Abstract
Proteinase A (PrA) is one of the most significant vacuolar proteinase in S. cerevisiae, and it plays an important role in S. cerevisiae physiology and metabolism, especially under unfavorable environment. In this study, the differences in pyruvate kinase (PYK) level under fructose-1,6-diphosphate (FDP) induction and ATP synthesis block among SC1 (the wild-type yeast that was industrial Saccharomyces cerevisiae WZ65), SC2 (PEP4 partial deletion) and SC3 (PEP4 complete deletion) were examined. Results showed that the induction caused by FDP clearly increased PYK expression no matter for which strain, but the increasing effect is more significant for SC2 (P < 0.05). The comparative results of intracellular ATP accumulation showed that the induction by FDP may be affected at the presence of PrA. The block experiment of ATP synthesis showed that PYK activities in PEP4-modified strains are lower than that of the wild type, but the intracellular ATP levels in the wild-type one are generally higher than the PEP4-modified strains after rotenone treatment (P < 0.01). This implies that the effect of PrA deficiency on intracellular ATP accumulation was much more pronounced than the effect of rotenone on oxidative phosphorylation. The cell morphology of three strains was comparatively examined by means of transmission electron microscopy (TEM). The PEP4-modified strains possessed more vacuoles, and cell structure were more integrated than the wild-type strain. Current data preliminarily indicated that the deletion of PEP4 gene in industrial S. cerevisiae WZ65 may not only affected PYK expression but also modulated the oxidative phosphorylation flux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteinase A (PrA), a crucial vacuolar enzyme in S. cerevisiae, is coded by yeast PEP4 gene. This enzyme is essential to the S. cerevisiae vacuolar proteolytic system under nutritional stresses, sporulation, and vegetative growth conditions, and it is implicated in the activities of other hydrolases, including proteinase B (PrB), carboxypeptidase (CPY), and aminopeptidase I [1]. Yeast PrA is negatively related to the stability of beer foam, because it can hydrolyze lipid transport protein (LTP1), which is the major protein that can keep beer foam stability [2]. The overexpression of gene Pdr5 ATP-binding cassette (ABC) multidrug transporter has significant effect on the formation of multidrug resistance (MDR) in S. cerevisiae. Normally, Pdr5 is a short-lived membrane protein with a half-life of about 60–90 min; whereas in ΔPEP4 mutant cells, Pdr5 accumulates in vacuoles of stationary-phase ΔPEP4 mutant cells, which means that the degradation of Pdr5 needs vacuolar proteolysis [3]. In previous studies, we found that yeast strains with PEP4 modification have been found to grow slower than the wild-type one [4, 5]. As reported previously, the expression of PEP4 was increased under H2O2 stress; this change could help yeast cells degrade harmful oxidized protein. Additionally, the activity of PrA enzyme increases during chronological aging, and cells lacking PEP4 displayed a shortened lifespan [6]. Thus, it is conceivable that there exist some relationships between PrA and survival ability in S. cerevisiae.
Glycolysis is one of the most basic life activities in in vivo yeast cells, which provides energy for cell growth and division. In this metabolism pathway, yeast pyruvate kinase (PYK) catalyzes the final step in glycolysis flux. This enzyme therefore represents an important control point. In almost every cell type, PYK controls the metabolism flux through the glycolysis pathway, together with the phosphofructokinase-1 (PFK1) [8]. PYK also serves as a switch between the glycolytic and gluconeogenic pathways in certain tissues. The regulation of PYK is important for controlling levels of ATP, GTP, and glycolytic intermediates. The transient inhibition of PYK leads to the accumulation of most glycolytic intermediates and an enhancement of gluconeogenesis [7]. PYK is allosterically activated by fructose 1,6-bisphosphate (FDP)[7, 10]. Pyruvate kinase 1 (Pyk1) of S. cerevisiae is demonstrated to be associated with an immunoprecipitate of yeast protein kinase A holoenzyme and to be phosphorylated in a cAMP-dependent process [9]. Though the regulatory mechanism of S. cerevisiae Pyk1 was complex, there was general agreement that FDP appeared to be a primary regulator of S. cerevisiae Pyk1 [10, 11]. HK (hexokinase), PFK (phosphofructokinase), and PYK are the three key enzymes of glycolytic pathway in yeasts [12]. However, no matter in yeast or in bacterial cells, individual or combined overexpression of genes encoding key glycolytic enzymes did not improve the glycolytic flux [13, 14]. The significant increase in the expression level of PYK did not improve the overall glycolytic flux, which, on the contrary, was slightly reduced. The results show that PYK is an important bottleneck to carbon flux only when glucose becomes limited [15]. Thus, the exact evaluation of PYK’s role in the wild-type and PrA-modified strains should consider the effect of different cultivation conditions. It has been demonstrated that ATP plays an essential role in the two crucial processes of substrate utilization: trans-membrane transportation and substrate phosphorylation [16]. Glycolytic flux is conditionally correlated with ATP presence in S. cerevisiae. It has been reported that the rate of glycolysis is negatively affected by intracellular ATP, indicating that the intracellular ATP is also involved in the regulation of this pathway [17].
