Abstract
To quantify the effect of oxygen concentrations on the quality and antioxidant enzyme system of stored golden needle mushroom, modified atmosphere packaging (MAP) with low and initial superatmospheric oxygen was applied during mushroom storage, and physiological changes associated with postharvest deterioration, activities of antioxidant enzymes, and of cellulase, were monitored during subsequent storage for 0–34 days. Golden needle mushrooms stored in MAP without oxygen or 20–50% oxygen rate had a poorer sensory quality because of chilling injury and physiological injury. These injuries included increased levels of malondialdehyde and superoxide anion whereas some extent of browning was observed. The antioxidant enzyme system, including superoxide dismutase, catalase, peroxidase, and polyphenol oxidase, was activated, to scavenge the reactive oxygen species to reduce injury during the initial storage period. However, these injuries also induced senescence of the stored golden needle mushroom during later storage, followed by a decrease in activities of the antioxidant enzymatic system. The activities of the antioxidant enzymatic system of the mushroom stored in MAP with 80% oxygen rate were the most favorable to delay the senescence process in the later period of storage, and the mushrooms had the best quality until the end of storage. MAP with high oxygen concentrations (e.g., 80% oxygen rate) can induce relatively high antioxidant capacity, significantly decrease postharvest quality loss and improve shelf life of fresh mushrooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Golden needle mushrooms (Flammulina velutipes), having a delicious taste and high nutritional value, is grown extensively, and its consumption in China has increased substantially in recent years [1]. Fresh golden needle mushrooms are highly perishable, so decreasing losses of fresh mushrooms is the main objective of postharvest technology. Fresh mushrooms are still alive and require oxygen for their metabolism. The metabolism may cause some physical and chemical changes such as losses of weight and firmness, browning, veil-opening, sensorial, and nutritional changes, which reduce the value of mushroom especially at room temperature [2].
Modified atmosphere packaging (MAP) and controlled atmosphere (CA) storage can potentially reduce respiration rates [3], but the designs of MAP and CA strongly depend on the respiration process, so it is necessary to optimize fresh-keeping conditions to reduce the respiration rate. Optimal conditions for MAP and CA depend on the metabolic characteristics of the specific product to be stored. Low oxygen levels reduce the rate of metabolic conversion and maintain fruit quality longer than in normal air storage. However, low oxygen levels also have the potential to induce undesirable effects. Undesirable responses include the induction of fermentation, development of disagreeable flavors, reduction in aroma biosynthesis, induction of tissue injury, and alteration in the makeup of microbial flora [4]. Fermentation occurs when ambient oxygen concentrations fall below a critical level, which is typically shown by an increase in respiratory quotient (RQ), ethanol production, or both [5].
Mushroom senescence is an oxidative process that involves degradation of the cellular and sub-cellular structures and macromolecules. Oxidative stress has been implicated in damages in mushroom, fruit, and vegetables during harvesting and storage [6]. Susceptibility to oxidative stress depends on the overall balance between oxidants and antioxidant capability of the cell [7, 8].
Reactive oxygen species (ROS), such as superoxide radical (O2·−), hydrogen peroxide (H2O2), hydroxyl radical (OH·), and singlet oxygen (1O2), have been suggested to play an important role in lipid peroxidation, damage to membranes, oxidization of proteins, and DNA, eventually resulting in senescence [9]. Environmental stress, including exposure to low or high oxygen levels, chilling, and water stress [6, 10], can increase the stationary levels of reactive oxygen–free radicals in cells, and lead to oxidative stress, which has been associated with damage to the stressed mushrooms and plants [11, 12]. Reduced activity of the cell’s antioxidant system causes extensive senescence of plant tissues [13]. The above mechanism forms the basis of physiological changes that can occur in stored mushrooms and may affect their postharvest shelf life. Enzymatic antioxidant systems provide protection against the toxic effects of ROS in the cell, and antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) are involved in the scavenging of reactive oxygen radicals [6, 14]. Of these, SOD is hypothesized to play a crucial role in antioxidant defense because it catalyzes the dismutation of O2·− into H2O2, whereas CAT and POD decompose H2O2 [15].
