Abstract
In the present study, the use of gas chromatography mass spectrometry (GC–MS)-based metabonomics to characterize blood serum in an intervention study of patients suffering from the common gastrointestinal disorder irritable bowel syndrome (IBS) was investigated. The patients included in the study consumed an acidified milk product with (n = 30) or without probiotics (n = 31) (Lactobacillus paracasei F19, Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12) for an 8-week period, and blood serum samples were collected before and after the intervention. Acidified milk is commonly used as a delivering vector for probiotics in commercial consumer settings. The serum samples were extracted and derivatized using N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA), and GC–MS analysis was carried out. Multivariate data analysis including principal component analysis (PCA), orthogonal partial least squares-discriminant analysis (OPLS-DA), and S-plot was applied on the obtained GC–MS data, which revealed higher serum lactate, glutamine, proline creatinine/creatine, and aspartic acid levels and lower serum glucose levels after the intervention period for both treatment groups. Consequently, the present study indicated an effect of acidified milk consumption on the plasma metabolite profile, which was independent of a concomitant intake of probiotics. In addition, the present study demonstrates that GC–MS is a useful analytical technique for metabonomics studies of blood serum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolomics is a rapidly growing discipline concerned with analyzing the entire measurable array of low-molecular-weight compounds, metabolites, in a given biological system [1, 2]. Measurements are performed on both biofluids (e.g., plasma/serum, urine, and cerebrospinal fluid) and tissues (liver, brain, gut, and muscles), providing vast amounts of data and highly complex metabolite profiles describing the system. Through the study of time variation of metabolites, complex responses to different perturbations or inputs such as disease [3], stress [4, 5], drug administration [6], environmental stimuli [7] and diets [8–10] can be analyzed, thereby providing very useful knowledge of affected pathways. Nuclear magnetic resonance (NMR) is a widely applied method due to the non-selective detection of multiple metabolites with minimum sample preparation and high reproducibility [11]. Mass spectrometry-based methods in combination with chromatography, such as gas chromatography—mass spectrometry (GC–MS) [12–14] offers, on the other hand, much higher sensitivity and the ability to detect a larger number of metabolites. The main drawback is that metabolites need to be derivatized before GC–MS analysis, which is labor intense. Nonetheless, the low detection limit and small sample volume required make it feasible for analysis of subtle metabolic changes induced by a diet intervention as pursued in this study.
Consumption of a probiotic-containing diet is believed to have beneficial effects on the gastro-intestinal tract and general wellbeing. Indeed, probiotics have been shown to have positive effects on gastrointestinal inflammation, thereby conferring beneficial effects to patients suffering from irritable bowel syndrome (IBS) and inflammatory bowel disease [15–20]. IBS is a common bowel disorder characterized by abdominal pain and disturbed bowel habit, i.e. diarrhea and/or constipation. Many patients can keep symptoms at a minimum by “over-the-counter” medication, control of diet and lifestyle; however, symptoms can be so severe that they in a proportion of patients can have a disabling effect on everyday life [21–23].
The aim of the present study was to elucidate the use of a GC–MS-based metabonomic application to examine the effect of an 8-week intervention with either an acidified milk product or an acidified milk containing probiotics on the blood serum metabolite profile in IBS patients.
Materials and methods
Study participants and experimental design
Based on the Rome II criteria [24], an initial group of volunteer IBS patients were enrolled in the study. Patients were then followed during a screening period for 2 weeks without study intervention to establish whether they truly matched the Rome II criteria and to study the severity of their symptoms. The IBS severity scoring system (SSI) [25] using Visual Analogue Scales (VAS) was used to assess individual symptom severity. VAS measures characteristics that cannot easily be directly measured, such as pain. The scale ranges across a continuum from none to extreme without any discrete jumps. In order to be included in the treatment phase of the study, patients had to score high on at least one of the five questions in the IBS SSI questionnaire. Furthermore, patients had to have a lack of adequate relief of the IBS symptoms the week before inclusion. Patients not fulfilling these criteria were excluded from the study, leaving 74 subjects. Subjects were randomized for one of two treatments. During the intervention period, seven subjects dropped out for reasons unrelated to the study. For different reasons, blood serum samples were not acquired from 6 subjects leaving the final group of subjects completing the study on 61 subjects. The total patient group consisted of 45 women and 16 men aging from 18 to 79 years with a mean of 42.5 years. Patient characteristics are described in previously published material [26, 27]. Informed consent was provided by all patients. The study was performed in accordance with the Declaration of Helsinki II and approved by the regional Ethical Committee at University of Gothenburg. The trial was registered at ClinicalTrial.gov with the registration number: NCT01127828.