In this study, three S. cerevisiae strains constructed in previous investigations [4, 5] were used to compare cell growth, glucose consumption, cell morphology, PYK activity, and intracellular ATP formation under FDP induction condition. Additionally, the block of ATP synthesis was also designed to examine the effect of PrA on the glycolytic pathway. The main objective, thus, is to provide more evidence for explaining the exact role of vacuolar PrA in cell metabolism.
Materials and methods
Reagents and chemicals
Phosphoenolpyruvate, ADP (potassium salts), and NADH (inhibitor free) were purchased from Boehringer; Tris and all phospholipids, and carboxymethyl cellulose were from Sigma. FDP (99.9% purity) was obtained from Fluka. Rotenone was obtained from Sigma Co. ELISA kit used for PYK determination was bought from Sigma Co. Other chemicals were obtained from Sigma Co. (USA) and were of analytical grade. Deionized water was used routinely for preparing all solutions.
Microorganisms
The yeast strains in this work were stored in our laboratory. The industrial brewing yeast, S. cerevisiae WZ65 (wild type, designated as SC1), was provided by China Lion Brewery Group. The mutant strain SC2 with partial deletion of PEP4 gene was previously constructed by Zhang et al. [4]. The PrA-negative mutant from SC2 was completed by Zhang et al. [5], which designated as SC3 in this work. All the three strains were preserved on YPD agar slants at 4 °C.
Culture medium and cultivation conditions
The YPD agar slant was used to be freshly inoculated to the culture medium. The YPD agar composition was composed of 1% yeast extract, 2% peptone, 2% glucose, and 2% agar (w/v). The culture medium having cell collection used YPD medium. The YPD liquid medium used contained 1% yeast extract, 2% peptone, and 2% glucose (w/v). The SD medium (0.67% (w/v) yeast nitrogen base, 2% (w/v) glucose) was used to examine the induction of FDP or rotenone treatment on cell metabolism. For FDP induction or rotenone treatment, FDP or rotenone was supplemented to the SD medium according to the designed concentration.
The three constructed yeast strains preserved on YPD agar slant (4 °C) must be transferred to fresh YPD agar slant (28 °C, 72 h) before use. The seed culture was inoculated with this well-grown yeast cells in the 250-mL flask containing 30 mL of YPD liquid culture medium; after 24 h culturing (28 °C, 120 rpm), the culture seed can be used for the following experiment. Thirty milliliters of SD culture medium (supplemented with FDP or rotenone for the experimental group), placed in 250-mL flasks, was inoculated with 10% (v/v) of the seed culture. The flask cultures were grown for 48 h at 28 °C, and the rotation rate was controlled at 120 rpm. The culture broth or the centrifuged cells sampled herein were used for the following comparison experiments.
Effect of FDP induction on intracellular PYK and ATP in designed industrial strains
Based on the preliminary study, FDP was used to add into SD fermentation broth at the middle stage of exponential growth phase; the final concentration was 0.5 mM/L, the culture broth without FDP was used as the control, and then the fermentation cultures were inoculated at a level of 10% (v/v) and cultivated at 28 °C, 120 rpm. The broth was sampled after 24, 48, and 72 h, respectively. The intracellular levels of ATP and PYK activity were determined as described below.
Effect of rotenone on glucose metabolism and glycolytic enzymes
Pre-cultured cells in the log phase were treated by different concentrations of rotenone (2, 5, 8 and 12 mg/L) that was previously dissolved in dimethyl phthalate; the cells were then cultivated for 48 h under aerobic conditions, and SD culture without rotenone served as the control. All cultivations were performed at 28 °C, 120 rpm,and 48 h [18]. After 48 h of cultivation, the culture broth was centrifuged and the supernatant was used for glucose determination. The collected cells were used for the determination of intracellular ATP level and glycolytic enzymes.
