-3-pentanone and 2-heptanone in the first case, and 3-methylbutanal, hexanal and heptanal in the second case. Net increases for both aldehydes and ketones throughout ripening have been observed for some varieties of cheese [8], while others reported a net increase over time for ketones and a decrease for total aldehydes [4, 40]. However, it was also referred an increase for ketones and aldehydes at early times during ripening, and a fast reduction for most of those compounds later [37, 38]. Aldehydes are considered as transitory compounds in cheese, as they can be rapidly reduced to primary alcohols or even oxidised to the corresponding acids [31]. In relation to this aspect, the increase for 3-methyl-1-butanol observed in this work may be attributed to the decrease in 3-methylbutanal. Once produced, ketones can also be precursors for other compounds. Methyl ketones can be converted to secondary alcohols [32], as it seems to be the case in this work for 2-heptanone, where a net decrease in this compound was simultaneous to the increase for 2-heptanol observed during ripening. Acetoin is usually referred, together with diacetyl, as responsible of milk or creamy attributes in cheeses. The decrease observed for acetoin may be related to the already observed diminution of the milk–creamy attribute in Reggianito cheese during ripening [14].
For ethyl butanoate, there was a reduction during the first 4 months of ripening. Commonly, esters increase during ripening, due to activity of esterase systems from lactic acid bacteria in cheese. Particularly, ethyl butanoate was found to increase over ripening in many cases [37, 38, 41, 42]. However, no changes have been reported in this group for Brie cheese during ripening [8] and esters decreasing with storage time were already found for Mahon cheese [43].
The fraction of alcohols, though being small, increased its area values throughout ripening, mainly because of increments for the two predominant compounds: 3-methyl-1-butanol and 2-heptanol. As previously stated, increases in 3-methyl-1-butanol and 2-heptanol can be explained by decreases in 3-methylbutanal and 2-heptanone, as this two carbonyl compounds are usually considered precursors of the latter. No significant changes in the area values for the hydrocarbons family were observed.
Effect of the different temperature–time combinations applied during Reggianito cheese ripening on volatile compounds
According to ANOVA performed using chromatographic area values for the 41 compounds detected, different results were observed with respect to significance of the factors analysed (ripening time and temperature–time combination) as shown in Table 1.
A total number of 11 compounds were not significantly affected by any of the main experimental factors evaluated, whereas 11 were significantly affected by both of them. Between the remaining 19 compounds, 11 were significantly affected by ripening time and 8 by the temperature–time combination factor. Within those compounds that were significantly affected by temperature–time combination are included many of which had an important contribution to total area value (acetic, isovaleric, 2-methylbutanoic and caprylic acids, acetoin, ethyl butanoate, 2-heptanone, heptanal and benzaldehyde). As a consequence, there was a statistically significant effect of the temperature–time combination factor for total area values, but the importance of this fact may be limited, as no clear tendency was observed for the individual volatile compounds. In fact, these changes may have not altered Reggianito cheese flavour, as a characteristic sensory profile was described in a previous work for these cheeses under the experimental conditions mentioned [19].
Due to the number of detected compounds and the diversity of behaviours with respect to the factors evaluated, PCA was performed using the area values of all the 41 identified compounds for C, E 1 and E 2 cheeses at 61, 124 and 180 days of ripening (Fig. 2). Analysis showed that the first two principal components, PC1 and PC2, explained 52.9 % of total variability. From sample scores distribution, it can be inferred that PC1 (29.6 % VAR) can be associated with the ripening time factor, and a clear separation was observed for PC1 scores associated with samples at 61 days from those corresponding to 124 and 180 days of ripening. Second principal component PC2 (23.0 % VAR) was in relation to temperature–time combination.
Regarding loadings distribution in the biplot, it can be observed that among compounds in the left side of the graph (negative PC1 values) are included those with a decreasing tendency with time, as in the right side are included those increasing with time. At the same time, on the upper side of the graph were placed loadings for those compounds in which lower area values were observed for experimental cheeses with respect to control cheeses and in the lower side, those for which higher area values could be observed for experimental than for control cheeses.
Loadings for some aldehydes and ketones (3-methylbutanal, heptanal, 2-pentanone, 2-hydroxy-3-pentanone and acetoin), ethyl butanoate and acetic acid are localised in the left part of the graph, together with scores associated with C, E 1 and E 2 cheeses at 61 days of ripening, allowing to associate these compounds (that shows a tendency to decrease) with young cheeses. On the other side of the graph, most of acids and methyl ketones, 2-heptanone, 2-nonanone and 2-undecanone are placed near scores for cheeses at 124 and 180 days and may be associated with ripened cheeses.
