Abstract
To identify chemical parameters that might be used as discriminators, pH, soluble solids, caffeine, trigonelline, total caffeoylquinic acids, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, total dicaffeoylquinic acids, 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, total feruloylquinic acids, 3-O-feruloylquinic acid, and 5-O-feruloylquinic acid were measured in Arabica and Robusta coffees submitted to three roasting levels. It was found that the fraction of soluble solids increased with roasting level, being slightly higher in Robusta roasted coffee. The contents of caffeine did not vary significantly between roasting degrees within the Arabica and Robusta samples, respectively, revealing a considerable stability during browning. The contents of trigonelline in Arabica and Robusta coffee decreased significantly with browning intensification. Overall, the levels of chlorogenic acids remained higher in Robusta roasted coffee beans but decreased sharply with roast increase. With roasting intensification, the ratio of total caffeoylquinic acids, total dicaffeoylquinic acids, and total feruloylquinic acids varied markedly in both species, with the proportion of total caffeoylquinic acids and total feruloylquinic acids increasing significantly, whereas the opposite occurred with dicaffeoylquinic acids. One can conclude, through the application of a multivariate analysis, that these chemicals form four clusters, constituting caffeine, trigonelline, total dicaffeoylquinic acids, and total feruloylquinic acids a relevant group for T3 roasting level discrimination, in both coffee species. Additionally, detailing discriminators for roasting intensity in Arabica coffee might be caffeine, trigonelline, 3-O-caffeoylquinic acid, and 4-O-caffeoylquinic acid, whereas in Robusta roasted coffee are trigonelline, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, 3-O-feruloylquinic acid, and 5-O-feruloylquinic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In green beans of C. arabica and C. canephora (Arabica and Robusta coffees, respectively), pH may vary between 5.26 and 6.13 [1], while soluble solids represent 23.85–27.31 and 26.07–30.60% of biomass, respectively [2]. In coffee beans, caffeine prevails in the cytoplasm of parenchyma cells, associated with chlorogenic acids, forming a potassium chlorogenate complex. Caffeine usually varies between 0.8 and 1.4% in C. arabica seeds and 1.7–4.0% in C. canephora (considering both Robusta and Conilon cultivars) [3]. Plants that produce seeds almost completely free of caffeine (0.08%) were also reported for C. arabica [4], while exceptionally high content values (5.5%) were detected in C. canephora [5].
Moreover, trigonelline content ranges between 0.3 and 1.3% d.b. [6]. Trigonelline has little direct influence (weak bitter taste) but its thermal degradation products contribute for final coffee aroma [7, 8]. The chlorogenic acids further accumulate in the cytoplasm of epidermal cells, but larger quantities can also be found in the periplasm. In green coffee beans, caffeoylquinic acids, dicaffeoylquinic acids, and feruloylquinic acids account for about 98% of total chlorogenic acids [9].
After roasting, the amount of caffeine in the seed slightly decreases, while chlorogenic acids and trigonelline are particularly degraded [3, 10]. Indeed, a large number of aromatic compounds result from the thermal decomposition of chlorogenic acids, namely phenolic esters, carbonyl compounds, esters, and polycyclic compounds [11], which contribute to flavor, acidity, and astringency of coffee drink [8]. In this context, hydroxycinnamic acids, which are particularly common in coffee beans, especially as chlorogenic acid in the double form of caffeine and potassium chlorogenate [10, 12], are significantly destroyed during roasting, with the release of alkaloids [13]. Additionally, 50–80% of trigonelline can be degraded during roasting [7, 14], with the formation of other compounds, namely nicotinic acid, pyridine, 3-methyl-pyridine, methyl ester of nicotinic acid, and the decarboxylated derivative 1-methylpyridinium as a major reaction product of trigonelline subjected to pyrolytic conditions [15].
It has long been known that the chemical composition of roasted coffee beans mostly depends on the temperature and duration of the roasting procedure [13]. Accordingly, this work aims to identify chemical discriminators that might be applied to differentiate the roasting intensity of commercial Arabica and Robusta coffee beans. The chemical composition of Arabica (from Brazil) and Robusta (from India) coffee beans after applying three levels of roasting levels was analyzed. For that was used a multivariate analysis in order to identify chemical clusters that might be usefully used as discriminators of roasting intensity and quality.