Dietary treatment and sample collection
The two different patient groups were administered two different dietary treatments. One treatment consisted of a fermented dairy product containing probiotics. The probiotic product (Cultura©, Arla Foods amba, Denmark) was produced by fermentation of homogenized, high-pasteurized, low-fat (1.5%) milk by the use of two fermentation starter cultures, Lactobacillus delbruckeii ssp. bulgaricus and Streptococcus thermophilus and the inclusion of three probiotic strains, Lactobacillus paracasei F19, Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12, in concentrations of 5 × 107CFU/mL (Proximate composition: protein 40 g/kg, fat 15 g/kg, carbohydrate 45 g/kg, lactose 35 g/kg). The final probiotic concentration was found to be 107–109 CFU/g. The other treatment was a non-probiotic chemically acidified milk product produced by acidification of homogenized, high-pasteurized, low-fat (1.5%) milk by the addition of d-(+)-gluconic acid δ-lactone (≥99.0%) (GDL) (Sigma-Aldrich, Seelze, Germany) (Proximate composition: protein 40 g/kg, fat 15 g/kg, carbohydrate 65 g/kg, lactose 45 g/kg). Both products had a pH value of 4.5. Over a period of 8 weeks, the two patient groups were instructed to consume 0.4 L/day, divided in two intakes per day of either the GDL-acidified milk product (n = 30) or the probiotic milk product (n = 31). In the study, the two products are referred to as the GDL milk product and the probiotic milk product. Patients were instructed not to consume any other probiotic containing dairy products during the intervention period. One day before the intervention period commenced (baseline), body weight measurements were performed and blood samples were collected from each subject. This procedure was repeated on the last day of the intervention period (post-treatment). Furthermore, dietary intake for each patient in 3 days before both baseline and post-treatment sample collection was recorded. Dietary records and weight measurements are reported in previously published material [26]. Patients had been fasting overnight and then consumed a fiber-rich non-dairy meal (Content: 540 kcal; 36% fat, 15% proteins, 49% carbohydrates; 9.2 g fiber) 1 h before collection of blood. Blood serum for GC–MS analyses was prepared by collecting 5 mL blood from the antecubital vein in silicon-treated Vacutainer® tubes. Clotting time was 30 min. followed by 20-min. centrifugation at 1,600g. The serum fraction was collected and stored in aliquots at −70 °C.
Chemicals and reagents
Methanol:water (8:1 v/v), methoxyamine, pyridine, MSTFA (N-Methyl-N-(trimethylsilyl) trifluoroacetamide) with 1% TMCS (trimethylchlorosilane) were purchased at Sigma-Aldrich (Germany). Standard compounds for d-glucose, glutamine, aspartic acid, proline, creatinine and lactic acid were purchased at Sigma-Aldrich (Germany). d-proline-1-13C was purchased at Cambridge Isotope Laboratories (United Kingdom).
Serum derivatization and GC–MS analysis
Extraction of metabolites from serum was performed using a modified method based on Jiye et al. 2005 [12]. From each sample, metabolites were extracted using 100 μL diluted serum (1:4 v/v in ddH2O) and 900 μL methanol:water (8:1 v/v). The solution was mixed and followed by a centrifugation at 19,600g for 10 min. at 4 °C. 200 μL supernatant was collected, transferred to a GC vial and evaporated to dryness in a vacuum centrifuge. Each residue was then dissolved in 30 μL methoxyamine (15 mg/mL) in pyridine. Methoxymation was performed at room temperature for 16 h. After methoxymation, silylation was carried out by adding 30 μL MSTFA with 1% TMCS to the mixture. This solution was vortexed and incubated for 1 h at room temperature. After silylation, 40 μL heptane was added to each vial. Derivatized serum extracts were analyzed with an Agilent gas chromatograph. A 1-μL-extract aliquot of the extracts was injected into a HP-5MS capillary column coated with 5% phenyl cross-linked 95% dimethylpolysiloxane (30 m × 250 μm i.d., 0.25-mm film thickness; Agilent J&W Scientific, Folsom, CA) in the split mode (10:1). MS quadropole temperature was set to 150 °C with inlet temperature at 270 °C and interface temperature at 280 °C. Initial GC temperature was 70 °C. After injection, the temperature was raised to 230 °C with 15 °C/min. Subsequently, the temperature was raised to 300 °C with 10 °C/min and held there for 4 min. Flow for helium carrier gas was set at 1.2 mL/min, and measurements were performed with an electron impact ionization (70 eV) in a full scan mode of 50–500 m/z. Reproducibility was tested by running specific samples two times with a span of 12 h between. Also samples were run with a 13C isotope labeled proline as internal standard.
