Abstract
Aspergillus subolivaceus dextranase is immobilized on several carriers by entrapment and covalent binding with cross-linking. Dextranase immobilized on BSA with a cross-linking agent shows the highest activity and considerable immobilization yield (66.7%). The optimum pH of the immobilized enzyme is shifted to pH 6.0 as compared with the free enzyme (pH 5.5). The optimum temperature of the reaction is resulted at 60 °C for both free and immobilized enzyme. Thermal and pH stability are significantly improved by the immobilization process. The calculated K m of the immobilized dextranase (14.24 mg mL−1) is higher than that of the free dextranase (11.47 mg mL−1), while V max of the immobilized enzyme (2.80 U μg protein−1) is lower than that of the free dextranase (11.75 U μg protein−1). The immobilized enzyme was able to retain 76% of the initial catalytic activity after 5.0 cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dextran is a homoglycan of α-D-1,6-glucopyranose molecules with α-1, 6 linkages with side with side-chains of α-1,2, α-1,3, and α-1,4 to the main chains [1]. Leuconostoc, Lactobacillus, and Streptococcus catalyze a primary reaction for enzymatic synthesis of dextran from sucrose, maltose, and isomaltose producing insoluble glucans [2]. Beside the important of dextran, it has harmful effects on sugar cane and sugar beets containing sucrose in food and sugar industries [3, 4]. Dextrans represent also a structural component of dental plaque that causes the development of dental caries [1]. Simultaneous use of dextran hydrolyzing enzymes could be advantageous to overcome these problems [5, 6].
Dextranase is commonly used to release d-glucose and shorter oligosaccharides causing decrease the de-branching degree and increase the solubility of higher molecular weights glucans removing the undesirable sliming in sugar industry [7] or as possible mouthwash ingredients [8]. Dextranolytic enzymes are also being used in the synthesis of potentially valuable prebiotic oligosaccharides [9]. Dextranase can also be used as universal targeting method for therapeutic agents to activate the cancer antibodies and delayed the efficiency of penicillin and temafloxacin to be active for a long time [1]. Furthermore, hydrolysis of dextran by microbial dextranase is of significant interest in drug formulation, vaccines, cosmetics, and other food industries [1, 10].
Dextranases are produced by various microorganisms, including bacteria [11, 12], yeast [13] and filamentous fungi [1, 5, 14]. Fungal dextranase has attracted much attention due to higher enzyme activity and due to the synthesis of isomaltooligosaccharides (IMOs) [15].
Due to the potential applications of dextranase, the objective of this paper is aimed to immobilize dextranase to improve its thermal, pH and operational stabilities as compared with free enzyme.
Materials and methods
Microorganism and culture maintenance
The fungal strain used in the present study was locally isolated from soil sample on dextran containing medium and identified as Aspergillus subolivaceus by Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, EGYPT. The strain was sub-cultured on modified agar media containing 1.0% dextran as a sole carbon source at 30 °C for 7.0 days and maintained at 4 °C. Induced slant of A. subolivaceus is mixed with 10 mL basal medium for preparing spore suspension. The spore count in the suspension was 2.0 × 107 spore mL−1.
Mode of fermentation and dextranase preparation
According to Shereif et al. [16], extracellular dextranase from A. subolivaceus was obtained through submerged fermentation medium (pH 5.5) containing 1.0% dextran; 0.3% NH4H2PO4; 0.05% KCl; 0.1% KH2PO4; and 0.05% MgSO4–7H2O. In 250 mL Erlenmeyer flask, 49 mL of medium was transferred and autoclaved at 121 °C at 15 lbs for 20 min. The medium after cooling is inoculated by 1.0 mL spore suspension (2.0 × 107 spores) and incubated at 30 °C for 4.0 days in incubating shaker (150 rpm). For obtaining extracellular dextranase, mycelial pellets were filtrated through Gough No. 1 under water pump. The filtrate was collected and stored at deepfreeze (−20 °C) as crude enzyme preparation till used for enzyme assay.
