Abstract
Methamidophos (MAP) is a highly efficient and broad-spectrum organophosphate pesticide. In this study, a rapid method for detecting MAP in vegetables using surface enhanced Raman scattering (SERS) spectroscopy has been established. Density functional theory calculations were performed with Gaussian 03 at RB3LYP level and with the 6-311G (d) basis set. SERS, normal and theoretical Raman spectroscopy were compared to investigate the mechanism of Raman scattering enhancement. The SERS signal of MAP was improved in alkaline conditions with optimum scattering efficiency at pH of 13.46. Furthermore, MAP detection in vegetables by SERS method had a good linear relationship between 0.01 and 1,000 μg/mL. The concentration of MAP in vegetables was detected and chosen for recovery test with three levels: 4, 8, and 15 μg/mL. The results of three level tests were 86.7–96.6 % and their relative standard deviations were between 1.2 and 2.5 %, which shows the good repeatability of this method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamidophos (MAP), in which molecular structure is C2H8NO2PS as indicated in Fig. 1A, is a highly efficient and broad-spectrum organophosphorus pesticide and is mainly used as an insecticide and acaricide. It has been widely applied around the world to control a number of pest insects such as trips, leaf hoppers, aphids, and spider mites, which are harmful to different plants such as fruits, vegetables, and other crops [1]. It has been reported that MAP with high solubility in water (>2 kg/L) and alcohol is chronically toxic in different test species and is sprayed with an average of preharvest at 0.5–1.5 kg/ha [2]. It has been argued by many researchers that even a trace of residual amount of MAP is harmful to human and many animals, because MAP influences acetylcholinesterases in the central nervous system, which are responsible for the degradation of acetylcholine at nerve junctions [3]. Due to this highly toxic property, while acting as acetyl-cholinesterase inhibitor, MAP has significant consequences on non-target biological communities including human, animals, plants, insects, and generally on the environment. For that reason, although still being used in agriculture, MAP has been recently prohibited from being applied on some crops by many countries including the USA, Germany, and China [3]. Therefore, the safety of MAP becomes a public concern and draws increasing attention to food controllers.
Currently, the determination of MAP has been done with many methods including chromatographic methods [4, 5], flow injection [6], and biosensors [7]. All these methods are commonly applied or progressively being integrated for food quality control analysis. However, most of these methods are generally complicated and require the investment in costly instruments, skills, and elaborative special sample preparation as compared. On the contrary, surface enhanced Raman scattering (SERS) technology is easy, simple, and rapid and provides useful information regarding chemical bands and metal adsorbate interactions [8]. In spite of these advantages, SERS application on MAP quantification is not widespread, and matching research studies have not been reported so far.
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system, which can be used in food analysis [9]. The “fingerprint-like” Raman spectrum is able to provide overall and specific information on various chemical and biochemical components. However, signals of conventional Raman spectroscopy are very weak, because only one photon in one million will scatter inelastically (Raman shift). For SERS, when target molecules attach to metallic nano-structures (typically, Au, Ag, Cu, Pt, or Pd), Raman signals can be significantly enhanced by millions of times in high localized optical fields of these structures due to ‘‘electromagnetic field enhancement’’ and ‘‘chemical or electronic enhancement’’ of signals. Electromagnetic enhancement illumination intensity is enhanced due to sharp metal edges or surface plasmon effects at the metal substrates [10], while the chemical enhancement mechanism works through molecular polarizability [11].
SERS is a highly effective technique for the detection of molecular vibrations from species present at trace concentration down to the single molecule detection level [12]. Its high sensitivity and potentiality in providing useful information regarding metal adsorbate interactions make SERS a versatile technique for application in diverse fields such as food analysis [13], surface chemistry [5], biomolecular [14], environmental monitoring [15], biomedical [16], and medical sciences.
Rapid and accurate detection and identification of trace amounts of chemical species is of the utmost importance in food control to prevent hazards to mammals and to the environment. SERS has been widely used in food analysis, where it specially solves the problem of trace analysis by high sensitivity. SERS detection method of food contaminants has been reported in numerous studies, such as microbial studies [17], detection of pesticide residues [18], polycyclic aromatic hydrocarbons [19], and melamine [20].
This study demonstrates a novel approach to detect MAP in vegetables using SERS technology on silver colloids. With this SERS substrate, the comparison between density functional theory (DFT) calculation, Raman, and SERS spectroscopy has been made. Moreover, the optimal solution and pH value have been derived to get improved SERS signals. This study will show that the SERS methodology provides a rapid, sensitive, and real-time measurement of MAP.