Data processing and multivariate data analysis
Inspection of Total Ion Chromatograms (TIC) showed errors in two GDL milk group samples and two probiotic milk group samples caused by errors in either derivatization or data acquisition. These samples along with their complementary baseline or post-treatment samples were excluded from the study leaving a data set comprised of 28 GDL and 29 probiotic samples. Each sample was represented by a GC–MS-TIC, and peaks with a signal-to-noise ratio higher than 5:1 was detected. Out of 69 initially automatically selected peaks, 14 were excluded from the original selection due to problems with low signal-to-noise ratio or shoulder separation. The intensities of the 55 selected peaks were calculated and used for multivariate data analysis (MVA). Normalization to total intensity of the spectrum was performed before further data analysis. Cross-validated principal component analysis (PCA) was performed on mean centered data to identify discrete patterns within the data set. PCA is an unbiased algorithm that lowers data dimensionality in large data set while retaining the variation within the data set. The algorithm identifies principal components (PC) in which the variation are at maximum, thereby large data sets containing many variables can be explained by a relatively low number of PCs. Analysis of the produced component plots can then identify similarities, differences, and discrete patterns in the data. Patterns detected in PCA are subsequently analyzed by cross-validated orthogonal partial least squares discriminant analysis (OPLS-DA) [28]. For the OPLS-DA, data were scaled using mean centered variable stability (VAST) scaling. VAST was used to obtain unit variance within each treatment [29]. The OPLS-DA algorithm decomposes the variation between two identified groups into three parts; the variation in X (the data matrix) related to Y (the discriminant matrix), the systemic variation in X and the residual variation in X. Thereby a model with minimum predictive components defined by the number of degrees of freedom between group variances was produced. S-plots [30] were used to sort out relevant metabolites. The S-plot incorporates both the covariance and the correlation of variables, thereby minimizing the possibility of false positives. MVA was performed using the Simca-P+12.0 software (Umetrix, Umeå, Sweden). One-way ANOVA was performed on principal component scores, and paired Student’s t test was performed on peak areas. All tests were performed on the 0.05 significance level.
Results
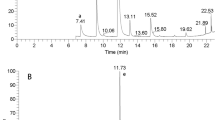
After GC–MS analysis of all samples, the TICs were inspected individually and by overlay plots. No discrepancies in peak appearance were found between different samples. A peak detection limit was imposed on the TIC for selection of metabolites, and 55 peaks were found to have a signal-to-noise ratio within the imposed detection limit. Figure 1a shows a chromatogram for a representative sample. The chromatograms showed a very high degree of alignment. The reproducibility was also found to be very high when comparing chromatograms recorded from a sample that was run twice with 12 h between the analyses (Fig. 1b). The 55 selected peaks were used for the subsequent MVA.
Total ion chromatograms of representative human serum samples. a TIC from RT 4.0–14.0 min retention time (RT) of a post-treatment sample. b Overlay plot from 10.0–14.5 min RT of two spectra collected from two identical post-treatment samples from a probiotic-milk-treated IBS patient run with a time span of 12 h in between
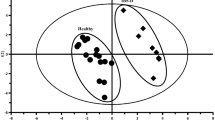
Peak intensity was calculated, and data were normalized to the total intensity of each spectrum. Baseline and post-treatment samples were analyzed by PCA for groupings caused by age, body mass index, gender, response to treatment, and type of IBS, respectively. No groupings were found within either the GDL-milk-treated patient group or the probiotic-milk-treated group of patients (data not shown). We then tested for groupings in PCA when comparing baseline and post-treatment samples for each treatment (Fig. 2) and found that there was a distinct separation between baseline and post-treatment for both GDL-milk (Fig. 2a)- and probiotic-milk (Fig. 2b)-treated patient groups.