Measurement of dry cell weight and residual reduced glucose
Cell growth was determined spectrophotometrically as optical density (OD) at 600 nm (Lambda 11, Perkin Elmer, Bodenseewerk, Germany) and converted to dry cell weight (DCW) per liter using a calibration curve (1 OD = 0.282 g/L DCW). The culture sample (10 mL) was centrifuged at 4,000×g for 10 min, cell pellets were washed twice with distilled water, dried to constant weight at 80 °C, and then weighed. Cell-free culture broth was used for the determination of residual glucose.
The concentration of residual glucose in the culture broth was measured using dinitrosalicylic acid (DNS) method [19]. Briefly, 1 mL of the culture supernatant was added into 1.5 mL DNS solution and then boiled for 5 min. The reaction solution was cooled down to room temperature to stop the reaction quickly, and then the solution was diluted to 25 mL and measured at the wavelength of 520 nm. The blank control was set using distilled water instead of culture supernatant.
Preparation of crude enzyme solution and determination of the three key enzymes
Yeast cells were harvested when the middle exponential phase was reached (OD600 nm = 2–3), and were washed with 0.85% (w/v) ice-cold saline and resuspended in 4 mL of 0.1 M ice-cold potassium phosphate buffer (pH 7.5). Crude extracts were prepared by sonication with working 3 s intervals 5 s in ice bath; the same deal was carried out 40 times. Then centrifuged at 4 °C, 10,000g for 5 min; the supernatant was conserved at −20 °C no longer than 30 days. For subsequent determination, the conserved supernatant must be thawed in cold water bath and hold at 0–4 °C [20].
HK was determined according to the method of Barnard with minor modifications [18]. In brief, the reaction mixture (3 mL) consisted of 0.1 M triethanolamine (pH 7.6), 2 mM MgCl2, 8 mM ATP, 15 mM glucose, 0.3 mM NADP+, and 2 U of glucose-6-phosphate dehydrogenase. The mixture was equilibrated at 30 °C for 5 min, and the reaction was started by adding 0.1 mL of cell-free extract solution. The reaction was monitored by measuring the increase in absorbance at 340 nm due to NADPH formation. PFK was assayed according to the method of Davies and Brindle with minor modifications [21]. The reaction mixture (5 mL) contained 5.2 mM glycyl-glycine (pH 7.6), 2 mM MgCl2, 8 mM ATP, 3 mM fructose 6-phosphate, 0.3 mM NADH, 1 U aldolase, 7 U triosephosphate isomerase, and 1 U glycerol-3-phosphate dehydrogenase. The mixture was equilibrated at 30 °C for 5 min, and the reaction was started by adding 0.2 mL of CFE. The rate of NADH oxidation was measured at 340 nm at 30 °C. The determination of PYK activity was performed according to the literature [18]. Briefly, PYK was measured spectrophotometrically (340 nm) at 30 °C in 1 mL of mixture containing 1 mM phosphoenolpyruvate, 2 mM ADP, 5 mM MgCl2, 0.14 mM NADH, 0.5 U lactate dehydrogenase, 50 mM Hepes, pH 7.0. One unit of enzyme is the amount that catalyzes the formation of 1 μmol of product per minute under the designed conditions.
Determination of intracellular ATP concentration
The concentration of intracellular ATP was analyzed using the sensitive bioluminescent luciferase ELISA. This assay is optimized for a sensitive detection of small amounts of ATP in various samples. The luciferase bioluminescent assay includes thermostable firefly luciferase as the substrate and appropriate buffer solutions optimized for the sensitive quantification [22]. Briefly, one milliliter of ATP-releasing agent Ec was added to 1 mL of cell suspension and reacted for 1.5 min. One hundred microliter of ATP standard sample must be added into a bioluminescent tube with 0.8 mL tricine buffer (25 mmol/L). After 0.1 mL luciferase/luciferin mixture was injected into it, the tube must be stirred as soon as possible and put in the light-tight reaction compartment at 25 °C. Then the light output was counted in a liquid scintillation counter for 1 min, using readings presented as counts per minute (CPM). The concentration of ATP is expressed as the count pulse per minute in this work.
Transmission electron microscopy
The sample preparation of transmission electron microscopy (TEM) was conducted as described previously [23]. Briefly, cells were fixed with 2.5% (v/v) glutaraldehyde in phosphate-buffered saline (PBS) (pH 7.0) for 45 min at 4 °C. Cells were further fixed with 2% potassium permanganate in water for 1 h at room temperature and then stained with 2% uranyl acetate for 120 min. Fixed cells were dehydrated with 50, 70, 80, 90, and 95% ethanol and then embedded in Spurr resin (ElectronMicroscopy Sciences, Pa). A diamond knife was adopted to cut 70–90 nm of ultrathin sections, then these sections were stained with lead citrate for 25 min, and examined using a Hitachi JEM-1230 electron microscope, equipped with a high-resolution (4 K × 4 K)-cooled CCD digital camera (Gatan, Inc., Pa, USA).