Experimental section
Materials and Reagents
MAP (99 %) and AgNO3 (99 %) were obtained from Alfa Aesar company, USA. All other reagents used were of analytical purity grade and obtained from Sinopharm Chemical Reagent Co. Ltd., trisodium citrate, methanol, ethanol, acetone, nitric acid, sodium hydroxide, hydrochloric acid, sodium phytate, activated carbon, and the carbon support film (Beijing emerging Berry Co. Ltd.). All above reagents were used without further purification, and ultra pure water was used to prepare all aqueous solutions. All glassware used were washed with aqua regia and rinsed with ultrapure water prior to use.
Methods
Instrument
A portable Raman spectrometer RamTracer®-200-HS (OptoTrace Technologies Co., Ltd.) was used with the following parameters: an excitation light source of 785 nm, spectral scanning range of 100–3,300 cm−1, and resolution of less than 6 cm−1.
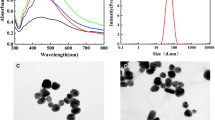
Transmission electron microscopy (TEM) image was obtained using a Hitachi H-8100 microscope with an acceleration voltage of 200 kV.
Silver nanoparticle preparation
The Ag nanoparticles were prepared by the reduction of AgNO3 with sodium citrate, referencing the method of Lee and Meisel cited by House and Schnitzer [21] with modification: A 5 mL and 0.001 mol/L of sodium phytate solution was added to a 0.0255-g portion of AgNO3 dissolved in 150 mL of ultrapure water, and the solution was quickly heated to boiling temperature. Then slowly, 3 mL l % sodium citrate was added. The solution was kept for 6 h by boiling and stirring it vigorously. Initially, the color of the solution during the reaction changed from colorless to light yellow and orange, and finally, a brown silver solution was obtained. The silver colloid was cooled to room temperature before storage at 4 °C.
The optical properties of the silver nanoparticles were obtained using TU-1900 dual-beam UV–visible light spectrophotometer, using a 1-cm-thick quartz cuvette. The UV–visible absorbance spectra were obtained from 200 to 900 nm. The maximum absorption was observed at 408 nm, with a corresponding average size of about 50 nm (Fig. 2).
Samples preparation
Six different vegetables were purchased randomly from Wuxi City, Jiangsu Province, P. R. China (Red amaranthus and Chinese little greens from Helie Street, little cabbage from Lihu Street, Chinese cabbage from Oudefu open market, leek from Huarui open market, and Spinach from Carrefour supermarket).
Extraction of MAP from vegetables for SERS detection
We dried the vegetables and then mashed them. Samples of 10 g were weighed accurately and grinded to dry powder with Na2SO4 of ration between 50 and 80 g, and then 0.2–0.4 g of activated carbon and 80 mL of acetone were added. The solution was shaked for 30 min and then filtrated. The filtrate was concentrated to 5 mL, and the solution was finally passed through 0.45 μm membrane for SERS detection.
Quantum chemical calculations
Density functional theory (DFT) is based on multi-electron quantum mechanical electronic structure methods that can directly reflect the molecular vibration information, with ab initio quantum chemistry being the most common method [22]. In this study, quantum chemical calculations were carried out with the Gaussian 03 program package and density functional methods RB3LYP; the hybrid functional combined 6-311G basis function set; and heavy atom polarization functions added to the d function. After analyzing the vibration corresponding to the imaginary frequencies designed to be the variables for the MAP molecular with the keyword for “opt = (gdiis, tight)” and “freq = Raman”, Gaussview was used to check the results. A geometry optimization calculation for each molecular configuration was followed by a frequency calculation with the same basis set. Modifications were done by computation functions and basis set combination of the 0.9613 correction factor [22].
Effects of the solvents to the SERS of MAP
Four different solvents, that is, acetone, methanol, ethanol, and ultrapure water with different polarity have been chosen to dissolve the MAP powder with the following concentration of 100 and 10 μg/mL. Silver colloid has been added to these solutions, respectively, and then NaOH was used to adjust the pH. The best solvent was then chosen by comparing the SERS intensity.
Effects of pH on SERS of MAP
After choosing the best solvent, HCl and/or NaOH solutions were used to adjust pH of the system in order to find the best pH convenient for SERS result.
SERS detection for MAP detection
To find the best proportion, the following three factors have been altered on an orthogonal experimental: sample volume, silver colloid amount, and HCl or NaOH amount (Table 1).