Materials and methods
Materials
Sampling from green coffee bags of Coffea arabica (from Brazil) and Coffea canephora (from India), selected because they are the two major trading species, was carried out according to the Instrução Normativa No 8 [16], NP 1666 [17], and ISO 4072 [18] and as recommended by the ICO (International Coffee Organization). The sampling process began with the selection of green coffee bags [19], at random (a minimum of 10% of the lot). The selected bags were separated from the lot, and ca. 30-g samples of coffee were collected in triplicate from three different points (top, middle, and bottom) of each bag. After extraction and homogenization, the portions were joined, for an overall take of green coffee, with a minimum mass of 1.5 kg. Arabica and Robusta green coffee samples were then submitted to three levels of roasting, T1, T2, and T3, at 220 ± 10 °C, for 7, 9, and 11 min, respectively. These samples were then used for all subsequent studies.
Chemical characterization
Soluble solids and pH were measured according to AOAC [20, 21], at 25 °C. Ground roasted coffee (10 g ± 0.1 mg) mixed with water (200 mL) was boiled for 5 min and cooled at room temperature and the weight adjusted by adding water. After filtration (Whatman no 1 filter), the pH was measured at ca. 25 °C. For the quantification of soluble solids, 25 mL of the filtrate was evaporated to dryness and the residue placed in an oven at 105 °C and finally in a desiccator for cooling and weighed. Data are the average of triplicates for each sample of roasted coffee.
Caffeine and trigonelline contents were measured according to ISO 10095 [22]. Ground roasted coffee (1 g ± 0.1 mg) and magnesium oxide (4.5 ± 0.5 g) were homogenized in water (100 mL) and placed in a water bath at 90 °C for 20 min, with continuous stirring. After cooling, the volume was restored to the original level and the mixture was filtered (Whatman no 1 filter) without washing the solid residue. A 2-mL aliquot of the mixture was added to 10 mL of distilled water and filtered through a 0.45-μm filter. Caffeine and trigonelline were quantified in an integrated HPLC system (Waters, equipped with UV–VIS detector, model 440, column Lichrosorb 100 RP-18 from Merck, 5 μm particle size, 4 mm × 250 mm), using the 32 Karat Software (version 7.0, Beckman Coulter, Inc). The elution of 20-μL aliquots was performed with 20 mM phosphate buffer (pH 4.3) and acetonitrile solution (9:1), using a 1 mL min−1 isocratic flow rate and a column temperature of 25 °C. Detection was performed at 254 nm, and the retention times for trigonelline and caffeine were 2 and 13 min, respectively (Fig. 1). For identification and quantification, standard curves were built with concentrations ranging between 8 and 1,000 μg mL−1 for caffeine and between 8 and 500 μg mL−1 for trigonelline. All extractions and chromatographic analysis were performed in triplicate.
Chlorogenic acids were analyzed as described elsewhere [20]. Briefly, after mixing 2 g ± 0.1 mg of ground roasted coffee with 10 mL of methanol/water (40:60), and mechanical agitation for 30 min, the mixture was centrifuged (9,400×g, 5 min, 25 °C). The supernatant was decanted, 1 mL of Carrez solutions I (aqueous solution of zinc acetate dihydrate and glacial acetic acid, 10.95 g and 1.5 mL, respectively, to a final volume of 50 mL) and II (aqueous solution of 5.3 g of potassium hexacyanoferrate II trihydrate in a final volume of 50 mL) was added for clearing, and thereafter, a methanol/ water (40:60) solution was added to a final volume of 100 mL. After 15 min, the mixture was filtered (Whatman filter no 1) and an aliquot of 10 mL was taken and filtered (PVDF filter, 0.45 μm) prior to a reversed-phase HPLC analysis (Beckman System Gold system) using an end-capped, C18, 5-μm Spherisorb S5 ODS-2 column (250 × 4.6 mm) and the 32 Karat Software (version 7.0, Beckman Coulter, Inc). The elution of 20-μL aliquots was performed at ca. 25 °C, over 45 min with a 1 mL min−1 flow rate, using a linear gradient of 20–70% methanol in 100% tripotassium citrate buffer solution (10 mM, pH 2.5). The column was allowed to re-equilibrate for 10 min. Detection was carried out at 325 and 330 nm using a diode-array detector (mod.168, Beckman). Chromatograms showing the retention times of chlorogenic acids are shown in Fig. 2.