PCA of baseline and post-treatment samples. a GDL milk group treated IBS patients (n = 28). Model composed of 4 principal components. R 2 X(1) = 0.630, R 2 X(2) = 0.172 and Q 2(cum) = 0.658. b Probiotic milk group treated IBS patients (n = 29). Model composed of 5 principal components. R 2 X(1) = 0.736, R 2 X(2) = 0.133 and Q 2(cum) = 0.745. One-way ANOVA was performed on baseline and post-treatment samples in all PC’s showing P ≤ 0.01 for PC1 in both the GDL-and the probiotic-milk-treated patient groups and PC4 for the GDL-milk product-treated patient group. The ellipse indicates the Hotelling T2 95% limit
Both PCA models, composed of 4 principal components for the GDL patient group and 5 principal components for the probiotic group, resulted high R 2(X) values and Q 2(cum) values indicating that nearly all the variation in the spectra were explained, and that the predictability of the models were good. Furthermore, one-way ANOVA on principal component scores showed a significant difference between baseline and post-treatment samples for PC1 for both groups and PC4 for the GDL patient group. The differences between baseline and post-treatment samples were identified using OPLS-DA. OPLS-DA models explained 42.5% (GDL, n = 28) and 48.2% (probiotic, n = 29) of the total variance with Q 2 values of 0.708 (GDL) and 0.767 (probiotic). Q 2 is the cross-validated predictive ability which is related to the statistical robustness of the model. Models were constructed with one predictive component and 4 orthogonal components. Consequently, both PCA and OPLS-DA showed a difference between baseline and post-treatment for both treatment groups. A PCA model was also constructed on post-treatment samples including both the probiotic-milk-treated patient group and the GDL-milk-treated patient group; however, no difference between the two groups could be observed (data not shown).
To sort out which metabolites were responsible for the observed differences between baseline and post-treatment groups, we investigated the S-plots obtained for both OPLS-DA analyses (Fig. 3). The resulting S-plots show the covariance and correlation of the different TICs included in the model. The two S-plots displayed a high degree of similarity. Metabolites showing a correlation of |R| > 0.5 and a covariance above 0.18 or below −0.18 were selected. This selection was based on the appearance of the S-plot, where three metabolite clusters were separated from the rest. Identification of the peaks revealed that the same metabolites were affected in both treatment groups. Even though metabolite no. 63 appeared to have a higher covariance and correlation in the probiotic-milk-treated patient group than in the GDL-milk-treated patient group, the difference was not high enough to discriminate between the two treatments. Metabolites with negative correlations and covariances had lower levels in the post-treatment samples, and those with positive values had higher levels.
Identification of metabolites important for baseline and post-treatment discrimination. a S-plot for VAST scaled OPLS-DA of baseline and post-treatment samples from GDL-milk-treated IBS patients. b S-plot for OPLS-DA of baseline and post-treatment samples from probiotic-milk-treated IBS patients. Metabolites having a correlation to the discrimination between baseline and post-treatment of |R| > 0.5 coupled with a covariance either above 0.18 or below −0.18 are marked
The changes in the peak areas of the selected peaks (Table 1) between baseline and post-treatment levels were significant (P < 0.04). The mass spectra of the selected peaks were compared with spectra in the National Institute of Standards and Technology (NIST) and metabolite identifications obtained from NIST were confirmed by using chemical standards for the specific metabolites (Table 1). We found several derivates originating from d-glucose to decrease with the treatments, and lactate, aspartic acid, proline, creatinine, glutamine, and one unidentified metabolite to increase. The creatinine found encompasses both blood serum creatinine and creatine, since the condition during derivatization causes creatine to form the cyclic creatinine [31].
Discussion
In the present study, the use of GC–MS for a metabonomics application on human serum was explored. Blood serum samples from irritable bowel syndrome (IBS) patients collected before and after an 8-week intervention period with a daily consumption of 0.4 L acidified fermented milk products with or without probiotics were examined. Independent of the fermented milk product contained probiotics or not, higher levels of lactate, glutamine, proline, creatine/creatinine, and aspartic acid were detected in the serum after the diet intervention period. In addition, comparison of pre- and post-intervention serum samples revealed a concomitant lower level of serum glucose for both treatment groups after the intervention period.
We utilized S-plots for the identification of significant metabolites, and the identification of the responsible metabolites for discrimination between baseline and post-treatment in both treatment groups was quite unambiguous with only one glucose derivative in one treatment group falling outside the selection criteria. Lowering the covariance limit could induce the risk of identifying metabolites varying only because of analytical variation and noise [30]. The simple data analysis method used here is useful when performing studies with a large number of samples with good stability and few variables. Correlation and covariance depends on both sample amount and stability; thus, analyses such as the one performed here, with many stable samples, offers a setup ideal for employing OPLS-DA coupled with S-plots for metabolite detection.