Liesbeth reported that high O2 atmospheres (exceeding 70% O2) were particularly effective in inhibiting enzymatic browning of fresh-cut vegetables [16]. Strawberries stored at high oxygen atmospheres pressure (>40 kPa) had higher antioxidant capacity, total phenolics, less decay, generally emitted lower levels of volatile compounds and had longer postharvest life than strawberries stored traditionally in air. High oxygen atmospheres (70% O2/30% CO2) resulted in reduced tenderness, juiciness, and proteolysis of meat stored during 14 days [17]. Although several studies have been conducted on production of oxidants and antioxidant activity during senescence, little is known about the effects of low and superatmospheric oxygen on antioxidative mechanisms in stored mushrooms. In our previous studies, a combination of MAP with high oxygen levels and moisture absorbers was effective for fresh-keeping and maintaining the quality of golden needle mushrooms [1]. The present paper investigates the effects of MAP with low and superatmospheric oxygen on the antioxidant enzyme activities and reactive-free radicals of stored mushrooms, specifically on the relationship between antioxidant enzyme activities and quality changes of mushrooms during storage, and the potential value of high oxygen atmosphere packaging (HOAP) storage as a practical technique for extending mushroom shelf life.
Materials and methods
Chemicals
Polyvinylpolypyrrolidone (PVPP), nitroblue tetrazolium (NBT), 2-thiobarbituric acid (TBA), titanium sulfate, diethyl thiocarbamate, bovine serum albumin, and xanthine oxidase were purchased from Sigma St. Louis, MO, USA. Trichloroacetic acid (TCA), polyethylene glycol (PEG), ascorbic acid, guaiacol, and catechol were purchased from Merck Chemicals Co. (Beijing, China). Acetonitrile, alcohol, ammonia, H2O2, and H2SO4 were obtained from Sinopharm Chemical Reagent Beijing Co., Ltd (Beijing, China). The other chemicals used were of analytical grade.
Preparation, packaging, and storage of golden needle mushrooms
Golden needle mushrooms (F. velutipes), produced by a local farm in Beijing (China) and harvested at a commercially mature stage, were purchased and transported to the laboratory within an hour. After cooled for 3 h at 3 °C, 120 g of fresh mushrooms were packed in polyethylene bags (PE package, 30 × 40 cm2, thickness 550 μm) containing 10 g bentonite (a moisture absorber) and 8 g active carbon (a deodorant), which was an optimized condition [1]. Each pack was at atmospheric pressure and supplied with an initial gas mixture [either 100% N2 (without oxygen) or 20% O2 (balanced by N2: 80%, similar to air as a control group) or high oxygen modified atmosphere with 50, 70, 80, 90, 100% O2 (balanced by N2: 50, 30, 20, 10%, 0)]. Subsequently, the packs were heat-sealed (Huhua packing machinery CO., LTD, Wenzhou, China) and stored in darkness at optimum storage temperature 3 °C and RH of 85%. Sampling was performed on days 0, 7, 14, 19, 24, 29, and 34. For each product analysis of quality and antioxidant system, triplicate containers were used in the storage period for each treatment.
Headspace gas analysis and respiration rate measurement
For each treatment, the gas composition during storage was analyzed by sampling 10 μL of the in-package atmosphere at different times, using a syringe. Gases were injected into a gas chromatograph (Agilent GC-6890A, Agilent Technologies, USA), consisting of two manifolds fitted with GC112A-TCD: a molecular sieve 5A PLOT (4 m, 0.32 mm) thermo stated at 40 °C for O2 detection, and warmed up to 80 °C for CO2 analysis. Helium was used as the carrier gas at 550 kPa.
The respiration rate, expressed as mg O2 consumed h−1 g−1 fw (fresh weight) or mg CO2 produced h−1 g−1 fw, could be calculated by the measured depletion of O2 and production of CO2. The respiration measurements were performed in triplicate at different O2-levels.
Ethanol in the headspace was measured using an Agilent GC-6890A gas chromatography equipped with a flame ionization detector (FID) and pp-20000 column. N2 was used as a carrier gas, the flow rate was 2 mL min−1, and the column temperature was 100 °C.
Sensory quality
The sensory quality was evaluated by the method of Liesbeth with some modifications [18]. A panel of ten trained judges evaluated the sensory characteristics of all mushrooms. The typical characteristics of the mushrooms and the possibilities of deterioration were explained before the evaluation began. The general appearance was evaluated on a scale of 9–1, where 9 represents excellent without any rotten or water-soaked spots, 7 represents good without any rotten or water-soaked spots, 5 represents fair and limited marketability with 1–2 rotten or water-soaked spots of 0.5 mm2, 3 represents poor and limited usability with 3–4 rotten or water-soaked spots of 0.5 mm2, and 1 represents very poor and inedible with 5 or more rotten or water-soaked spots of 0.5 mm2.