Statistical analysis
All tests were performed in triplicate. Analysis of variance (ANOVA) was performed using the Duncan’s multiple range tests to compare treatment means. Significance was defined at P < 0.05.
Results and discussion
Comparison of the effect of FDP on cell growth among three strains
The effects of FDP on cell growth by SC1, SC2, and SC3 are comparatively examined (Fig. 1). Data presented in Fig. 1a demonstrated that the induction by FDP can significantly improve cell growth (P < 0.05), especially after 24 h culture broth. Cell density of SC1 was greater than those of SC2 and SC3 after 24 h of cultivation. Without FDP induction (Fig. 1b), cell growth in SC1 was still better than those of SC2 and SC3. The results herein demonstrated that FDP may play an important role in regulating cell growth at the middle and later phases. However, the effect of FDP on cell growth was rarely reported in available literatures. The possible correlation between cell growth and FDP induction is mainly involved in improvement of PYK formation.
Regulatory effect of FDP on PYK in PEP4-modified S. cerevisiae strains
PYK controls the flux of glycolysis in almost every cell type; it catalyzes the essentially irreversible transphosphorylation from phosphoenolpyruvate (PEP) and ADP to pyruvate and ATP, respectively. The product pyruvate feeds into a number of metabolic pathways that places this enzyme at a primary metabolic intersection [11]. PYK showed cooperative kinetics toward the essential activating monovalent cations K+, NH4 +, Mg2+, and phosphoenolpyruvate [11, 25]. In vivo, FDP affects the affinity of pyruvate base for phosphoenolpyruvate. This, in turn, appears to regulate the intracellular content of phosphoenolpyruvate and phosphoglyceric acids [24, 26]. Although the structure and function of PYK in S. cerevisiae cells are well known, the effects of vacuolar PrA on these key glycolytic enzymes are limitedly understood. As two yeast strains are constructed with PEP4 modification, the elucidation of their correlation is investigated in this work (Fig. 1a, b). As PYK is evaluated among three strains, the data indicated that PYK is highly improved in SC2 under FDP induction. As cultivation time lasted longer (48 h), the induction effect of FDP on PYK measured in SC1 and SC2 strains are more significant than that in SC3, which displayed a negative impact of FDP on PYK at 48 h culture time. The deletion of PEP4 (strain SC3) in S. cerevisiae WZ65 may lead to a harmful influence on PYK or/and its enzyme regulation pathway. The present data imply that PrA may have indirect influence on the key glycolytic enzyme, especially for PYK.
Influence of FDP on intracellular ATP formation among three strains
As reported earlier, the concentrations of intracellular ATP and pyruvate were considered to be indicators of physiological state of the yeast in sake mash. The intracellular ATP indicates the vitality and energy charge of cells. Under anaerobic condition, the intracellular ATP in microorganisms is generated by the glycolytic pathway [27]. The dissociation of ATP from hsp70 of S. cerevisiae is stimulated by both Ydj1p and peptide substrates. In the yeast S. cerevisiae, polypeptide substrates and Ydj1p both serve to stimulate ATPase activity of Ssa1p [28]. Glycolytic flux is conditionally correlated with ATP concentration in S. cerevisiae. But Larsson et al. [17] considered that there exists a strongly negative correlation between intracellular ATP and the rate of glycolysis; this suggests that ATP level is involved in the regulation of this pathway. Cronwright et al. [29] have constructed a kinetic model of the glycerol synthesis pathway to gain a clear understanding of and to quantify the extent to which parameters of the pathway affect glycerol flux in S. cerevisiae. The metabolism analysis showed that ATP has the strongest negative response on this system. An increase in the ratio of ATP/ADP to fivefold results in a 10% decrease in glycerol flux. As the ratio of ATP/ADP is halved, the flux through this pathway is increased by approximately 10% [29]. As a consequence, it is necessary to elucidate the relationship between the vacuolar PrA and intracellular ATP. As the results presented in Fig. 2, the concentration of ATP was significantly increased by FDP induction in SC2 as compared to SC1 and SC3, especially at culturing time of 48 h. Nevertheless, without FDP induction, the intracellular ATP level in SC1 was higher than PEP4-modified strains (P < 0.05). The findings herein suggest that PrA modification affects formation of the intracellular ATP. It is well acknowledged that FDP is the inner activator of PYK in higher organisms. As FDP induces the activation of PYK, the overall glycolytic flux was certainly improved. Considering the above data of PYK, we try to make the supposition that the positive relationship is observed between PYK and intracellular ATP after FDP induction. HK and PFK are key enzymes in the control of glycolytic flux. PFK is subject to a number of allosteric regulators, including an inhibition by ATP [17]. Reibstein et al. [30] reported that PFK activity is decreased with the variation of ATP level from 1.5 to 3.1 mM. The data in this study indicated that the partial deletion of PEP4 in industrial S. cerevisiae caused positive effect on PYK activation and ATP formation, when 0.5 mM/L FDP was used as the regulator. In contrast, the reported data demonstrated that the increase in intracellular ATP level may block the glycolytic flux [17, 18]. In a word, the findings derived from this work imply that glycolytic pathway can be regulated through the activation and block of intracellular ATP. Thus, further elucidation on the relationships between glycolytic flux and ATP formation and vacuolar PrA are completely needed.