For the isomerization of chlorogenic acid (5-CQA), in order to obtain 3-CQA and 4-CQA standards, 200 mg of 5-CQA standard was solubilized in 20 mL of distilled water and the pH was adjusted to 8 with ammonium hydroxide (4 M). Then, the solution was boiled for 30 min in a water bath and cooled and the pH adjusted to 2.5 with HCl (4 M). After filtration, samples were used for quantification. The identification of chromatographic peaks and quantification of results were carried out using standard solutions of 5-CQA. To identify the isomers 3-CQA and 4-CQA, the standard 5-CQA isomer was subjected to isomerization, as already described. The peaks appeared with the following sequence: 3-CQA, 3-FQA, 4-CQA, 5-CQA, 4-FQA, 5-FQA, 3,4-diCQA, 3,5-diCQA, and 4,5-diCQA. The calibration curve was obtained from 5-CQA with readings at 325 and 330 nm. The quantification was done assuming the peak areas as a reference and comparing them with the standard 5-CQA. To quantify each compound, the following equation [23, 24] was used: \( {\mathbf{c}} = \frac{{{\text{Fr}} \times \varepsilon_{1} \times {\text{Mr}}_{2} \times {\rm A}}}{{\varepsilon_{2} \times {\text{Mr}}_{1} }} \) (being: c, the concentration of the isomer to quantify, in mg L−1; Fr, the response factor of the standard 5-CQA in mg L−1 per unit area; ε1, the molar absorption coefficient of the standard 5-CQA in L mol−1 cm−1; ε2, the molar absorption coefficient of the isomer to quantify, in L mol−1 cm−1; Mr2, the molecular weight on the isomer under study—CQA = 354.31 g mol−1, FQA = 368.28 g mol−1, diCQA = 516.44 g mol−1; Mr1, the molar mass of acid 5-CQA; A, the peak area of the isomer to quantify). The molar absorption coefficients (3-CQA = 18,400, 4-CQA = 18,000, 5-CQA = 19,500, 3,4-diCQA = 31,800, 3,5-diCQA = 31,600, 4,5-diCQA = 33,200, with λ = 330 nm; 3-FQA = 19,000, 4-FQA = 19,500, 5-FQA = 19,300, with λ = 325 nm) indicated in [23, 24], in L mol−1 cm−1, were used. Data were within the detection limits of the method. All extractions and chromatographic analysis were performed in triplicate.
Statistical analysis
Data were statistically analyzed using a two-way ANOVA (p ≤ 0.05). Based on the ANOVA results, a Tukey’s test was performed for mean comparison, for a 95% confidence level. Different letters in the tables indicate significant differences for 95% confidence level.
The multivariate analysis was carried out with STATISTICA 6.0 software Copyright StatSoft, Inc., following several authors [25–29].
Results and discussion
The generation, volatilization, and degradation of acids occur simultaneously during roasting [11]. However, depending on the amount of acids formed during the pyrolysis of the coffee beans, the pH of different coffee genotypes can vary in a similar roasting [2]. In this context, it was found that pH reached a minimum value both for Arabica and for Robusta coffees at T2 roasting level, whereas the highest value was developed at T3 roasting level (Table 1). Such highest values would eventually result from the lower proportion between total aliphatic acids and bean mass, as inferred from [30]. Independently of the roasting level applied, all pH values fall in the characteristic range observed among different genotypes [2, 11, 31].
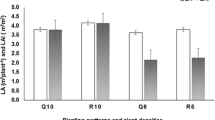
The fraction of soluble solids also increased with roasting level, with a tendency to display values slightly higher in Robusta roasted coffee (Table 1). Although organic acids losses and chemicals volatilization during pyrolysis might trigger soluble solids losses, the raise of the later might prevailed after roasting due to the increased cell breakage of coffee beans, which implicates re-solubilization of celluloses and carbohydrates as well as protein denaturation [2].
During coffee roasting, the bean temperature rises up to about 200 °C, mostly due to a combination of external with internal heating (as temperature changes inside the roaster between 170 and 230 °C). Therefore, as temperature value surpasses the temperature of sublimation of caffeine (178 °C), considerable losses of this compound would be expected [32]. However, during roasting, the sublimation point of caffeine also increases due to the internal pressure rise, triggered by carbon dioxide synthesis and low vapor diffusion produced through the outer layers of the grain [32]. In this context, it was found that the contents of caffeine in Arabica and Robusta roasted coffees, although significantly different among them (Table 1), revealed a considerable stability during browning, close to values observed in other beans from different geographical origins [32–34]. Nevertheless, the caffeine levels were above the minimum (0.7%) required for marketing [35]. Caffeine contents of roasted coffees are similar to others [35, 36].