Firmness measurement
Firmness and softening of mushrooms were determined by penetration test using a Texture Analyser CT3 (Brookfield, USA). After removal of the stem of the mushroom, the cap was compressed with a probe (diameter 50 mm) at 5 mm s−1. The peak stress at 70% compression and the maximum force for extrusion were used to determine firmness of the mushrooms [19].
Analysis of antioxidant enzymes
SOD activity
SOD was extracted from 1 g mushroom tissue, which was sampled from the packages with oxygen rate of 0, 20, 50, 70, 80, 90, 100% on storage days 0, 7, 14, 19, 24, 29, and 34, respectively, ground (used sea sand to deal with samples for 5 min) in 10 mL of 50 mM phosphate buffer, pH 7.8, containing 1% polyvinylpolypyrrolidone (PVPP) at 4 °C and then centrifuged at 15,000g for 15 min at 4 °C. The supernatant was used to determine SOD activity using the method reported by Sujatha [20]. The superoxide radicals were generated by xanthine oxidase and NBT was used as indicator of superoxide radical production. One unit of enzyme activity is defined as the amount of enzyme that gave half-maximal inhibition. In all enzymatic preparations, protein was determined by the method of Lowry using bovine serum albumin (BSA, Sigma) as standard [21].
CAT activity
CAT was extracted from 2.5 g fresh weight of mushroom tissue ground in 25 mL of 50 mM phosphate buffer, pH 7.8 at 4 °C and then centrifuged at 15,000g for 15 min at 4 °C. The supernatant was used to determine CAT activity by the method of Candan [22]. CAT activity was assayed in a reaction mixture containing 50 mM phosphate buffer (pH 7.0) 10 mM H2O2 and enzyme with a Gold S54T UV–Vis Spectrophotometer (Shanghai Lingguang Technology CO. LTD, China) at 240 nm. The unit of CAT activity was defined as the amount of enzyme, which decomposes 1 mmol H2O2 per minute at 25 °C.
POD activity
POD was extracted from 50 g fresh weight of mushroom tissue ground in 50 mL of 50 mM phosphate buffer (pH 7.0) and then centrifuged at 10,000g for 10 min. For POD activity, the guaiacol was used as an electron donor for oxidation [23]. To 0.1 mL of supernatant, 0.09 mL of 30 mM H2O2, 50 μL of 1.5 mM of guaiacol, and 2.75 mL of 50 mM phosphate buffer (pH 7.0) was added. The mixture was shaken gently and the absorbance reading was read after 15 and 30 s at 470 nm. Enzyme activity was evaluated as follows: APOD = A470 × (V × t)−1, where, A470—absorbance at 470 nm, V—volume of the sample, and t—time of the reaction.
PPO activity
PPO was extracted from 20 g mushroom homogenized in 100 mL of 100 mM of phosphate buffer (pH 7.0) containing 10 mM ascorbic acid and polyethylene glycol (1% of mushroom in weight). After filtration of the homogenate through muslin, the filtered material was centrifuged at 10,000g for 20 min at 4 °C; the supernatant was used to determine PPO activity. PPO activity was assayed with catechol as a substrate by a spectrophotometric procedure [24]. The increase in absorbance at 420 nm was recorded for 5 min. One unit of enzyme activity was defined as the amount of the enzyme, which caused a change of 0.001 in absorbance per minute.
Cellulase activity
Cellulase activity (Fpase activity) was determined according to the method of Emtiazi [25]. One microliter of the supernatant (duplicated by section “POD activity”) was added to a test tube containing 0.05 g Whatman No.1 filter paper strip (1 × 6 cm2), and 1 mL 0.05 M citrate buffer pH 4.8 and incubated at 50 °C for 1 h. Then, 2 mL DNS were added to test tube and incubated at 100 °C for 15 min. Then, 1 mL Sodium Potassium tartrate and 5 mL water were added to the test tube, followed by measuring the released glucose by optical density at 575 nm. Enzyme activity was expressed as U mL−1 (the amount of reducing sugars (mM) released mL−1 filtrate/hour).
MDA content determined
Malondialdehyde (MDA) content was determined according to the method of Shah [26]. Fresh tissue (1 g) was ground in 0.25% 2-thiobarbituric acid (TBA) in 10% TCA using a mortar and pestle, which were precooled at 0 °C. After heating at 95 °C for 15 min, the mixture was quickly cooled in an ice bath and centrifuged at 10,000g for 10 min. The absorbance of the supernatant was read at 532 nm and corrected for unspecific turbidity by subtracting the absorbance of the blank at 600 nm. The blank was 0.25% TBA in 10% TCA. The concentration of lipid peroxides together with oxidatively modified proteins of plants was thus quantified in terms of MDA level using an extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 fw (fresh weight).