Assay of dextranase
Dextranase activity was determined by detecting the amount of liberated reducing sugar from the hydrolysis of dextran (MW 260 kDa; BDH) according to Nelson [17] and Somogyi [18] method using Spectro UV–VIS RS spectrophotometer. Unless specified otherwise, the assay mixture consisted of 0.5 mL dextran (2.0% w/v) in acetate buffer (0.1 M, pH 5.5) and 0.5 mL of enzyme solution or weighed amount of the immobilized enzyme. The reaction was incubated at 60 °C for 20 min. One unit of enzyme activity (U) is defined as the amount of the enzyme that releases 1 μmol of reducing sugars (d-glucose) per minute under assay conditions.
Protein determination
Soluble protein was determined according to Bradford method [19], by measuring optical density of developed color at 595 nm. Optical density was measured against blank. The μg of protein was estimated using standard curve of bovine serum albumin (BSA).
Immobilization methods of dextranase
Carriers for enzyme immobilization
Chitins, gelatin and BSA were from Sigma, and Na-alginate was from BDH. All other chemicals were of analytical grade.
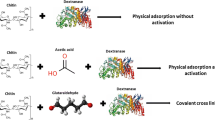
Covalent binding with cross-linking
Chitin (1.0 g) was shaken with 10 mL of 2.5% glutaraldehyde. Chitin was then collected by filtration using a sintered glass funnel and washed with distilled water to remove the excess glutaraldehyde. The wet chitin was mixed with the enzyme solution (80 U; A. subolivaceus dextranase) for 1.0 h at room temperature (25 °C). The unbound enzymes were removed by washing with 0.1 M acetate buffer pH 5.5 [20].
One gram of gelatin or BSA was mixed with 80 units of A. subolivaceus dextranase (50 °C for melting gelatin). Then, 0.7 mL (50% v/v) glutaraldehyde was added, the net concentration of prepared gel was 10% (w/v). The mixture was incubated overnight at 4.0 °C. The resulting gel was washed as described above and then cut into small 0.2 cm3 cubes [21].
Entrapment in Ca-alginate
Eighty units of A. subolivaceus dextranase were mixed with different concentrations of sodium alginate (Pharmacia chemicals) with a final concentration of 3.0, 5.0, and 7.0%. The entrapment was carried out by dropping each alginate solution in 0.1 M CaCl2 solution. The resulting beads were collected, washed with acetate buffer (0.1 M; pH 5.5), and kept in the same acetate buffer at room temperature for 2.0 h to remove unbounded enzymes [20].
Properties of the free and immobilized dextranase
Optimum pH
The optimum pH for free and immobilized dextranase was estimated by incubating enzyme at different pH values (0.1 M acetate buffer was used at a range of pH 4.0–5.5 and 0.1 M phosphate buffer for a range of pH 6.0–8.0 at 45 °C for 20 min using 2.0% dextran as substrate with different controls). Dextranase activity was then assayed as described above.
Optimum temperature
The effect of temperature was studied by incubating both soluble and immobilized dextranase in their respective optimum pH at different temperatures (ranging from 30 to 80 °C) with different controls for 20 min using 2.0% dextran as substrate.
Activation energy (E a )
The activation Energy was determined from the slope of a linear plot of the log of the enzyme activity (v) versus 1/T, according to the Arrhenius law:
The enzyme activity (v) was expressed in U μg protein−1, the temperature (T) in Kelvin (K), the gas constant (R = 1.987), and the activation energy (E a ) in Kcal mol−1.
pH stability
The pH stability of the free and immobilized enzyme was examined after pre-incubating enzyme samples at 25 °C for 30 min at different pH values, followed by adjusting the pH to the value of the standard assay system. The residual activity was assayed under the standard conditions.
Thermal stability
The enzyme samples were incubated in 0.1 M acetate buffer at designated temperatures of 60, 70, and 80 °C for times ranging from 5.0 to 90 min. The residual activity was assayed under the standard conditions for free and immobilized dextranase.
Determination of the half-life (t 1/2)
The half-life of the enzyme activity (t 1/2), which corresponds to the time necessary for the residual enzyme activity to decrease to 50% of its initial value, can be calculated from the equation:
Determination of the deactivation energy (E d )
The deactivation energies of free and immobilized dextranase were determined by plotting the activity data [log of the ratio of Ar (residual activity)/A 0 (initial activity)] as a function of time to obtain the deactivation rate constant (K d ) at each temperature. From Arrhenius equation:
plotting the log of K d as a function of the inverse of the absolute temperature, the energy of deactivation (E d ) is obtained as the product of the slope of the resultant straight line times R, the universal gas constant.