Symptom evaluation is subjective and is based on the patient’s own perception of discomfort, and in studies concerning bowel disorder, a high degree of placebo response has been reported [32–34]. The present GC–MS analysis of blood serum revealed that the GDL and the probiotic-milk-treated patient groups responded in a very similar way to the intervention. Although the evidence for efficacy of probiotic treatment in IBS patients is weak, overall symptom improvements have been shown using approximately the same intervention time and probiotic load as we have used in the present study [35]. We therefore believe that the similar response from the two treatment groups indicates an independency of the probiotics added to one of the products. During the intervention period, a subgroup of the probiotic-milk-treated patients reported a faster symptom relief compared with the GDL-treated patients. However, at the end of the diet intervention period, symptom relief was balanced out between the two treatment groups [27]. Comparing this with our results could indicate that the change in the blood serum metabolite profile is correlated with symptom relief. However, based on our analysis, it is not possible to conclude whether the blood serum metabolic response is directly linked to symptom relief, and to further investigate this, samples should be acquired during the intervention period.
In accordance with a previous NMR-based metabonomic study [26], we were not able to detect any influence of the probiotics administered to the patients. Irrespective of probiotics we can conclude that both responders and non-responders in both treatment groups displayed a similar blood serum metabolite response after 8 weeks of intervention.
We have previously used 1H-NMR metabonomics and a standard enzymatic assay to identify lactate and 3-hydroxybutyric acid as metabolites involved the separation between baseline and post-treatment samples [26]. However, by utilizing the higher sensitivity of the GC–MS analysis, we could also identify glucose, glutamine, proline, aspartic acid, and creatine/creatinine to be affected by intake of the fermented milk products. Glucose signals are evident in the 1H NMR spectrum, in particular the anomeric CH protons from α- and β-glucose give rise to characteristic signals. However, unfortunately, the residual water signal interferes with these signals, which may be the reason that glucose was not identified by 1H NMR-based metabonomics. In addition to anomeric glucose signals, the 1H NMR spectrum contains a large region around ~3–4 ppm, where various CH protons arising from carbohydrates and amino acids are overlapping. This may distort the detection of proline, which appear in the same region. Aspartic acid and creatine/creatinine were less concentrated, and therefore, the higher sensitivity in metabolite detection together with a better resolution offered by GC–MS probably explains the reason for detection of changes in these metabolites by GC–MS and not by NMR. In contrast to the 1H-NMR analysis, we were not able to detect 3-hydroxybutyrate by the applied GC–MS method. The reason for this remains unknown.
The present GC–MS metabonomic approach revealed that glucose levels were lower after the 8-week intervention compared with baseline. Both at baseline and after the intervention, the patients had been fasting overnight and consumed a standardized meal 1 h before sample collection. Consequently, the effect on glucose could indicate a difference in blood serum glucose clearance, since blood serum glucose would increase after the consumed meal before sample collection. The release of insulin following nutrient consumption increases the rate of blood serum glucose removal, and after approximately 2 h, postprandial blood serum glucose should have reached a normal level again [36, 37]. We analyzed 1 h postprandial samples; thus, our measurements were performed as blood serum glucose is being removed. Therefore, the lower level of blood serum glucose could be due to an increased rate of removal [38]. Schenk and colleagues reported a more effective clearance of plasma glucose as an effect of an earlier and marked insulin response in the initial 20-min postprandial period [39], and it has also been shown that addition of fermented products in a mixed meal lowers the postprandial glycemia and insulinemia [40]. Further research is needed to clarify whether short-term acidified milk treatment can lead to an earlier postprandial insulin response.
Glutamine is the major energy source for enterocytes in the intestinal tissue and when diet resources are consumed, intestinal tissues rely on circulating glutamine [41]. Intestinal tissues are affected in IBS patients, and the higher levels of glutamine and proline could indicate a change in the energy status of the intestinal tissue as a result of intake of the acidified milk products, as catabolism of glutamine in the intestine produces proline as an end product [42, 43]. Furthermore, the role of aspartic acid as a precursor for oxaloacetate, which is an important intermediate in the TCA cycle, and involvement of creatine and creatinine in muscle energy consumption [31], could indicate that intake of the acidified milk products induces a change in metabolism [44, 45].