Comparison of the influence of rotenone on glucose metabolism among three strains
It has been proposed that the control of glycolysis may be executed by certain rate-limiting or flux-controlling glycolytic enzymes, mainly those catalyzing irreversible reactions. Oxidative phosphorylation plays a central role in ATP formation [18, 31, 32]. To compare the differences in glucose consumption rate among three strains of S. cerevisiae, we hypothesize that impairing ATP production by disrupting oxidative phosphorylation might enhance substrate-level phosphorylation, and thus lead to the increased glycolytic flux.
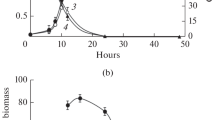
The effects of rotenone, a specific inhibitor of complex I (NADH dehydrogenase), on the glycolytic flux of S. cerevisiae strains are presented in Fig. 3, and the differences in key enzymes with regard to glycolytic flux after shock by rotenone were analyzed (Table 1). In terms of glucose consumption rate among three strains, SC2 had the greatest increased effect on glucose metabolism at the shock of 5 mg/L rotenone, as compared to those of SC3 and SC1 (P < 0.05). At 8 mg/L of rotenone, SC2 had the lowest GCR value. However, the increasing effect of glucose consumption rate is generally insignificant after rotenone treatment (2–12 mg/L). The present study indicated that the block of ATP formation had no significantly improved effect on glucose metabolism, whether PEP4 gene was present or not. Rotenone causes the greatest improved effect on glucose utilization for SC1. ATP had been reported to result in the negative effect on glycolytic flux and key enzymes in S. cerevisiae [17, 33]. The data indicated that the block of ATP formation did not improve glucose metabolism, whether PEP4 gene was present or absent. Nevertheless, more studies are needed in order to further illustrate the intrinsic mechanism underlying the effect of PEP4 on glucose metabolism by industrial S. cerevisiae.
Effect of rotenone on key enzymes with regard to the glycolytic pathway
In previous investigation, the intracellular ATP had a strong negative correlation with the glycolytic flux [20]. Similar results were reported in permeabilized cells of S. cerevisiae [33], in which the main targets of ATP inhibition were PFK and PYK. Recent studies also show that glucose consumption rate is primarily regulated by PFK [18]. However, lower level of intracellular ATP did not actually increase PFK activity and improve glycolytic flux in industrial S. cerevisiae WZ65. But evidences reported herein, that the glycolytic enzymes significantly affect glycolytic flux in industrial yeast, are not yet enough. Results presented in Table 1 showed the differences in key enzymes (HK, PFK, and PYK) with regard to the glycolytic flux among three strains. In terms of PFK variation in Table 1, PFK activity in SC2 was not increased by rotenone treatment in comparison to the untreated, but PFK activities in SC1 and SC3 were both increased as the adding concentration of rotenone was 8 mg/L. This suggests that the inhibition of oxidative phosphorylation in SC2 cannot improve PFK biosynthesis in glycolytic pathway, but inconsistent with that as early reported [18]. Indeed, in a similar pattern, the increase in ATP demand through the inhibition of oxidative phosphorylation leads to an increased glycolytic flux in Torulopsis glabrata [18].
As HK activity was comparatively analyzed, SC2 seemly revealed no improved HK activity by rotenone shock in comparison to those of SC1 and SC3. The activity of HK in SC1 was significantly spurred by the addition of 5 mg/L rotenone, when SC3 was used as the referee (P < 0.05). Current results imply that the glycolytic flux in SC2 is not positively regulated by the block of oxidative phosphorylation. Whatever, it remains to be further demonstrated whether PFK and HK are controlled by cAMP-dependent phosphorylation or through other unknown mechanisms.