Kinetic values (K m and V max)
Different concentrations of pure dextran (BDH) (1.0–50 mg mL−1) were respectively prepared for dextranase assay. The enzyme activity was determined after 20 min incubation at 60 °C for free and immobilized enzyme. The kinetic values of enzyme (K m and V max) were investigated through Lineweaver–Burk Plot by plotting the relation between different substrate concentrations against the corresponding rate reciprocals using Graph-Pad Prism 5 software.
Operational stability of immobilized dextranase
BSA-immobilized dextranase (1.5 g, wet) was incubated with 5.0 mL 2.0% (w/v) dextran in acetate buffer (0.1 M, pH 6.0) at 60 °C for 20 min. At the end of the reaction, the immobilized enzyme was collected by filtration, washed with distilled water, and re-suspended in 5.0 mL freshly prepared substrate to start a new run. The supernatant fluid was assayed for d-glucose.
Reproducibility
All the experiments were repeated at least four times, and the results were reproducible. The data points represent the mean values within ±5.0% of the individual values.
Results and discussion
Dextranase from A. subolivaceus was immobilized by two methods including: (1) covalent binding with cross-linker on chitin, bovine serum albumin (BSA) or gelatin; and (2) entrapment in Ca-alginate (Table 1). The immobilized enzyme prepared by covalent binding with cross-linker to bovine serum albumin had the highest specific activity (1.56 U μg proteins−1) compared to other carriers with immobilization yield about 66.7%; therefore, it was used in the succeeding part of this work. In this connection, immobilization by covalent binding using a cross-linking agent (glutaraldehyde) probably increases the local surface area, which contributes to minimizing the steric effect around the active site of the immobilized enzyme [22]. In addition, these results are similar to those reported during immobilization of P. funiculosum dextranase [23] and A. aculeatus tannase [24].
The immobilization yields of the immobilized enzyme by entrapment on 3.0, 5.0, and 7.0% Ca-alginate were 55, 73.3, and 83.3%, respectively, while the specific activities were lower as compared to the other used carriers, reaching to 0.4, 0.59, and 0.62 U μg proteins−1. The lower values of dextranase activity with entrapment may be due to enzyme leakage [22]. Similar observations were also reported [23, 25].
The initial specific activity exhibited by free dextranase is 8.13 U μg protein−1, while the specific activity of immobilized dextranase on BSA (1.56 U μg protein−1) was retained about 19.43% of specific activity exhibited by free dextranase. This drop in the specific activity after immobilization may be due to diffusion limitation (i.e., resistance to diffusion of the substrate into the immobilization matrix and resistance to diffusion out of the products), as reflected by the lower apparent activation energy for immobilized dextranase (1.27 kcal mol−1 vs. 2.31 kcal mol−1). Lower activation energy for the immobilized enzyme has been reported to be an indication of diffusional limitations [26]. On the other hand, the immobilization of the enzyme by covalent binding could lead to a decrease in the flexibility of the enzyme molecule, which is commonly reflected by a decrease in catalytic activity [27]. A decrease in specific activity after dextranase immobilization is previously reported [23].
The optimum pH of immobilized dextranase was increased at 5–7 range of pH, reaching its maximal activity at pH 6.0 as compared to pH 5.5 as the optimum for free dextranase (Fig. 1). This result may be attributed to an ionic change around the enzyme active site as result of the immobilization process [28]. The shift of pH optima has been previously reported for other immobilized dextranase [29] and for other immobilized enzymes [25]. Furthermore, both immobilized and free dextranase of Brevibacterium fuscum have the same optimum pH [30].
The profile of pH stability of immobilized and free dextranase (Fig. 2) shows that the activity of immobilized dextranase is shifted to alkaline range and significantly stable at wide range of pH (5.5–8.0) than free enzyme (pH; 5.5–6.0). This result means that immobilized dextranase would be more resistant to pH changes and could be used industrially. This effect may have been caused by the micro-environmental pH of the BSA matrix [22]. On the other hand, immobilized dextranase from P. lilacinum is more stable at low pH (3.5–4.0) and higher pH (6.0–7.5) ranges [31].
The effect of temperature on the activity of the immobilized and free dextranase (Fig. 3) shows that the optimum temperatures are similar namely, 60 °C for free and immobilized dextranase. Similarly, the optimum temperature of P. aculeatum dextranase was 50 °C for both free and immobilized enzyme [32]. On contrary, optimum temperature was shifted from 60 °C for free P. funiculosum dextranase to 80 °C for immobilized enzyme [23].