Superoxide anion assays
The rate of superoxide anion (O2·−) generation was measured following the method of Chaitanya [27]. About 1 g of F. velutipes fresh sample was homogenized in 50 mM sodium phosphate buffer (pH 7.8) containing 1 mM diethyl dithiocarbamate to inhibit SOD activity. After centrifugation at 10,000g for 20 min, O2·− in the supernatant was measured by their ability to reduce nitroblue tetrazolium (NBT). The assay mixture (total 3 mL) contained 100 mM sodium phosphate buffer (pH 7.8), 1 mM diethyl thiocarbamate, 0.25 mM NBT, and the supernatant. The absorbance of the end product was measured at 540 nm in a Gold S54T UV–Vis Spectrophotometer (Shanghai Lingguang Technology CO. LTD), and the O2·− production rate was expressed as nmol g−1 on a fresh weight basis. A standard curve with NO2 was used to calculate the O2·− production rate from the reaction equation of O2·− with hydroxylamine.
Browning degree
Mushroom quality was assessed by the extent of browning of the cap and measured using the reflectometer function of a Hunter Colormeter (Shanghai precision instrument Co. Ltd., Shanghai, China). Each mushroom was measured at three equidistant points from the cap of the mushrooms. To analyze the reflectance data (L), they were first transformed by the function: Y = log n (100 L), where Y can be described as the degree of browning [28]. All experiments were replicated thrice, and the mean values were used in the analysis. Data were subjected to ANOVA and LSD values at P = 0.05.
Statistical analysis
The storage experiment was performed in triplicate per treatment, where each pack constituted a replicate. Results of the quality and antioxidant system were presented as the mean and standard deviation (mean ± SD) and analyzed by a Tukey and Duncan test by applying SAS/STAT® Version 8.2 software (SAS Institute Inc., USA) for Windows XP in order to determine significant differences (P < 0.05) between the applied modified atmosphere packs.
Results and discussion
Respiration rate and gas change in headspace of packs
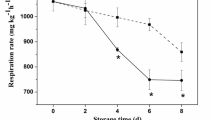
Figure 1 shows the change of respiration rate (RR) in all packs during storage of golden needle mushrooms. The RRs increased rapidly and reached a peak after 7 days storage, dropped rapidly from day 7–14, and remained relatively stable. The respiration was enhanced during the storage and the breathing peak occurred at day 7; this has also been called “breathing promotion period” [29]. In these high oxygen packs, the RRs of initially oxygen rate of 50 and 80% reached a peak value of 0.093 and 0.043 mg h−1 g−1 fw on the day 7, respectively. There was significant difference (P < 0.05) in the RRs between high oxygen treatment (exceeding 50% oxygen) and the control groups (20% oxygen and without oxygen). Among these treatments during the initial 2 weeks storage, the RRs of 50% oxygen packs were higher than that of others. Interestingly, the RR of 80% oxygen packs was the lowest among the oxygen rates of 70% O2, 90% O2, 100% O2. Moreover, the O2 depletion rate of 50% oxygen rate packs showed a significant increase and was 1.5–2 times higher than that of 70–100% oxygen packs (data not shown).
Respiration rate (RR) of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
CO2 concentrations in the headspace of all packs increased gradually with increasing storage time and reached a highest value at the end of storage (Fig. 2). The CO2 concentration of 50% oxygen packs was the highest and reached a highest value of 31.3 mL L−1, whereas the CO2 concentration in the packs of 80% oxygen was the lowest among the high oxygen packing during the storage time. These results were in accordance with those of the RRs. The changes in ethanol concentration were also detected in the mushroom storage packages. No ethanol was detected in the MAP with the high oxygen treatment (>70% oxygen rates), but ethanol was detected in the MAP without O2 and with 20% O2 rate after the day 7. The ethanol concentration of two treatments was found to increase with increasing storage time. This indicates that anaerobic respiration occurred in mushroom packs at the low O2 levels (below 20% oxygen).
CO2 concentrations of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
Compared with our previous work [1], in all packs the trends of changing RRs, O2 depletion and CO2 production in headspace were similar. The results indicated that mushroom respiration maybe inhibited by high oxygen (>70% oxygen), with the strongest inhibition by 80% oxygen. Wszelaki also found that the oxygen atmospheres that are most effective for decay control were those close to 80% or those in combination with carbon dioxide [30]. Ayala confirmed that applying a high O2 atmosphere did not induce fermentation, but had a beneficial effect on the microbial composition, and prolonged the shelf life of raspberries and strawberries [31].