Most importantly, the key glycolytic enzyme, PYK was compared among three designed strains. Rotenone had detrimental effect on PYK in glycolytic pathway whether for SC1 or SC2 or SC3. The block of oxidative phosphorylation may have no positive regulation on PYK. Interestingly, a theoretical study by Torres also suggests that, in addition to sugar transport, HK might be an important factor in controlling the flux through the pathway [34]. It remains to make clear, but in any case, it is interesting that the present results are consistent with the low flux control in the PFK and PYK steps, which was suggested by the metabolic model of Torres. Considering the above results, three key enzymes that connected to the glycolytic pathway in SC1 possessed much higher activity than those of SC2 or SC3. Thus, PEP4 modification in industrial S. cerevisiae may cause the delay of cell metabolism.
Effect of oxidative phosphorylation inhibitor on intracellular ATP formation among three yeast strains
The regeneration of ATP in microbial cells occurs in two ways: substrate-level phosphorylation and oxidative phosphorylation. The manipulation of oxidative phosphorylation seems to be a more efficient way to regulate intracellular ATP, because under aerobic conditions, most ATP production comes from oxidative phosphorylation pathway [35]. Further elucidation of the effect of PEP4 on intracellular ATP formation is necessary. Thus, the ATP-blocking experiment was designed to investigate this correlation. Results in Fig. 4 revealed that the formations of intracellular ATP in SC2 and SC3 were significantly inhibited by rotenone treatment in contrast to SC1. The PEP4-modified strains are likely more sensitive to rotenone than the wild-type one. As presented in Table 1, the inhibition of rotenone leads to the damaged effect on key glycolytic enzymes. Glycolysis is activated during the aerobic–anaerobic transition by a drop in ATP, which de-inhibits both PFK and PYK [11]. But the present data seemly show the opposite regulative effect of ATP on glycolytic flux. It speculates that vacuolar PrA may affect not only glycolytic enzymes but also regulate the oxidative phosphorylation that involved into electron formation and transfer.
In this work, the data obtained imply that PrA plays an important role in three key enzymes synthesis by industrial S. cerevisiae, especially for PYK enzyme. It has been shown that PEP4 gene is important for the survival of stationary cells, as it recycles nitrogen in starved cells [36]. The dramatic shortening of the chronological lifespan in cells lacking PEP4 was possibly due to the sum of two detrimental effects: nitrogen starvation and accumulation of damaged proteins [6]. However, the intrinsic mechanism underlying the effect of PrA on the glycolytic flux still remains to be uncovered in future study.
Comparative characterization of cell morphology by transmission electron microscope
Transmission electron microscopy (TEM) has the advantage over SEM that cellular structures of the specimen can be viewed at very high magnifications. The differences in cell structure among three designed yeast cells were examined using TEM (Fig. 5). The cell structure of SC1 possessed less vacuole than the PEP4-modified strains (SC2 and SC3); moreover, some vacuoles in SC1 decomposed and lost the complete structure. Furthermore, the vacuole morphology of SC2 and SC3 remained complete and stable, especially for SC3. The observations in this study imply that PEP4 gene encoding PrA certainly affects the presence and stability of morphology of vacuole in yeast cells. As reported previously, PrA plays a role in the cell endurance that can degrade the vacuole to provide nutritional components under nutritional stress, sporulation, and vegetative growth conditions [1]. Thus, the obtained results in this study show the observed direct correlation between PrA and vacuole structure.
Conclusions
In this study, the influence of PEP4 modification on PYK enzyme and intracellular ATP formation is further examined and compared in the industrial S. cerevisiae WZ65. Results derived from this work clearly indicated that PYK enzyme is directly affected at the presence of PrA. FDP induction led to the improvement of cell growth, but without influence on glucose metabolism. Moreover, the intracellular ATP formation was induced by FDP treatment, especially for SC2. This implies that the partial deletion of PEP4 gene in industrial S. cerevisiae is favorable for ATP synthesis. Though FDP is beneficial for PYK activation in yeast cells, the positive relationship between PYK and PrA was not observed. The inhibition effect was most pronounced for PEP4-modified strains. The block of oxidative phosphorylation reported previously shows the increase in glycolytic flux, which also includes key glycolytic enzymes. However, the effect of PrA on the glycolytic pathway is likely much more pronounced than the regulation of intracellular ATP formation. Possibly, PrA in S. cerevisiae has direct linkage with the glycolytic pathway. Thus, the modification of PEP4 in industrial S. cerevisiae may lead to the delay in cell metabolism. As a consequence, the intrinsic mechanism underlying the regulation of vacuolar PrA on oxidative phosphorylation and substrate phosphorylation remains to be further elucidated.