The temperature data are replotted in the form of Arrhenius plots (Fig. 4). The plots of both free and immobilized dextranase were linear, and the activation energy of immobilized dextranase was lower (1.27 kcal mol−1) than the activation energy of free enzyme (2.31 kcal mol−1). These results are more or less similar to those reported for immobilized and free P. funiculosum dextranase [23]. This decrease in the activation energy may be due to the diffusion limitations of immobilized enzyme [25].
The results in Fig. 5a, b and Table 2 indicate that the immobilization process improved the thermal stability of dextranase relative to free enzyme. For example, the free dextranase was completely inhibited after 10 min incubation at 80 °C, while the immobilized form retained 74.8% of its original activity after the same treatment. The calculated half-lives of free dextranase at 60, 70, and 80 °C are 1.3, 1.43, and 7.2 times faster than those of immobilized enzyme, respectively. Similarly, increase in half-lives times of P. funiculosum immobilized dextranase compared with free enzyme is reported [23]. Results also showed that the immobilized dextranase had the highest thermal stability translated as the longest half-life and the lowest deactivation rate constant at 60 °C. Furthermore, the Arrhenius plot (Fig. 6) for the deactivation energies (E d ) of free and immobilized enzymes raveled that the value of E d of immobilized dextranase (69.8 kcal mol−1) is lower as compared with the value of free enzyme (113.1 kcal mol−1). This decrease in the deactivation energy of immobilized dextranase may be attributed to diffusion limitation at different treated temperatures. In contrast, the deactivation energy of other immobilized enzyme was increased compared with free form [23, 24].
K m (Michaelis constant) and V max (maximum reaction velocity) of free and immobilized dextranase were estimated under optimal pH and temperature by incubating each enzyme at different concentrations of pure dextran (BDH) ranged from 1.0 to 40 mg mL−1. Linweaver Burk plots (Fig. 7) showing that K m of the immobilized dextranase (14.24 mg mL−1) is higher than the value of free dextranase (11.47 mg mL−1), while V max (1/slope) of the immobilized enzyme (2.80 U μg protein−1) is lower than that of free dextranase (11.75 U μg protein−1). This increase in the K m value after the immobilization may be partially due to mass transfer resistance to diffusion into the immobilization matrices and/or to low substrate accessibility to the enzyme active site. On the other hand, fixation of the enzyme on the immobilization matrix could lead to a decrease in the flexibility of the enzyme molecule, which is commonly reflected by a decrease in the catalytic activity [22]. Consequently, the maximum rate of the reaction catalyzed by the immobilized enzymes was lower than that of the free enzyme. Several researchers reported an increase in K m and decrease in V max for dextranase due to immobilization [23, 33]. On the other hand, little increases in K m value after immobilization of dextranase of Chaetomium erraticum is recorded [34].
In this experiment, free and immobilized enzymes were incubated with different metal ions in solution at room temperature for 30 min. Then the residual activity was measured at optimum conditions. The results in Table 3 show that Mg2+, K+, and Ca2+ ions are slightly activate both free and immobilized dextranase. While Hg2+ was completely inhibited the activities of both free and immobilized dextranase. BSA as a carrier appears to protect dextranase against the inhibitory effect of other metal ions; hence, it was generally observed that the inhibitory effects of the ions were less pronounced in immobilized dextranase compared with the free enzyme. This protection may be due to the following: (1) structural changes in the enzyme molecule introduced by the immobilization procedure, lower the accessibility of inhibiting ions to the active site of the enzyme and (2) the chelating effect of BSA, which is known to be a very powerful chelating agent, especially when cross-linked with glutaraldehyde forming glutaraldehyde-cross-linked BSA particles [35].
Enzyme inactivation by heavy metals, including mercury (Hg2+), proceeds by the reduction in the thiol group in cysteine residues, with the formation of mercaptides, or the reduction in disulfide bridges, leading to S–Hg–S bonds [36]. These results are in agreement with those obtained for other immobilized dextranase [23]. In this connection, more stability of immobilized Streptomyces anulatus dextranase to Pb+2, Cu+2, Al+3 ions than free enzyme is reported [37].