Quality evaluation
Figures 3 and 4 show the sensory evaluation of the mushroom stored in MAP with different oxygen rates. The scores for appearance decreased with increasing storage time, irrespective of treatments. However, there were significant effects (P < 0.05) on the appearance scores between 80% oxygen and others after 19 days of storage, and the differences were associated mostly with the oxygen concentration. The appearance scores of stored mushrooms decreased slowly until day 19, and then decreased strongly because of chilling injury (water-soaked appearance). Among these treatments, the appearance score in MAP with 80% oxygen had the slowest decrease during storage. Based on the acceptable score (5.0), only mushrooms in MAP with 80% oxygen rate could be stored for 29 days. Li found that poor atmosphere (under 10 mL L−1 O2 and over 159 mL L−1 CO2) resulted in physiological injury and senescence, which caused another mushroom (A. chaxingu) to have fragile caps and soft and spongy stems [32]. Several works reported some beneficial effects of gas concentration with low O2 content (less than 10%) and limited CO2 content (5% maximum) on controlled atmosphere (CA) storage potential of fresh mushrooms (Agrocybe chaxingu, Agaricus bisporus) [11, 33]. In this study, golden needle mushrooms stored at low oxygen rates suffered chilling injury. The gas composition in the headspace of all packs with low oxygen conditions was O2 under 40 mL L−1 and CO2 above 147 mL L−1, which indicates anaerobic respiration and unfavorable atmosphere, which further caused physiological injury and senescence, and rapid loss of mushroom quality. It was confirmed that mushrooms stored in MAP with 80% oxygen rate at 3 °C had the best sensory quality as shown in Fig. 4, whereas the mushrooms stored in MAP without oxygen had the worst sensory quality.
Appearance scores of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
Odor scores and weight loss followed similar trends as the general appearance (data not shown).
The odor scores of 80% oxygen rate packs remained above the acceptable value of 5.0 after 24 days of storage, and weight loss of 80% oxygen rate packs after 30 days of storage was less than 3.5%, which would be acceptable in commercial practice. However, the low O2 atmosphere packs were accompanied by off-odors; significant differences (P < 0.05) were observed between the odor scores of the mushrooms stored in MAP with 80% oxygen and in low oxygen packs. In addition, we found that bentonite (a moisture absorber) and active carbon (a deodorant) were beneficial in maintaining quality of mushroom under MAP with high oxygen atmosphere, because they absorbed water and smell substances, produced from respiration and metabolism of fresh mushroom, and could provide protection against microbial deterioration.
SOD activity and superoxide anion (O2·−) generation
Table 1 shows the effect of oxygen levels and storage conditions on total SOD activity. All packs had a similar SOD activity pattern. SOD activities all increased and reached their maximum after 14 days, and thereafter decreased in all groups. Different oxygen concentrations had significant effect on SOD activity of the golden needle mushrooms at the storage time (P < 0.05), especially, high oxygen rates had a positive effect on enhancing the SOD activity during the storage.
SOD is regarded as the key enzymatic antioxidant in mushroom responses to stress factors to eliminate superoxide radicals and is the first line of defense against damage caused by superoxide radicals [31, 32]. SOD catalyzes the dismutation of superoxide anion (O2·−) into H2O2, and this might be the primary step for the defense against chilling injury. Therefore, changes in SOD activity are induced by the substrate level-superoxide radical, which increases in stress conditions [34]. Our results correspond with the measured O2·− generation (Fig. 5). The O2·− generation increased slowly during the initial 7 days storage, and then increased quickly and reached a highest value at the end of storage. Therefore, maintaining a high SOD activity might be a more efficient factor of mushroom tolerance to unfavorable environmental conditions, and the antioxidant activity decreases with the progress of senescence. It was reported that a high degree of senescence of mushrooms (Agaricus bisporus, Agrocybe chaxingu), vegetables, and fruit resulted in low antioxidant activity, and that storage stress was caused by increased O2·− generation [11, 32]. Of all our experimental packs, those stored in 80% oxygen had the highest SOD activity and lowest O2·− generation throughout the storage time. Therefore, mushrooms in MAP with 80% oxygen rate having the highest SOD activity during the latter part of the storage period had a delayed progress of senescence and consequently had the best quality.