The contents of trigonelline found in the present work in Arabica and Robusta beans were similar to what was previously found in other coffees from different origins [34, 37]. These values strongly decreased with browning intensification (Table 1), showing 43% (Arabica) and 63% (Robusta) in T3 as compared to T1. That would lead to pyridines, N-methylpyrrole and, due to demethylation, niacin synthesis [32]. The degradation of trigonelline, which was more pronounced in roasted Arabica coffee (except roasting level T1), would result from the physical and chemical characteristics of grains of each type of coffee [32]. The values obtained for roasted coffees are similar to others [35].
The roasting also promoted severe decreases in CGA contents, as a result from various reactions, such as acyl migration, hydrolysis, oxidation, fragmentation, polymerization, and association with denatured proteins [38]. That led to lactone synthesis due to the dehydration of quinic acid residue, with the consequent formation of a ring, resulting from the participation of acid substituent groups at position C1 and alcohol in position C5. In this context, following the increase in roasting intensity, 3-CQA, 4-CQA, and 5-CQA decreased gradually to 17–20% in Arabica and to 27–37% in Robusta beans, thereby determining the sharp decrease in CQAtotal. These losses were stronger in Arabica, which showed simultaneously the lowest values (Table 2). 5-CQA contents are similar for Arabica, but lower for Robusta than those obtained by others [35].
Concerning 3,4-diCQA, 3,5-diCQA, and 4,5-diCQA, a even higher susceptibility to degradation was observed. In fact, in T3 treatments, the values ranged from 5 to 8% in Arabica and 10–14% in Robusta, when compared with T1, and also remained significantly higher in Robusta beans (Table 3).
Although less affected than CQAs and diCQAs, the FQA values were significantly reduced under higher roasting conditions, with 5-FQA being more reduced than 3-FQA, while 4-FQA can be considered irrelevant, as also found by others [10, 37–39]. As for the CQAs and diCQAs, the FQAtotal of Robusta was less affected and a significantly higher value persisted when compared with Arabica beans (Table 4).
Our data showed that among CGA compounds, the diCQAs are the most sensitive, being severely degraded with more intense roasting, while FQAs, although strongly affected, suffered a lower reduction, thus increasing its relative weight (Tables 2, 3, 4). Furthermore, in the overall CGAs of roasted coffees, the CQA fraction maintained the highest relative weight, prevailing 5-CQA.
To reduce the large number of variables (pH, soluble solids, caffeine, trigonelline, CQAtotal, di-CQAtotal, and FQAtotal) to a smaller number of factors for data modeling, a cluster analysis using a linkage distance around 1.97 and 2.08 (Fig. 3a, b) allowed a discrimination of roasting T3 from T1 and T2, in Arabica and Robusta samples. In this context, considering this critical design and the correlation across the hierarchical levels, in both coffee species, the differentiation of roasting T3 was related to higher values for pH and soluble solids (Table 1), whereas lower values were detected for trigonelline, CQAtotal, diCQAtotal, and FQAtotal (Tables 1, 2, 3, 4). The differentiation between these species was further linked to higher values of pH, soluble solids, caffeine, CQAtotal, diCQAtotal, and FQAtotal in Robusta roasted coffee, while the opposite occurred with trigonelline. To select a subset of variables from all variables set, (i.e., considering the highest correlations with the principal component factors), data suggest that, following the patterns in each roasting degree, the parameters can be subdivided into four groups in both coffee species, according to their importance for discrimination (Figs. 3, 4). Following this hierarchical clustering, in both species, although the relevance of pH, soluble solids, and CQAtotal content might be minimized (Figs. 4, 5), caffeine, trigonelline, di-CQAtotal, and FQAtotal constitute a relevant group for roasting T3 discrimination (Fig. 4). Moreover, further assessing the significant variations (Tables 1, 2, 3, 4), T3 roasting treatment can be differentiated in both species, as the detailing discriminators in Arabica roasted coffee are caffeine, trigonelline, 3, CQA, and 4-CQA (Fig. 4b), whereas in Robusta roasted coffee are trigonelline, 3-CQA, 4-CQA, 5-CQA, 3,4-diCQA, 3,5-diCQA, 4,5-diCQA, 3-FQA, and 5-FQA (Fig. 3b).