Based on SERS spectral data, experimental Raman (ER) spectrum, and theoretical Raman (TR) spectrum of MAP, we provided a theory basis to the MAP detection with consideration of scattering enhancement theory.
A standard stock solution of MAP with a concentration of 104 μg/mL was prepared, followed by dilution to a concentration of 100, 10, 1, 0.1, 0.01, and 0.001 μg/mL from the standard solution. As far as the best system for pH adjusted, SERS spectra of MAP were detected by Raman spectrum scanning, and SERS spectra of different concentrations of MAP were recorded.
Determination of the MAP recoveries
To determine the recoveries of MAP in vegetables, low, medium, and high levels of MAP, standard solution has been added to known concentration of MAP in samples. The calculation of recoveries was done according to the analysis of samples of the same analytical procedure . The formula used was the following: X = A 1 CV/A 2 M with X = methamidophos in the sample (mg/kg), A 1 = the peak area of methamidophos in sample solution, A 2 = the peak of methamidophos standard solution, C = the concentration of methamidophos in standard solution (μg/mL), V = volume of the final sample solution (mL), and M = sample mass (g).
The data were analyzed using SPSS 16.0 software for Windows (SPSS Inc., USA), which applied a one-way variance analysis (ANOVA). Difference of P < 0.05 was considered significant.
Results and Discussion
Theoretical Raman (TR) and experimental Raman (ER) spectra of MAP
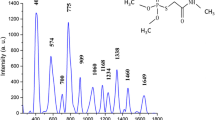
The DFT method is convenient for the calculation of molecular vibration spectra and its efficiency has been proven by the large number of studies in which experimental data were successfully reproduced [23]. In this study, we used the RB3LYP and 6-311G basis function set for the calculation and analysis of the MAP Raman spectrum. In Fig. 1A, the optimized molecular structure of MAP can be seen. And the main Raman peaks and corresponding assignments are listed in Table 2.
From the figure above, it can be seen that there are some deviations between the ER spectrum and TR spectrum of MAP. The calculations usually assumed an idealized model, which did not consider the intermolecular interactions from many MAP molecules, solvent effects and environmental impacts; hence, numerical agreement of any particular peak with the observed one may involve a little deviation.
Effect of the solvents on SERS of MAP
Generally, the choice of solvent can have appreciable effect on the SERS results. It has been shown that the solvent has a great influence on sensitivity, linear range of response, and shape of the calibration graph of SERS detection method. The best solvent does not react towards the reagents. To improve the quality of the spectra, the solvent is generally optimized for the adsorbate and with minimum interference. Moreover, molecules in solution could make very complex interactions with surrounding molecules. These effects are mainly from the Van der Waals force and hydrogen bonds between molecules and cause different spatial arrangements of different roles. These interactions may change the properties of the molecules in the liquid such as the molecular vibration frequency and spectral intensity.
In this study, the analysis of the recorded spectra revealed that the adsorption on the colloidal in the same system was different depending on the solvents. All four solvents were tested and evaluated according to their effect (Fig. 3). As to 102 μg/mL, MAP dissolved in different solvents, the Raman peak at 700 cm−1 was weak in methanol as shown in Fig. 3B, so SERS effect was worst when dissolved in the methanol. When MAP dissolved in ethanol or acetone, SERS signals were better but there were lots of interferential peaks, and when MAP dissolved in ultrapure water, the SERS signal was better but there were less interferential peaks. Therefore, the best solvent was chosen for ultrapure water, which was chosen to be used for the following experiments.
Effect of pH on SERS of MAP
The pH value of the colloidal solution has a great effect on the SERS intensity. Adsorbed chloride causes a decrease in the surface pH. This decrease is explained by a shift of the electrostatic potential at the outer Helmholtz plane caused by specific adsorption of chloride [24].
Figure 4 shows the SERS data at different pH adjusted by HCl and NaOH. The pH value of the Ag colloid was 7.88, which was a weak alkaline, but it changed to acidity after the addition of HCl. It can be seen from the Fig. 4A that there was no SERS signal in acidic condition. On the other hand, when array of pH was adjusted by NaOH (Fig. 4B), a maximum enhancement appeared when NaOH solution of 1 mol/L was added to the system. The obviously enhanced peaks were located at 668, 946, 1,302, 1,426, and 2,928 cm−1. The peak at 1,302 cm−1 displayed a shift to higher frequency compared to 1,318 cm−1 in ER spectrum. Due to the lack of SERS signals in acidic solution, NaOH has been chosen as the best pH adjuster in the following experiments.