Conclusions
From SPME–GC–MS methodology, volatile compounds profile in mature Reggianito cheese was studied, as well as its evolution during ripening and the influence of elevated temperatures applied at the beginning of the storage period. A total of 41 volatile compounds were identified and grouped as acids, ketones, aldehydes, esters, alcohols and hydrocarbons. The volatile profile of Reggianito cheese was clearly dominated by carboxylic acids (especially short chain FFA), as reported in previous works for this and other grana-type cheeses. The more notable changes in the volatile compounds profile occur during the first 2 months of ripening, and can be mostly explained by the increase in carboxylic acids. Other changes with a potential impact on Reggianito cheese flavour were observed for ketones, aldehydes, ethyl butanoate and alcohols. The different temperature–time combinations applied during Reggianito cheese ripening significantly affected the quantities of some of the volatile compounds, but the balance between the different groups of compounds remained roughly the same, and changes in total chromatographic area were small. Finally, potential relationships were established between the presence of some volatile compounds and the age of cheeses.
References
Plutowska B, Wardencki W (2007) Aromagrams—aromatic profiles in the appreciation of food quality. Food Chem 101(2):845–872
Ziino M, Condurso C, Romeo V, Giuffrida D, Verzera A (2005) Characterization of “Provola dei Nebrodi”, a typical Sicilian cheese, by volatiles analysis using SPME–GC/MS. Int Dairy J 15:585–593
Bellesia F, Pinetti A, Pagnoni UM, Rinaldi R, Zucchi C, Caglioti L, Palyi G (2003) Volatile components of grana Parmigiano-Reggiano type hard cheese. Food Chem 83:55–61
Delgado FJ, González-Crespo J, Cava R, García-Parra J, Ramírez R (2010) Characterization by SPME–GC–MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem 118:182–189
Frank DC, Owen CM, Patterson J (2004) Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT Food Sci Technol 37:139–154
Innocente N, Munari M, Biasutti M (2013) Characterization by solid-phase microextraction-gas chromatography of the volatile profile of protected designation of origin Montasio cheese during ripening. J Dairy Sci 96:26–32
Randazzo CL, Pitino I, De Luca S, Scifò GO, Caggia C (2008) Effect of wild strains used as starter cultures and adjunct cultures on the volatile compounds of the Pecorino Siciliano cheese. Int J Food Microbiol 122:269–278
Calzada J, del Olmo A, Picon A, Nuñez M (2014) Effect of high-pressure-processing on lipolysis and volatile compounds of Brie cheese during ripening and refrigerated storage. Int Dairy J 39:232–239
Endrizzi I, Fabris A, Biasioli F, Aprea E, Franciosi E, Poznanski E, Cavazza A, Gasperi F (2012) The effect of milk collection and storage conditions on the final quality of Trentingrana cheese: Sensory and instrumental evaluation. Int Dairy J 23:105–114
Ferreira IMPLVO, Pinho O, Sampaio P (2009) Volatile fraction of DOP “Castelo Branco” cheese: Influence of breed. Food Chem 112:1053–1059
Hannon JA, Kilcawley KN, Wilkinson MG, Delahunty CM, Beresford TP (2007) Flavour precursor development in Cheddar cheese due to lactococcal starters and the presence and lysis of Lactobacillus helveticus. Int Dairy J 17:316–327
Sihufe GA, Rubiolo AC, Zorrilla SE (2012) Reggianito cheese: hard cheese produced in Argentina. In: Hui YH (ed) Handbook of animal-based fermented food and beverage technology, 2nd edn. CRC Press, Boca Raton, pp 377–386
Candioti MC, Hynes E, Quiberoni A, Palma SB, Sabbag N, Zalazar CA (2002) Reggianito Argentino cheese: influence of Lactobacillus helveticus strains isolated from natural whey cultures on cheese making and ripening processes. Int Dairy J 12:923–931
Hough G, Martinez E, Barbieri T, Contarini A, Vega MJ (1994) Sensory profiling during ripening of Reggianito grating cheese, using both traditional ripening and in plastic wrapping. Food Qual Prefer 5:271–280
Hynes ER, Bergamini CV, Suárez VB, Zalazar CA (2003) Proteolysis on Reggianito Argentino cheeses manufactured with natural whey cultures and selected strains of Lactobacillus helveticus. J Dairy Sci 86:3831–3840
Perotti MC, Bernal SM, Meinardi CA, Zalazar CA (2005) Free fatty acid profiles of Reggianito Argentino cheese produced with different starters. Int Dairy J 15:1150–1155
Sihufe GA, Zorrilla SE, Perotti MC, Wolf IV, Zalazar CA, Sabbag NG, Costa SC, Rubiolo AC (2010) Acceleration of cheese ripening at elevated temperature. An estimation of the optimal ripening time of a traditional Argentinean hard cheese. Food Chem 119:101–107
Ceruti RJ, Zorrilla SE, Sabbag NG, Costa SC, Sihufe GA (2015) Acceleration of Reggianito cheese ripening. Effect of increased initial ripening temperatures on biochemical and sensory characteristics. Dairy Sci Technol 95:231–243