Arabica coffees dendogram of roasting treatments (T1, T2, and T3) of a pH, soluble solids, caffeine, trigonelline, CQAtotal, diCQAtotal, and FQAtotal based on Euclidean distances between them; b pH, soluble solids, caffeine, trigonelline, the 3-CQA, 4-CQA, 5-CQA, 3,4-diCQA, 3.5-diCQA, 4.5-DICQ, 3-FQA, and 5-FQA, based on the Euclidean distances between them
Robusta coffees dendogram of roasting treatments (T1, T2, and T3) of a pH, soluble solids, caffeine, trigonelline, CQAtotal, diCQAtotal, and FQAtotal based on Euclidean distances between them; b pH, soluble solids, caffeine, trigonelline, the 3-CQA, 4-CQA, 5-CQA, 3,4-diCQA, 3.5-diCQA, 4.5-DICQ, 3-FQA, and 5-FQA, based on the Euclidean distances between them
Considering that factor analysis might reduce space from a larger number of variables to a smaller number of factors and following the chemical characterization of Arabica and Robusta roasted coffee provided in this study (that in general closely follows what is observed worldwide for these parameters in these coffee species), we suggest that the fourth cluster (pH, caffeine, trigonelline, diCQAtotal, FQAtotal, CQAtotal, and soluble solids) might be used as a common discriminator for roasting intensity (Fig. 4a), further considering coffee species (Fig. 4b).
Abbreviations
- CGA:
-
Chlorogenic acid
- CQAtotal :
-
Total caffeoylquinic acids
- 3-CQA:
-
3-O-caffeoylquinic acid
- 4-CQA:
-
4-O-caffeoylquinic acid
- 5-CQA:
-
5-O-caffeoylquinic acid
- diCQAtotal :
-
Total dicaffeoylquinic acid
- 3,4-diCQA:
-
3,4-O-dicaffeoylquinic acid
- 3,5-diCQA:
-
3,5-O-dicaffeoylquinic acid
- 4,5-diCQA:
-
4,5-O-dicaffeoylquinic acid
- FQAtotal :
-
Total feruloylquinic acid
- 3-FQA:
-
3-O-feruloylquinic acid
- 5-FQA:
-
5-O-feruloylquinic acid
- ICO:
-
International coffee organization
References
Leroy T, Ribeyre F, Bertrand B, Charmetant P, Dufour M, Montagnon C, Marraccini P, Pot D (2006) Braz J Plant Physiol 18:229–242
Mendonça LMVL, Pereira RGFA, Mendes ANG (2005) Ciências Tecnologia Alimentar 25:239–243
Nestlé (1991) Coffee. Documentation Centre, Corporate Affairs Department, Nestec Ltd, Vevey, Switzerland, pp 1–60
Silvarolla MB, Mazzafera P, Fazuoli LC (2004) Nature 429:826
Ferrão RG, Ferrão MAG, Fonseca AFA, Pacova BEV (2007) Melhoramento genético de Coffea canephora. In: Ferrão RG et al. (ed) Café Conilon, pp 121–173
Stennert A, Maier HG (1994) Z Lebensm Unters Forsch 199:198–200
Macrae R (1985) Nitrogenous components. In: Clarke RJ, Macrae R (eds) Coffee I: Chemistry. Elsevier Applied Science Publishers Co Ltd, London & New York, pp 115–152
Morais SAL, Aquino FJT, Nascimento PM, Nascimento EA, Chang R (2009) Quim Nova 32:327–331
Clifford MN, Staniforth PS (1977) A critical comparison of six spectrophotometric methods for measuring chlorogenic acids in green coffee beans. In: Huitième Colloque Scientifique International sur le Café. ASIC, Paris, pp 109–114
Viani R (1993) The composition of coffee. In: Caffeine, coffee, and health. S Garatin Raven Press Ltd, New York, pp 17–41
Clifford MN (1987) Chemical and physical aspects of green coffee products. In: Clifford MN, Wilson KC (eds) Coffee, botany, biochemistry and production of beans and beverage. Croom Helm & Methuen Inc, New York, pp 305–374
Clifford MN (1999) J Sci Food Agric 79:362–372
Correia MNG (1995) Manual da tecnologia do café. Cultivar, Associação dos Técnicos de Culturas Tropicais, Porto, p 117