O2·− generation of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
CAT activity and Malondialdehyde (MDA) generation
The increases in CAT activity were found during the initial 14 days of storage, followed by a decrease (Table 1). No statistically significant differences were detected among the treatments on the initial 14 days of the storage (P > 0.05), but with the extension of storage time, small differences appeared. The significant differences of CAT activity were observed after 21 days. All of the above observations indicated that senescence induced a decline of CAT activities in golden needle mushroom during the later stage of storage. The initial enhancement of CAT activity might be an adaptive response to oxygen rates and cold storage.
The levels of lipid peroxides were measured in terms of malondialdehyde (MDA) content throughout the storage (Fig. 6). The MDA content increased slowly from the initial value of 2 nmol g−1 fw to values between the highest terminal value of 11 nmol g−1 fw (without oxygen) and the lowest value of 5 nmol g−1 fw (80% oxygen), respectively. There were significant differences (P < 0.05) between the outcomes of the two conditions at the end of storage. The results indicated that high oxygen rates (i.e., >70% O2) could prevent the existence of the mushroom oxidative injury to some extent.
MDA generation of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
The level of lipid peroxidation products in mushroom samples was expressed as MDA content, and CAT activity was relative to the level of H2O2 [11, 26]. CAT participates in the main defense system against accumulation and toxicity of hydrogen peroxide and can play a role in controlling the H2O2 level in cells, but more rapid increase in H2O2 content at later senescence phase might cause the SOD activity and CAT activity following decline [35]. In this work, unfavorable atmosphere resulted in a relatively high content of MDA and generation of superoxide anion which induced oxidative injury, and SOD and CAT scavenged the active oxygen species to alleviate these injuries. The MAP with 80% oxygen rate could alleviate the oxidative injury of golden needle mushroom to higher degree and helped maintain the quality of mushrooms.
POD activity and reactive-free radicals
POD activities of the golden needle mushrooms stored in MAP with different oxygen rates increased slowly until they peaked on day 7, followed by a decline from day 7–14 (Table 1). Although POD activities of all treatments peaked on the same day, mushrooms stored with 80% oxygen had higher peaks and declined faster, which indicated that they provided a more suitable environment for storing golden needle mushrooms. Free radical reactions have been suggested to play an important role in the degradation process of membrane polar lipids in senescence [36, 37]. POD belongs to enzymes involved in growth, development, and senescence processes of mushrooms, and it is an important active-free radical scavenging enzyme [32, 38]. Liu reported that the POD activity was highest in high oxygen at eighth day, then reduced. High oxygen, especially 100% O2, was effective at maintaining the quality of mushroom [31].
PPO activity and extent of browning
Mushroom browning is an important cause of loss quality during postharvest storage. Oxidation of phenol by polyphenol oxidase (PPO) and POD results in the formation of browning complexes. The influences of different oxygen concentrations on the activity of PPO, POD, and the extent of browning of stored mushrooms are shown in Table 1 and Fig. 7, respectively. Both PPO activities and browning increased slowly during the storage. After day 19, significant differences of PPO activity were found among the seven treatment groups (P < 0.05), whereas after day 24 significant differences of browning were found. The mushrooms in MAP with 80% oxygen showed a relatively lower PPO activity and browning degree, and a longer shelf life. Liu reported that high oxygen, especially 100% O2, was effective at inhibiting discoloration. The PPO activity of mushroom was significantly (P < 0.05) higher in 100% O2 compared with air treatment [31]. Application of high oxygen atmosphere packaging (i.e., >70% O2) for fresh-cut vegetables was evaluated as an alternative technique for low O2 modified atmosphere (3% O2—5% CO2—balance N2); high O2 concentrations (>70% O2) were found to be particularly effective in inhibiting enzymatic browning of fresh-cut vegetables [16].
Browning of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
Cellulase (Cx) activity and firmness
The Cx activities of the mushrooms increased with prolonged storage time and reached their peak on day 14 in packs with 50% oxygen rate and on day 19 in the other treatments, and then declined until the end of storage (Fig. 8). There were no significant differences (P > 0.05) in Cx activities in all packs during the initial 19 days storage, but in the period 19–34 days, significant differences (P > 0.05) between the treatments occurred.