Optimization of the experimental condition for best SERS signal for MAP
The orthogonal test table (Table 1) was designed to derive the most suitable ratio of the three following factors for SERS measurement: sample volume (factor A), the silver nanoparticle volume (factor B), and NaOH volume (factor C). The concentration of 100 μg/mL MAP solution was prepared with ultrapure water, and the peak selected for evaluation was at 668 cm−1.
According to the value of Range R (Table 3), factor A showed the most significant effect on SERS measurement, and the order of importance was found to be sample volume (A) > NaOH volume (C) > the silver nanoparticle volume (B). The most suitable volume ratio for SERS of MAP detection was MAP: Ag: NaOH = 20:500:50, respectively. However, the increase in the ratio of solvent to the system will reduce the enhancement intensity due to a limited pH enhancement effect. On the other hand, if the amount of the base NaOH added is not enough, the enhancement will not be very obvious due to pH effect.
The adsorption behavior of MAP on Ag nanoparticles
In Fig. 5, the SERS spectrum of the MAP solution in the concentration of 100 μg/mL MAP and the ER spectrum are presented. The significantly enhanced peaks are located at 686, 946, 1,430, and 2,928 cm−1, because the distance of nanoparticles was long before pH adjustment. After the addition of NaOH to the system, the nanoparticles became slightly more aggregated and the distance between them become smaller. This led to a stronger Raman scattering signal.
In Table 2, the band at 700 cm−1 of normal Raman spectrum (ER) can be assigned to C–S stretching vibration, which had a 14 cm−1 shift to lower frequency to 686 cm−1 in the SERS spectrum. Therefore, the likely adsorption mode of the MAP molecules absorbed on the silver nanoparticle was found along the S atom forming the connection of Ag–S, as schematically illustrated in inset of Fig. 5.
Quantitative analysis of MAP by SERS detection
From Fig. 5, it is obvious that the peaks suitable for a qualitative assessment of MAP are located at 686, 946, and 2,928 cm−1. For a quantitative analysis, the peak with highest intensity should be selected to ensure highest accuracy [25].Thus, we will focus on the maximum intensity peak at 686 cm−1 in the following quantitative analysis. A standard curve was made to show the relationship between MAP concentration and SERS intensity (Fig. 6). This figure shows that as the concentration of MAP increased, the relative SERS intensity also increased.
The linear working range between 0.1 and 1,000 μg/mL of MAP solution showed a good linearity with a correlation coefficient of 0.9852, and the detection limit was 0.1 μg/mL. For further study, SERS detection for MAP has been applied on real vegetables.
In Table 4, the results for detection of MAP concentration from six different vegetables using SERS are presented. It shows that the concentration of MAP in leek and Chinese cabbage was the highest; 24.68 and 14.64 mg/kg, respectively, and was the lowest in little cabbage (6.02 mg/kg). For each sample, six parallel detections have been done.
Recoveries were estimated by spiking the known concentration of little cabbage samples, with three different levels of MAP standard solutions, to bring them up to the range of 9.68, 12.56, and 18.23 μg/mL. Six replicates at all concentration levels were detected according to established procedures. Table 5 shows that the recovery was between 86.7 and 96.6 %, with relative standard deviation (RSD; n = 6) between 1.2 and 2.5 %, and the limit of detection was 0.1 μg/mL. The high recovery rate and low RSD suggested that rapid and reliable detection of MAP can be achieved by SERS.
Conclusion
In this study, we have developed a novel method of SERS detection for the qualitative and quantitative assessment of MAP in vegetables. Various experimental conditions including solvent and pH values have been compared to get best SERS effect of MAP. After an orthogonal test design, we detected the concentration of MAP in six different vegetable samples. The standard curve shows a good linear relationship, in the range of 0.1–100 μg/mL. SERS resulted quite precisely with RSD were between 1.2 and 2.5 %, and the limit of detection was 0.01 μg/mL. The SERS method serves an effective approach for rapid and reliable detection of MAP in vegetables and probably it can be used as the detection method for other food contaminants as well to ensure food safety.