Ceruti RJ, Zorrilla SE, Sabbag NG, Costa SC, Sihufe GA (2014) Effect of increased initial ripening temperature on the sensory characteristics of Reggianito cheese. Int J Dairy Technol 67:539–546
Wolf IV, Perotti MC, Bernal SM, Zalazar CA (2010) Study of the chemical composition, proteolysis, lipolysis and volatile compounds profile of commercial Reggianito Argentino cheese: characterization of Reggianito Argentino cheese. Food Res Int 43:1204–1211
Ceruti RJ, Zorrilla SE, Sihufe GA (2012) The influence of elevated initial ripening temperature on the proteolysis in Reggianito cheese. Food Res Int 48:34–40
Bianchi F, Careri M, Mangia A, Musci M (2007) Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J Sep Sci 30:563–572
Flavornet and human odor space (2015) http://www.flavornet.org/flavornet.html. Accessed 25 Aug 2015
LRI and odour database (2015) http://www.odour.org.uk/lriindex.html. Accessed 25 Aug 2015
NIST webbook of chemistry (2015) http://www.webbook.nist.gov/chemistry/. Accessed 25 Aug 2015
The Pherobase. Database of pheromones and semiochemicals (2015) http://www.pherobase.com/database/kovats. Accessed 25 Aug 2015
Barbieri G, Bolzoni L, Careri M, Mangia A, Parolari G, Spagnoli S, Virgili R (1994) Study of the volatile fraction of Parmesan cheese. J Agric Food Chem 42:1170–1176
Qian MC, Burbank HM (2007) Hard Italian cheeses: Parmigiano-Reggiano and Grana Padano. In: Weimer BC (ed) Improving the flavour of cheese. CRC Press, Boca Raton, pp 421–443
Wolf IV, Urgeghe P, Addis M, Zalazar C, Piredda G (2008) Aplicación de la microextracción en fase sólida al estudio de la fracción volátil de quesos de origen italiano [Application of solid phase microextraction to the study of the volatile fraction of Italian cheeses]. Revista Argentina de Lactología 25:55–83
Nagata Y (2003) Measurement of odor threshold by triangle odor bag method. Odor measurement review, Japan Ministry of the Environment, Government of Japan, pp 118–127
Curioni PMG, Bosset JO (2002) Key odorants in various cheese types as determined by gas chromatography–olfactometry. Int Dairy J 12:959–984
Collins YF, McSweeney PLH, Wilkinson MG (2003) Lipolysis and free fatty acid catabolism in cheese: a review of current knowledge. Int Dairy J 13:841–866
Moio L, Addeo F (1998) Grana Padano cheese aroma. J Dairy Res 65:317–333
Qian M, Reineccius G (2002) Identification or aroma compounds in Parmigiano-Reggiano cheese by gas chromatography/olfactometry. J Dairy Sci 85:1362–1369
Qian M, Reineccius G (2003) Potent aroma compounds in Parmigiano Reggiano cheese studied using a dynamic headspace (purge-trap) method. Flavour Frag J 18:252–259
Qian M, Reineccius G (2003) Static headspace and aroma extract dilution analysis of Parmigiano Reggiano cheese. J Food Sci 68:794–798
Boltar I, Čanžek Majhenič A, Jarni K, Jug T, Bavcon Kralj M (2015) Volatile compounds in Nanos cheese: their formation during ripening and seasonal variation. J Food Sci Technol 52:608–623
Delgado FJ, González-Crespo J, Cava R, Ramírez R (2011) Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME–GC–MS. Food Chem 129:1156–1163
De Angelis M, Gobbetti M (2011) Lactobacillus spp.: general characteristics. In: Fuquay JW, Fox PF, McSweeney PLH (eds) Encyclopedia of dairy sciences, vol 3, 2nd edn. Academic Press, London, pp 78–90
Bontinis ThG, Mallatou H, Pappa EC, Massouras Th, Alichanidis E (2012) Study of proteolysis, lipolysis and volatile profile of a traditional Greek goat cheese (Xinotyri) during ripening. Small Ruminant Res 105:193–201
Caporaso N, Armento V, Sacchi R (2015) Volatile profile of Conciato Romano cheese, a traditional Italian cheese, during ripening. Eur J Lipid Sci Technol 117:1422–1431
Carbonell M, Nuñez M, Fernández-García E (2002) Evolution of the volatile components of ewe raw milk La Serena cheese during ripening. Correlation with flavor characteristics. Lait 82:683–698
Mulet A, Escriche I, Rosello C, Tarrazó J (1999) Changes in the volatile fraction during ripening of Mahon cheese. Food Chem 65:219–225
Acknowledgments
This research was partially supported by the Universidad Nacional del Litoral (Santa Fe, Argentina), Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina) and Agencia Nacional de Promoción Científica y Tecnológica (Argentina). We thank Milkaut S.A. for the supply of cheeses and Lic. Juan C. Andini for his valuable advice in chromatographic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Roberto J. Ceruti, Guillermo A. Sihufe and Susana E. Zorrilla declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ceruti, R.J., Zorrilla, S.E. & Sihufe, G.A. Volatile profile evolution of Reggianito cheese during ripening under different temperature–time combinations. Eur Food Res Technol 242, 1369–1378 (2016). https://doi.org/10.1007/s00217-016-2640-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2640-1