Cx activities of golden needle mushrooms stored under MAP with different initial oxygen rates: (blue diamond without oxygen, pink square 20% oxygen rate, yellow triangle 50% oxygen rate, aqua cross 70% oxygen rate, purple cross 80% oxygen rate, brown square 90% oxygen rate, green plus 100% oxygen rate and balanced N2). Vertical lines represent the mean ± SD of replicates
Cellulase can degrade mushroom tissue and decrease its firmness. Abeles described that a sixfold increase in cellulase activity, measured as the reduction in viscosity of carboxymethyl cellulose, was associated with maturation and softening in strawberry fruit [39]. In addition, the improved firmness of calcium-treated vegetables could be due to enzymatic demethylation of cellulose and pectins followed by the formation of bridge bonds [40]. Perhaps high oxygen packs also had effect on enzymatic demethylation of cell framework and extending postharvest life, but the potential effects need further research.
Conclusions
Golden needle mushrooms stored in MAP without oxygen or 20–50% oxygen had a poorer sensory quality because of physiological injuries. These injuries induced a relatively high MDA content, O2·− generation, and browning. These injuries also induced senescence of the stored mushrooms, which was accompanied by lower activities of antioxidant enzyme system. Gas atmosphere of mushroom packs with greater than 40 mL L−1 O2 and lesser than 147 mL L−1 CO2 resulted in anaerobic respiration and physiological injury and a rapid loss of mushroom quality.
The antioxidant enzyme system, including SOD, CAT, POD, and PPO, was activated to scavenge ROS and reduce injury during the initial storage period. However, these injuries also induced senescence of golden needle mushrooms in the later part of storage period, which decreased the activities of antioxidant enzymatic system. The storage of golden needle mushrooms under initial 80% oxygen concentrations significantly affected the antioxidant capacity, overall quality, MDA content, and O2·− generation. Data presented in this study suggest that the high O2 increases the oxidative stress so induce the activities of the antioxidant enzymes. Especially, the activities of antioxidant enzyme system of the mushroom stored in MAP with 80% oxygen were the most favorable to delay the senescence process in the latter part of the storage period. This treatment resulted in mushrooms of the best quality until the end of storage.
References
Wang CT, Liu L, Wang J, Cao YP, Sun BG (2010) Effect of oxygen pressures on golden needle mushrooms fresh-keeping. Food Sci (China) 18:28–35
Azamkhan M, Ahmad I (2005) Morphological studies on physical changes in apple fruit after storage at room temperature. J Agri Soc Sci 1:1–10
Prange RK, DeLong JM, Leyte CJ (2002) Oxygen concentration affects chlorophyll fluorescence in chlorophyll-containing fruit. Postharvest Biol Tech 24:201–205
Golis J, Nemcova A, Canek A (2007) Storage of sweet cherries in low oxygen and high carbon dioxide atmospheres. Hortic Sci (Prague) 34(1):26–34
Heydari A, Shyesteh K, Naimeh E, Bordbar H (2010) Studies on the Respiration Rate of Banana Fruit Based on Enzyme Kinetics. Int J Agric Biol 12(1):145–149
Sala JM, Lafuente MT (2004) Antioxidant enzymes activities and rindstaining in ‘Navelina’ oranges as affected by storage relative humidity and ethylene conditioning. Postharvest Biol Tec 31:277–285
Rio LA, Pastori GM, Palma JM, Sandalio LM (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol 116:1195–1200
González-Aguilar GA, Villegas-Ochoa MA (2007) Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J Food Sci 72:197–202
Vicente AR, Martinez GA, Chaves AR (2006) Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol Tec 40:116–122
Sgherri CLM, Loggini B, Puliga S (1994) Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35:561–565
Li TH, Zhang M (2010) Effects of modified atmosphere package (MAP) with a silicon gum film window and storage temperature on the quality and antioxidant system of stored Agrocybe chaxingu. LWT - Food Sci Technol 43:1113–1120
Soto-Zamora G, Yahia EM, Brecht JK (2005) Effect of postharvest hot air treatments on the quality and antioxidant levels in tomato fruit. LWT-Food Sci Technol 38:657–663
Starzynska A, Leja M, Mareczek A (2003) Physiological changes in the antioxidant system of broccoli flower buds senescing during short-term storage, related to temperature and packaging. Plant Sci 165:1387–1395
Scandalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:7–12
Jung S (2004) Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol Bioch 42:225–231