References
Wang MC, Liu YH, Wang Q, Gong M, Hua XM, Pang YJ, Hu S, Yang YH (2008) Impacts of methamidophos on the biochemical, catabolic, and genetic characteristics of soil microbial communities. Soil Biol Biochem 40(3):778–788
WHO (1993) Methamidophos health and safety guide 79. World Health Organization, Geneva, Switerland
Yang L, Liu J, Wu YJ, Li W, Gao XW, Leng XF (2007) Oral administrations of methamidophos and TOCP have different effects on in vitro protein phosphorylation levels from subcellular fractions of hen brain. Pestic Biochem Physiol 88(1):20–25
Sharma D, Nagpal A, Pakade YB, Katnoria JK (2010) Analytical methods for estimation of organophosphorus pesticide residues in fruits and vegetables: a review. Talanta 82(4):1077–1089
Suh JS, Jang NH, Jeong DH, Moskovits M (1996) Adsorbate photochemistry on a colloid surface: phthalazine on silver. J Phys Chem 100(2):805–813
Granby K, Andersen JH, Christensen HB (2004) Analysis of pesticides in fruit, vegetables and cereals using methanolic extraction and detection by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 520(1–2):165–176
Ruiz TP, Martinez-Lozano C, Tomas V, Martin J (2001) Flow injection determination of methamidophos using online photo-oxidation and fluorimetric detection. Talanta 54(5):989–995
Gao J, Gu H, Dong X (2011) The determination of captopril in solution by Raman spectroscopy. J Phys: Conf Ser 277(1):012009
Fleischmann M, Hendra P, McQuillan A (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26(2):163–166
Utzinger U, Heintzelman DL, Mahadevan-Jansen A, Malpica A, Follen M, Richards-Kortum R (2001) Near-infrared Raman spectroscopy for in vivo detection of cervical precancers. Appl Spectrosc 55(8):955–959
Persson B (1981) On the theory of surface-enhanced Raman scattering. Chem Phys Lett 82(3):561–565
Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275(5303):1102
Han XX, Zhao B, Ozaki Y (2009) Surface-enhanced Raman scattering for protein detection. Anal Bioanal Chem 394(7):1719–1727
Han XX, Xie Y, Zhao B, Ozaki Y (2010) Highly sensitive protein concentration assay over a wide range via surface-enhanced Raman scattering of Coomassie brilliant blue. Anal Chem 82(11):4325–4328
Xie Y, Wang X, Han X, Xue X, Ji W, Qi Z, Liu J, Zhao B, Ozaki Y (2010) Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst 135(6):1389–1394
Kneipp K, Moskovits M, Kneipp H (2006) Surface-enhanced Raman scattering: physics and applications, vol 103. Springer, Berlin
Zhang X, Young MA, Lyandres O, Van Duyne RP (2005) Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J Am Chem Soc 127(12):4484–4489
Shende C, Gift A, Inscore F, Maksymiuk P, Farquharson S (2004) Inspection of pesticide residues on food by surface-enhanced Raman spectroscopy
Xie Y, Wang X, Han X, Song W, Ruan W, Liu J, Zhao B, Ozaki Y (2011) Selective SERS detection of each polycyclic aromatic hydrocarbon (PAH) in a mixture of five kinds of PAHs. J Raman Spectrosc 42(5):945–950
Zhang XF, Zou MQ, Qi XH, Liu F, Zhu XH, Zhao BH (2010) Detection of melamine in liquid milk using surface enhanced Raman scattering spectroscopy. J Raman Spectrosc 41(12):1365–1370
House PG, Schnitzer CS (2008) SERRS and visible extinction spectroscopy of copper chlorophyllin on silver colloids as a function of pH. J Colloid Interface Sci 318(2):145–151
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi L, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B.04; Gaussian, Inc., Pittsburgh
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree-Fock, Miller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100(41):16502–16513
Sarkar UK (2003) A pH-dependent SERS study of thiophene-2-carboxylic acid adsorbed on Ag-sols. Chem Phys Lett 374(3–4):341–347
Le Ru EC, Etchegoin PG (2008) Principles of surface-enhanced Raman spectroscopy: and related plasmonic effects. Elsevier Science, Amsterdam
Acknowledgments
This article is supported by National Key Technology R&D Program in the 11th Five-year Plan of China, No. 2009BADB9B04, the Fundamental Research Funds for the Central Universities No. JUSRP21124, JUSRP111A37, 30908, 31005, 31106 and 111A37, 111 Project-B07029 and PCSIRT0627.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Y., Mukamurezi, G., Sun, Y. et al. Establishment of rapid detection method of methamidophos in vegetables by surface enhanced Raman spectroscopy. Eur Food Res Technol 234, 1091–1098 (2012). https://doi.org/10.1007/s00217-012-1724-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1724-9