Abbreviations
- SC1:
-
Industrial Saccharomyces cerevisiae WZ65 (wild-type yeast)
- SC2:
-
Saccharomyces cerevisiae strain with PEP4 partial deletion
- SC3:
-
Saccharomyces cerevisiae strain with PEP4 complete deletion
- PrA:
-
Proteinase A
- PrB:
-
Proteinase B
- PEP4 :
-
A kind of gene that encodes proteinase A
- CYP:
-
Carboxypeptidase
- FDP:
-
Fructose-1,6-diphosphate
- HK:
-
Hexokinase
- PFK:
-
Phosphofructokinase
- PYK:
-
Pyruvate kinase
- ATP:
-
Adenosine triphosphate
- ADP:
-
Adenosine diphosphate
- NADH:
-
Reduced form of nicotinamide-adenine dinucleotide
- TEM:
-
Transmission electron microscopy
- GCR%:
-
Glucose consumption rate, it is the D-value between the original glucose (g/50 mL) in the culturing medium and the residual glucose in the broth (g/50 mL) after certain culturing time divided by the original glucose in the culturing medium (g/50 mL)
References
Parr CL, Keates RA, Bryksa BC, Ogawa M, Yada RY (2007) The structure and function of Saccharomyces cerevisiae proteinase A. Yeast 24:467–480
Wang ZY, He GQ, Liu ZS, Ruan H, Chen QH, Xiong HP (2005) Purification of yeast proteinase A from fresh beer and its specificity on foam proteins. Int J Food Sci Technol 40(8):835–840
Fegner R, Mahé Y, Pandjaitan R, Kuchler K (1995) Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol Cell Biol 15(11):5879–5887
Zhang Q, Chen QH, Fu ML, Wang JL, Zhang HB, He GQ (2008) Construction of recombinant industrial Saccharomyces cerevisiae strain with bglS gene insertion into PEP4 locus by homologous recombination. J Zhejiang Univ Sci B 9(7):527–535
Zhang HB, Zhang HF, Chen QH, Ruan H, Fu ML, He GQ (2009) Effects of proteinase A on cultivation and viability characteristics of industrial Saccharomyces cerevisiae WZ65. J Zhejiang Univ Sci B 9(7):769–776
Marques M, Mojzita D, Amorim MA, Almeida T, Hohmann S, Moradas-Ferreira P, Costa V (2006) The Pep4p vacuolar proteinase contributes to the turnover of oxidized proteins but PEP4 overexpression is not sufficient to increase chronological lifespan in Saccharomyces cerevisiae. Microbiology 152:3595–3605
Jurica MS, Mesecar A, Heath PJ, Shi WX, Nowak T, Stoddard BL (1998) The allosteric regulation of pyruvate kinase by fructose-1, 6-bisphosphate. Structure 15(6):195–210
Allert S, Ernes I, Poliszczak A, Opperdoes FR, Michels PA (1991) Molecular cloning and analysis of two tandemly linked genes for pyruvate kinase of Trypanosoma brucei. Eur J Biochem 200:19–27
Portela P, Howell S, Moreno S, Rossi S (2002) In vivo and in vitro phosphorylation of two isoforms of yeast pyruvate kinase by protein kinase A. J Biol Chem 277:30477–30487
Clive JB, Brian W, Rodney VB (1971) Regulation of pyruvate pinase by fructose 1, 6-diphosphate in Saccharomyces cerevisiae. Eur J Biochem 18:64–69
Muñoza Ma E, Ponce E (2003) Pyruvate kinase: current status of regulatory and functional properties. Compar Biochem Physiol Part B: Biochem Mol Biol 135(2):197–218
Fell D (1997) Understanding the control of metabolism. Portland Press, London
Schaaff I, Heinisch J, Zimmermann FK (2004) Overproduction of glycolytic enzymes in yeast. Yeast 5(4):285–290
Ruijter GJ, Panneman H, Visser J (1997) Overexpression of phosphofructokinase and pyruvate kinase in citric acid-producing Aspergillus niger. Biochim Biophys Acta 1334:317–326
Ramos A, Neves AR, Ventura R, Maycock C, López P, Santos H (2004) Effect of pyruvate kinase overproduction on glucose metabolism of Lactococcus lactis. Microbiology 150:1103–1111
Hearn EM, Patel DR, van den Berg B (2008) Outer-membrane transport of aromatic hydrocarbons as a first step in biodegradation. Proc Natl Acad Sci USA 105:8601–8606