Conclusions
Using GC–MS-based metabonomics, we showed that a short-term dietary intervention with acidified milk products resulted in elevated levels of serum lactate, glutamine, proline, creatine/creatinine and aspartic acid, and a decreased level of serum glucose in patients suffering from IBS. Our results confirm a previous report [26] using 1H NMR-based metabonomics where lactate was identified as the major metabolite responsible for the difference between baseline and post-treatment samples, but also unravel new information, as effects on glutamine, proline, creatine/creatinine, aspartic acid, and glucose was not identified by 1H NMR-based metabonomics. Changes in the array of metabolites could indicate a change in the energy metabolism. The effects were observed in both a probiotic and a non-probiotic acidified milk group. This is in good agreement with previously reported symptom relief in both groups after 8 weeks treatment and furthermore indicates that the delivering vector for probiotics, which was acidified milk in this study, can be very important for the study outcome.
References
Nicholson JK, Lindon JC, Holmes E (1999) ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29(11):1181–1189
Xiayan L, Legido-Quigley C (2008) Advances in separation science applied to metabonomics. Electrophoresis 29(18):3724–3736
Weljie AM, Dowlatabadi R, Miller BJ, Vogel HJ, Jirik FR (2007) An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J Proteome Res 6(9):3456–3464
Malmendal A, Overgaard J, Bundy JG, Sorensen JG, Nielsen NC, Loeschcke V, Holmstrup M (2006) Metabolomic profiling of heat stress: hardening and recovery of homeostasis in Drosophila. Am J Physiol Regul Integr Comp Physiol 291(1):R205–R212
Pedersen KS, Kristensen TN, Loeschcke V, Petersen BO, Duus JO, Nielsen NC, Malmendal A (2008) Metabolomic signatures of inbreeding at benign and stressful temperatures in Drosophila melanogaster. Genetics 180(2):1233–1243
Janis MT, Laaksonen R, Oresic M (2008) Metabolomic strategies to identify tissue-specific effects of cardiovascular drugs. Expert Opin Drug Metab Toxicol 4(6):665–680
Hines A, Oladiran GS, Bignell JP, Stentiford GD, Viant MR (2007) Direct sampling of organisms from the field and knowledge of their phenotype: key recommendations for environmental metabolomics. Environ Sci Technol 41(9):3375–3381
Bertram HC, Hoppe C, Petersen BO, Duus JO, Molgaard C, Michaelsen KF (2007) An NMR-based metabonomic investigation on effects of milk and meat protein diets given to 8-year-old boys. Br J Nutr 97(4):758–763
Bertram HC, Duarte IF, Gil AM, Knudsen KE, Laerke HN (2007) Metabolic profiling of liver from hypercholesterolemic pigs fed rye or wheat fiber and from normal pigs. High-resolution magic angle spinning 1H NMR spectroscopic study. Anal Chem 79(1):168–175
Bertram HC, Bach Knudsen KE, Serena A, Malmendal A, Nielsen NC, Frette XC, Andersen HJ (2006) NMR-based metabonomic studies reveal changes in the biochemical profile of plasma and urine from pigs fed high-fibre rye bread. Br J Nutr 95(5):955–962
Lenz EM, Wilson ID (2007) Analytical strategies in metabonomics. J Proteome Res 6(2):443–458
Jiye A, Trygg J, Gullberg J, Johansson AI, Jonsson P, Antti H, Marklund SL, Moritz T (2005) Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem 77(24):8086–8094
Pasikanti KK, Ho PC, Chan EC (2008) Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci 871(2):202–211
Qiu Y, Su M, Liu Y, Chen M, Gu J, Zhang J, Jia W (2007) Application of ethyl chloroformate derivatization for gas chromatography-mass spectrometry based metabonomic profiling. Anal Chim Acta 583(2):277–283
Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M (2003) Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 124(5):1202–1209
Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG (2004) Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum 47(6):876–884
Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R (2005) A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther 22(5):387–394
Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo SS, Rasmussen M, Jarvenpaa SS, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R (2007) Clinical trial: multispecies probiotic supplementation alleviates the symptoms of IBS and stabilises intestinal microbiota. Aliment Pharmacol Ther 27(1):48–57
Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR (2003) A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 17(7):895–904
Niedzielin K, Kordecki H, Birkenfeld B (2001) A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299 V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 13(10):1143–1147