Liesbeth J, Frank D (2001) Effect of high oxygen modified atmosphere packaging on microbial growth and sensorial qualities of fresh-cut produce. Int J Food Microbiol 71:197–210
Lund MN, Lametsch R (2007) High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci 77:295–303
Liesbeth J, Devlieghere F (2002) Temperature dependence of shelf-life as affected by microbial proliferation and sensory quality of equilibrium modified atmosphere packaged fresh produce. Postharvest Biol Tec 26:59–73
Master AM, Knott ER, Teunissen PGM (2004) Effects of high isostatic pressure on mushrooms. J Food Eng 5:11–16
Sujatha V, Korde JP, Rastogi SK (2010) Amelioration of heat stress induced disturbances of the antioxidant defense system in broilers. J Vet Med Anim Health 2(3):18–28
Lowry OH, Rosenbrough JJ, Farr AL (1951) Estimation of protein with the folin-phenol reagent. J Biol Chem 193:265–275
Candan N, Tarhan L (2003) Relationship among chlorophyll-carotenoid content, antioxidant enzyme activities and lipid peroxidation levels by Mg deficiency in the Mentha pulegium leaves. Plant Physiol Bioch 41:35–40
Ei-hilali F, Ait-Oubahou A (2003) Chilling injury and peroxidase activity changes in “Fortune” mandarin fruit during low temperature storage. Bulg J Plant Physiol 29(1–2):44–54
Jiang YM (1999) Purification and some properties of polyphenol oxidase of longan. Food Chem 66:75–79
Emtiazi G, Pooyan M, Shamalnasab M (2007) Cellulase activities in nitrogen fixing Paenibacillus isolated from soil in N-free media. World J Agri Sci 3(5):602–608
Shah K, Kumar RG, Verma S (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Chaitanya KSK, Naithani SC (1994) Role of superoxide lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Faertn. New Phytol 126:623–627
Rajarathnam S, Shashirekha MN, Rashmi S (2003) Biochemical changes associated with mushroom browning in Agaricus bisporus (Lange) Imbach and Pleurotus florida (Block & Tsao): commercial implications. J Sci Food Agri 83(14):1531–1537
Tucker ML, Laties GG (1995) The dual role of oxygen in avocado fruit respiration: kinetic analysis and computer modeling of diffusion-affected respiratory oxygen isotherms. Plant Cell Environ 8(2):117–127
Wszelaki A, Yehoshua SB, Mitcham B (1999) Do high oxygen atmospheres control postharvest decay of fruits and vegetables? Perishables Handling Quarterly 99:22–25
Zhanli Liu, Xiangyou Wang, Jiying Zhu (2010) Effect of high oxygen modified atmosphere on post-harvest physiology and sensorial qualities of mushroom. Int J Food Sci Technol 45(6):1097–1103
Li T, Zhang M, Wang S (2007) Effects of modified atmosphere packaging with a silicon gum film as a window for gas exchange on Agrocybe chaxingu storage. Postharvest Biol Tec 43:343–350
Guillaume C, Schwab I (2010) Biobased packaging for improving preservation of fresh common mushrooms (Agaricus bisporus). Innov Food Sci Emerg. doi:10.1016/j.ifset.2010.05.007
Tsang EWT, Bowler C, Herouart D, VanCamp W (1991) Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell 3:783–792
Kerdnaimongkol K, Woodson WR (1999) Inhibition of catalase by antisens RNA increases susceptibility to oxidative stress and chilling injury in transgenic tomato plants. J Am Soc Hortic Sci 124:330–336
Lin JN, Kao CH (1998) Effect of oxidative stress caused by hydrogen peroxide on senescence of rice leaves. Botanical Bulletin of Academic Sinica (Taipei, Botanical Studies) 39:161–165
Tao F, Zhang M, Yu H (2007) Effect of vacuum cooling on physiological changes in the antioxidant system of mushroom under different storage conditions. J Food Eng 79:1302–1309
Jiang TJ, Jahangir MM, Jiang ZH, XiY Lu (2010) Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Tec 56:209–215
Abeles FB, Takeda F (1990) Cellulase activity and ethylene in ripening strawberry and apple fruits. Scientia Hortic 42(4):269–275
Stute R, Eshtiagi MN (1996) High pressure chemical engineering. Elsevier, New York, pp 10–45
Acknowledgments
The authors would like to acknowledge the financial support from the Ministry of Science and Technology of China (Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period, Project No. 2008BADAIB07-11; National Basic Research Program, Project No. 2007CB707802; Project No. 31071593) and the Talents Cultivation Deepening Plan of Beijing Higher Institutions (Project No. 0142131301).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C.T., Wang, C.T., Cao, Y.P. et al. Effect of modified atmosphere packaging (MAP) with low and superatmospheric oxygen on the quality and antioxidant enzyme system of golden needle mushrooms (Flammulina velutipes) during postharvest storage. Eur Food Res Technol 232, 851–860 (2011). https://doi.org/10.1007/s00217-011-1453-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1453-5