The operational stability of immobilized dextranase is the most important factor affecting dextran biodegradation in many industrial applications such as food, beverage, and sugar cane industries and other undesirable effects of dextran [32]. The operational stability of immobilized dextranase is evaluated in repeated batch processes. After each run, the immobilized dextranase was washed and reused at optimum conditions for another reaction. The percent of residual dextranase activity was determined for 6 cycles. The immobilized enzyme was able to maintain a good yield of reducing sugars, while drop in activity was possible after many cycles due to the release of bound enzyme from the carrier. The results in Fig. 8 indicate that immobilized dextranase retains 76% of its original activity after 5 cycles. The result also indicated that the loss rate of immobilized dextranase activity was 4.6% cycle−1. These results are more or less similar with that obtained for P. funiculosum immobilized on chitosan [23].
Conclusions
In this study, A. subolivaceus dextranase produced by submerged fermentation on dextran was efficiently immobilized by two methods of entrapment and covalent bonding with cross-linking. Cross-linking within BSA in the presence of glutaraldehyde gave immobilization yield (66.7%) and highest specific activity (1.56 U μg protein−1) compared with the other carriers used. The immobilized dextranase remained stable for longer periods of time and also at higher temperatures as compared to the free enzyme. In addition, pH studies indicated that the enzyme remained significantly active over a broader pH range (5.5–8.0) compared to the free enzyme in solution. The kinetic properties of dextranase revealed a lower affinity of the immobilized enzyme with a higher K m (14.24 mg mL−1) compared to free dextranase. In addition, after five operational cycles, it was observed that the immobilized dextranase retained 76% of its original activity. The properties of the immobilized dextranase described here suggest its value for industrial applications that would not be feasible with the free enzyme system. Further studies will be subject in future works are needed to use immobilized and thermal-stable dextranase in the continuous removal of undesirable dextran molecules in food, juice, sugar cane industries, and oligosaccharide synthesis.
References
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69(2):306–324
Patel S, Goyal A (2011) Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol 27:1119–1128
Eggleston G, Monge A, Montes B, Stewart D (2007) Factory trials to optimize the industrial application of dextranase in raw sugar manufacture part II. Intern Sugar J 109:757–764
Milintawisamai N, Niamsanit S, Ngasan C, Pliansinchai U, Weerathaworn P (2009) Dextran producing microorganisms from Mitr Phuveing sugar factory, Thailand. Sugar Tech 11(2):196–199
Jiménez ER (2009) Dextranase in sugar industry: a review. Sugar Tech 11(2):124–134
Eggleston G, Monge A, Montes B, Stewart D (2009) Application of dextranases in sugarcane factory: overcoming practical problems. Sugar Tech 11(2):135–141
Lee JH, Kim SH, Cho DL, Day DF, Kim D (2006) Treatment of a glucanhydrolase from Lipomyces starkeyi for the removal of soluble polysaccharides in sugar processing. J Microbiol Biotechnol 16:983–987
Kim D, Ryu SJ, Son EJ, Chung HJ, Kim SH, Kim DW, Day DF (2002) Glucanhydrolase from Lipomyces starkeyi KSM 22 as potential mouthwash ingredient. J Microbiol Biotechnol 12:993–997
Chen L, Zhou X, Fan W, Zhang Y (2007) Expression, purification and characterization of a recombinant Lipomyces starkey dextranase in Pichia pastoris. Protein Expres Purif 58:87–93
Goulas AK, Cooper JM, Grandison AS, Rastall RA (2004) Synthesis of isomaltooligosaccharides and oligodextrans in a recycle membrane reactor by the combined use of dextransucrase and dextranase. Biotechnol Bioeng 88:778–787
Kim Y, Ko E, Kang H, Kim D (2009) Construction, expression and characterization of fusion enzyme from Arthrobacter oxydans dextranase and Klebsiella pneumoniae amylase. Biotechnol Lett 31:1019–1024