Martin MJ, Pablos F, Bello MA, González AG (1997) Fresenius J Anal Chem 357:357–358
Stadler RH, Varga N, Hau J, Vera FA, Welti DH (2002) J Agric Food Chem 50:1192–1199
Instrução Normativa no 8 (2003) Regulamento técnico de identidade e de qualidade para a classificação do café beneficiado grão cru. Ministério de Estado da Agricultura, Pecuária e Abastecimento, Brasil
NP 1666 (1980) Café—Colheita de amostras de café verde em sacos. Direcção Geral de Qualidade, Lisboa
ISO 4072 (1982) Green coffee in bags-sampling. International Organization for Standardization
PSCB no 36/02 (2002) Code of practice: enhancement of coffee quality through prevention of mould formation. International Coffee Organization
AOAC (1996a) Coffee and tea. AOAC International—Association of Official Analytical Chemists, vol. 2, chapter 30
AOAC (1996b) Definition of terms and explanatory notes. AOAC International—Official methods of analysis, vol. 1, note 11
ISO 10095 (1992) Coffee. Determination of caffeine content. Method using high-performance liquid chromatography. International Organization for Standardization
Trugo LC, Macrae R (1984) Analyst 109:263–266
Farah A, De Paulis T, Trugo LC, Martin PR (2005) J Agric Food Chem 53:1505–1513
Neto JMM, Moita GC (1998) Quim Nova 21:467–469
Alvarenga N, Silva P, Rodriguez-Garcia J, Sousa I (2008) J Dairy Res 75:233–239
Chapman KW, Lawless HT, Boor KJ (2001) J Dairy Sci 84:12–20
Maeztu L, Andueza S, Ibañez C, de Peña MP, Bello J, Cid C (2001) J Agric Food Chem 49:4743–4747
Da Silva AFS, Minim VPR, Chaves JBP, Stringheta PC, Ribeiro MM (2004) Ciências Tecnologia Alimentar 24:468–472
Woodman JS (1985) Carboxylic acids. In: Clarke RJ, Macrae R (eds) Coffee I: Chemistry. Elsevier Applied Science Publishers Co Ltd, London and New York, pp 266–289
Siqueira HH, Abreu CMP (2006) Ciências Agrotecnológicas 30:112–117
Toci A, Farah A, Trugo LC (2006) Quim Nova 29:965–971
Daglia M, Cuzzoni MT, Dacarro C (1994) J Agric Food Chem 42:2273–2277
IC AB (2007) Revisão 15—Norma de qualidade recomendável e boas práticas de fabricação de cafés torrados em grão e cafés torrados moídos. Associação Brasileira da Indústria do Café, Brasil, pp 1–20
Alves ST, Dias RCE, Benassi MT, Scholz MBS (2006) Quim Nova 29:1164–1168
Mori EEM, Bragagnolo N, Morgano MA, Anjos VDA, Yotsuyanagi K, Faria EV, Iyomasa JM (2003) Food Food Ingred J Jpn 208:416–423
Clifford MN (1985) Chlorogenic acids. In: Clarke RJ, Macrae R (eds) Coffee I: Chemistry. Elsevier Applied Science Publishers Co Ltd, London and New York, pp 153–202
Ky LC, Louarn J, Dussert S, Guyot B, Hamon S, Noirot M (2001) Food Chem 75:223–230
Viani R (1986) Coffee. In: Ullmann's Encyclopedia of industrial chemistry, vol A7. VCH Verlagsgesellschaft, Weinheim, pp 315–339
Acknowledgments
The authors wish to thank Drs. Joel I. Fahl and Maria Luíza Carelli (IAC, Brazil) for the supply of seed material, Prof. Dr. Santos Oliveira (FCT/UNL) for his scientific suggestions and NOVADELTA for technical facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bicho, N.C., Leitão, A.E., Ramalho, J.C. et al. Identification of chemical clusters discriminators of the roast degree in Arabica and Robusta coffee beans. Eur Food Res Technol 233, 303–311 (2011). https://doi.org/10.1007/s00217-011-1518-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1518-5