Akehurst R, Kaltenthaler E (2001) Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut 48(2):272–282
Drossman DA, Camilleri M, Mayer EA, Whitehead WE (2002) AGA technical review on irritable bowel syndrome. Gastroenterology 123(6):2108–2131
Foxx-Orenstein A (2006) IBS—review and what’s new. MedGenMed 8(3):20
Ersryd A, Posserud I, Abrahamsson H, Simren M (2007) Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment Pharmacol Ther 26(6):953–961
Francis CY, Morris J, Whorwell PJ (1997) The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 11(2):395–402
Pedersen SMM, Nielsen NC, Andersen HJ, Olsson J, Simrén M, Ohman L, Svensson U, Malmendal A, Bertram HC (2010) The serum metabolite response to diet intervention with probiotic acidified milk in irritable bowel syndrome patients is indistinguishable from that of non-probiotic acidified milk by 1H NMR-based metabonomic analysis. Nutrients 2(11):1141–1155
Simren M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H (2010) Clinical trial: the effect of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome (IBS)—a randomized, double-blind, controlled study. Aliment Pharmacol Ther 31(2):218–227
Trygg J, Wold S (2002) Orthogonal projections to latent structures (O-PLS). J Chemometr 16(3):119–128
Keun HC, Ebbels TMD, Antti H, Bollard ME, Beckonert O, Holmes E, Lindon JC, Nicholson JK (2003) Improved analysis of multivariate data by variable stability scaling: application to NMR-based metabolic profiling. Anal Chim Acta 490(1–2):265–276
Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J (2008) Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 80(1):115–122
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80(3):1107–1213
Enck P, Klosterhalfen S (2005) The placebo response in functional bowel disorders: perspectives and putative mechanisms. Neurogastroenterol Motil 17(3):325–331
Kreisberg RA (1980) Lactate homeostasis and lactic acidosis. Ann Intern Med 92(2 Pt 1):227–237
Pitz M, Cheang M, Bernstein CN (2005) Defining the predictors of the placebo response in irritable bowel syndrome. Clin Gastroenterol Hepatol 3(3):237–247
Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P (2009) A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol 9:15
Daly M (2003) Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr 78(4):865S–872S
Daly ME, Vale C, Walker M, Littlefield A, Alberti KG, Mathers JC (1998) Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet. Am J Clin Nutr 67(6):1186–1196
DeFronzo RA, Ferrannini E (1982) Influence of plasma glucose and insulin concentration on plasma glucose clearance in man. Diabetes 31(8 Pt 1):683–688
Schenk S, Davidson CJ, Zderic TW, Byerley LO, Coyle EF (2003) Different glycemic indexes of breakfast cereals are not due to glucose entry into blood but to glucose removal by tissue. Am J Clin Nutr 78(4):742–748
Ostman EM, Liljeberg Elmstahl HG, Bjorck IM (2001) Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr 74(1):96–100
Watford M (2008) Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr 138(10):2003S–2007S
Bertolo RF, Brunton JA, Pencharz PB, Ball RO (2003) Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284(5):E915–E922
Fujita T, Yanaga K (2007) Association between glutamine extraction and release of citrulline and glycine by the human small intestine. Life Sci 80(20):1846–1850
Burtscher M, Brunner F, Faulhaber M, Hotter B, Likar R (2005) The prolonged intake of l-arginine-l-aspartate reduces blood lactate accumulation and oxygen consumption during submaximal exercise. J Sports Sci Med 4(3):314–322
Lancha AH Jr, Recco MB, Abdalla DS, Curi R (1995) Effect of aspartate, asparagine, and carnitine supplementation in the diet on metabolism of skeletal muscle during a moderate exercise. Physiol Behav 57(2):367–371
Acknowledgments
We acknowledge The Danish Dairy Council for funding the project “A nutrigenomic approach in the elucidation of basic molecular mechanisms for the action of probiotic bacteria”. In addition, the authors acknowledge support from the Danish National Research Foundation, Arla Foods and the Interdisciplinary Nanoscience Center (iNANO) at Aarhus University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, S.M.M., Nebel, C., Nielsen, N.C. et al. A GC–MS-based metabonomic investigation of blood serum from irritable bowel syndrome patients undergoing intervention with acidified milk products. Eur Food Res Technol 233, 1013–1021 (2011). https://doi.org/10.1007/s00217-011-1599-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1599-1