Lee JH, Nam SH, Park HJ, Kim YM, Kim N, Kim G, Seo ES, Kang SS, Kim D (2010) Biochemical characterization of dextranase from Arthrobacter oxydans and its cloning and expression in Escherichia coli. Food Sci Biotechnol 19(3):757–762
Millson SH, Evans IH (2007) Multiple dextranases from the yeast Lipomyces starkeyi. Antonie van Leeuwenhoek 92:399–404
Erhardt FA, Stammen S, Jordening H (2008) Production, characterization and (co-) immobilization of dextranase from Penicillium aculeatum. Biotechnol Lett 30:1069–1073
Thitaram SN, Chung CH, Day DF, Hinton A, Bailey JS, Siragusa GR (2005) Isomaltooligosaccharide increases cecal Bifidobacterium population in young broiler chickens 1. Poult Sci 84(7):998–1003
Sherief AA, El-Tanash AB, El-Baz E (2010) Production and characterization of dextranase from some local isolated fungi. Mansoura J Biol 36(2):33–49
Nelson N (1944) A photometric adaptation of Somogyi method for determination of glucose. J Bio Chem 153:375–380
Somogyi M (1952) Notes on sugar determination. J Bio Chem 195:19–23
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Abdel-Naby MA (1993) Immobilization of Aspergillus niger NRC 107 xylanase and β-xylosidase and properties of the immobilized enzymes. Appl Biochem Biotechnol 38:69–81
Ghosh M, Nanda G (1993) Thermostability of β-xylosidase from Aspergillus sydowii MG49. Federation of European Biochemical Societies (FEBS) 330(3):275–278
Cao L (2005) Carrier-bound immobilized enzymes: principles, application and design. WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim. ISBN:3-527-31232-3
Abdel-Naby MA, Ismail S, Abdel-Fattah AM, Abdel-Fattah AF (1999) Preparation and some properties of immobilized Penicillium funiculosum. Process Biochem 34:391–398
El-Tanash AB, Sherief AA, Nour A (2011) Catalytic properties of immobilized tannase produced from Aspergillus aculeatus compared with the free enzyme. Braz. J Chem Eng 28(3):381–391
Yu X, Li Y, Wang C, Wang D (2004) Immobilization of Aspergillus niger tannase by micro encapsulation and its kinetic characterization. Biotechnol Appl Bioc 40:151–155
Kitano H, Nakamura K, Ise N (1982) Kinetic studies of enzyme immobilized on anionic polymer lattices. J Appl Biochem 4:34–40
Gottschalk N, Jaenicke R (1991) Authenticity and reconstitution of immobilized enzymes: characterization and denaturation/renaturation of glucoamylase 11. Biotechno Appl Bioc 14:324–335
Krajewska B, Leszko M, Zaborska W (1990) Urease immobilized on chitosan membrane: preparation and properties. J Chem Technol Biot 48:337–350
Ramesh V, Singh C (1980) Bacterial dextranase immobilized on zirconia coated alkylamine glass using glutaraldehyde. Biochem Biophys Res Commun 97:779–786
Sugiura M, Ito A (1975) Studies on dextranase. VIII. Some enzymatic properties of immobilized dextranase from Brevibacterium fuscum var. dextranlyticum. Chem Pharm Bull 23(12):3223–3227
Aslan Y, Tanriseven A (2007) Immobilization of Penicillium lilacinum dextranase to produce isomaltooligosaccharides from dextran. Biochem Eng J 34:8–12
Madhu GLS, Prabhu KA (1985) Studies on dextranasa from Penicillium aculeatum. Enzyme Microb Technol 6:217–220
Rogalski J, Szczodrak M, Pleszczyhka J, Fiedurek J (1997) Immobilisation and kinetics of Penicillium notatum dextranase on controlled porous glass. J Mol Catal B-Enzym 3:271–283
Erhardt FA, Jördening HJ (2007) Immobilization of dextranase from Chaetomium erraticum. J Biotechnol 131(4):440–447
Kennedy JF, Kalogerakis B (1980) Immobilization of glucoamylase on gelatin by transition-metal chelation. Biochimie 62(8–9):549–561
Vallee BL, Ulmer DD (1972) Biochemical effects of mercury, cadmium, and lead. Ann Rev Biochem 41:91–128
Mahmoud D, Helmy W (2009) Application of cold-active dextranase in dextran degradation and isomaltotriose synthesis by micro-reaction technology. Aust J Basic Appl Sci 3(4):3808–3817
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
El-Tanash, A.B., El-Baz, E. & Sherief, A.A. Properties of Aspergillus subolivaceus free and immobilized dextranase. Eur Food Res Technol 233, 735–742 (2011). https://doi.org/10.1007/s00217-011-1570-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1570-1