Larsson C, Nilsson A, Blomberg A, Gustafsson L (1997) Glycolytic flux is conditionally correlated with ATP concentration in Saccharomyces cerevisiae: a chemostat study under carbon- or nitrogen-limiting conditions. J Bacteriol 179:7243–7250
Liu LM, Li Y, Li HZ, Chen J (2006) Significant increase of glycolytic flux in Torulopsis glabrata by inhibition of oxidative phosphorylation. FEMS Yeast Res 6:1117–1129
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–427
Chen QH, Liu XJ, Fu ML, Zhang HB (2010) Effect of PrA encoding gene-PEP4 deletion in industrial S. cerevisiae WZ65 on key enzymes in relation to the glycolytic pathway. Eur Food Res Technol 231(6):943–950
Davies SEC, Brindle KM (1992) Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochem 31:4729–4735
Ronner P, Friel E, Czerniawski K, Fränkle S (1999) Luminometric assay of ATP, phosphocreatine, and creatine for estimation of free ADP and free ATP. Anal Biochem 275(2):208–216
Yang H, Ren Q, Zhang Z (2006) Chromosome or chromatin condensation leads to meiosis or apoptosis in stationary yeast (Saccharomyces cerevisiae) cells. FEMS Yeast Res 8(6):1254–1263
Adah A, Benghuzzi H, Tucci M, Huang D, Franklin L, Adah F (2006) Metabolic effects of fructose 1,6-bisphosphate in normoxic and hypoxic states of MG63 osteosarcoma cells. Biomed Sci Instrum 42:120–125
Hunsley JR, Swelter CH (1969) Yeast pyruvate kinase. J Biol Chem 244:4819–4822
Barwell CJ, Woodward B, Brunt RV (1971) Regulation of pyruvate kinase by fructose 1,6-diphosphate in Saccharomyces cerevisiae. Eur J Biochem 18(1):59–64
Kazuo S, Yukinar Y, Toshiyuki H, Toshiyuki O (2000) On-line measurement of intracellular ATP of Saccharomyces cerevisiae and pyruvate during sake mashing. J Biosci Bioeng 3(90):294–301
Ziegelhoffer T, Lopez BP, Craig EA (1995) The dissociation of ATP from hsp70 of Saccharomyces cerevisiae is stimulated by both Ydj1p and peptide substrates. J Biol Chem 18(270):10412–10419
Cronwright GR, Rohwer JM, Prior BA (2002) Metabolic control analysis of glycerol synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 68(9):4448–4456
Reibstein D, den Hollander JA, Pilkis SJ, Shulman RG (1986) Studies on the regulation of yeast phosphofructo-1-kinase: its role in aerobic and anaerobic glycolysis. Biochemistry 25:219–227
Beauvoit B, Rigoulet M, Bunoust O, Raffard G, Canioni P, Guérin B (1993) Interactions between glucose metabolism and oxidative phosphorylations on respiratory-competent Saccharomyces cerevisiae cells. Eur J Biochem 214:163–172
Senior AE (1988) ATP synthesis by oxidative phosphorylation. Physiol Rev 68:177–231
Larsson C, Pahlman IL, Gustafsson L (2000) The importance of ATP as a regulator of glycolytic flux in Saccharomyces cerevisiae. Yeast 16:797–809
Torres NV (1994) Modeling approaches to control of carbohydrate metabolism during citric acid accumulation by Aspergillus niger: II. Sensitivity analysis. Biotechnol Bioeng 44:112–118
Zhou JW, Liu LM, Shi ZP, Du GC, Chen J (2009) ATP in current biotechnology: regulation, applications and perspectives. Biotechnol Adv 27:94–101
Teichert U, Mechler B, Muller H, Wolf DH (1989) Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J Biol Chem 264:16037–16045
Acknowledgments
This work was financially supported by the National Hi-Tech Research and Development Program (863) of China (No. 2007AA10Z315).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, XJ., Feng, Y., Fu, ML. et al. The shock of vacuolar PrA on glycolytic flux, oxidative phosphorylation, and cell morphology by industrial Saccharomyces cerevisiae WZ65. Eur Food Res Technol 233, 941–949 (2011). https://doi.org/10.1007/s00217-011-1586-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1586-6