Abstract
The shrimp processing byproducts were hydrolyzed by various proteases, and calcium binding activity of the hydrolysates was examined. Among the digests, trypsin digest showed the most potent calcium binding activity (0.294 mmol/g-protein) and highest degree of hydrolysis (18.4%). The trypsin hydrolysate was fractionated according to the molecular weights using ultrafiltration membrane system. The lowest molecular weight fraction (<1 kDa) showed the highest calcium binding activity. Then, the lowest molecular weight fraction was isolated and purified by ion-exchange chromatography, gel filtration, and ODS reversed high-performance liquid chromatography. The purified peptide showed the highest calcium binding activity of 2.70 mmol/g-protein, and its structure was identified as Thr-Cys-His by ESI/MS/MS. Therefore, these results suggested that the peptide derived from shrimp processing byproducts protein hydrolysates is responsible for higher calcium binding properties and may be as natural functional additive in food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal ions play a role in many, if not most, metabolic processes, such as, triggering mechanisms, stabilizing structure, and controlling redox behavior. Of all the divalent metallic cations, calcium plays the most important role, such as, providing rigidity to the skeleton, acting as a secondary messenger in regulating numerous intracellular events, taking advantage of its large membranous concentration gradient. Calcium deficiency causes osteoporosis, and calcium supplements from the diet are essential. To enhance the bioavailability of calcium, it is important to consider not only the amount of calcium ingested but also the substances coexisting with it. Milk or dairy products are the most common and trusted source of calcium. Casein phosphopeptides (CPP) derived from the intestinal digestion of casein has the capacity of chelating calcium, increasing the amount of soluble calcium availability for absorption, and then enhancing bone calcification [1]. However, some oriental people do not drink milk because of lactose indigestion or intolerance. Thus, many other sources of calcium supplements as alternatives have been studied over recent years, for examples, soybean protein [2], fructo-oligosaccharides [3], fish meal [4], etc. Recently, some small peptides have been reported to posses the capacity of calcium binding and enhance the calcium bioavailability from fish bone [5–8], whey protein [9], egg yolk [10], and soybean protein [11]. However, studies on the utilization of shrimp processing byproducts hydrolysates as source of calcium binding peptides are scarce.
World production of shrimp, both captured and farmed, is around six million tonnes, about 60 percent of which enters the world market. Annual exports of shrimp are currently worth more than US$10 billion, or 16 percent of all fishery exports [12]. Considerable amounts of shrimp processing byproducts, mainly composed of head and body carapace, are discarded each year, but the shrimp processing byproducts are important source of bioactive molecules. The shrimp processing will generate solid biowaste ranging from 48 to 56% of the whole shrimp depending on the species [13]. Only a little few shrimp processing byproducts are actually used in some way, mostly for animal feed or chitin production; the remainder is discarded and represents an environmental problem. These byproducts are highly perishable material and seasonally produced, which deteriorate fast due to bacterial contamination resulting in decomposition before any possible use.
Investigations have therefore been conducted into generating value-added products from these shrimp processing byproducts, including extraction of chitin [14, 15], production of protein hydrolysates for use in food and animal feed [14, 16, 17], and extraction of carotenoids [13, 18]. The potential for producing functional bioactive peptides through enzymatic hydrolysis of shrimp texture or shrimp processing byproducts has also been suggested by several recent research reports, for example, antioxidant peptides [19], angiotensin-I-converting enzyme (ACE) inhibitory peptides [20], and antimicrobial peptides [21]. In our previous studies [22], iron-binding peptides were isolated and purified from shrimp processing byproducts hydrolysates. Now, we will discuss the purification of a calcium binding peptide from shrimp processing byproducts hydrolysates expecting to be a calcium supplement as the alternative for dairy products.
Materials and methods
Materials
Shrimp processing byproducts were bought from aquatic product market in Wenzhou City, Zhejiang Province, China, and stored at −18 °C until use. Flavourzyme (500 LAPU/g), protamex (1.5 AU/g), and alcalase (2.4 AU/g) were purchased from Novzyme China Investment Company. Pepsin (12.5 U/mg) and trypsin (200 U/mg) were purchased from Huadong Pharmaceuticals Company, China. Chemicals and solvents were of analytical and high-performance liquid chromatography (HPLC) grade. All other reagents were of the highest grade available commercially.
Preparation of shrimp processing byproducts hydrolysates
Shrimp processing byproducts were crushed into powder after dried at 80 °C under vacuum. The shrimp processing byproducts protein solution (20 mg protein/mL) was hydrolyzed with various enzymes at optimal temperature (flavourzyme, 50 °C; protamex, 50 °C; alcalase, 55 °C; pepsin, 37 °C; trypsin, 40 °C). The enzyme to substrate ration (E/S) was 1:1000 (w/v). The pH of the protein solution was adjusted to the optimal values (flavourzyme, pH 7.0; protamex, pH 6.5; alcalase, pH 8.0; pepsin, pH 2.0; trypsin, pH 8.2) before hydrolysis was initiated, and it was readjusted to the optimal values every 15 min during hydrolysis with 1 M NaOH or 1 M HCl.
After hydrolysis for 6 h, the pH of the solution was adjusted to 7.0, and then the solution was heated at 95 °C for 10 min to inactive the enzyme. The hydrolysate was filtered through a filter paper and centrifuged at 6,000g for 20 min, then stored at −18 °C until analysis. Degree of hydrolysis (DH) and protein content of the hydrolysates were determined using trinitrobenzensulfonic acid (TNBS) method [23] and Lowry method [24], respectively.
Calcium binding activity analysis
The calcium binding activity analysis was performed according to the method of Jung [7] with some modifications. After demineralization by D751 Chelex resin, the protein content of the hydrolysate was adjusted to 1.0 mg/mL, and then the hydrolysates were mixed with 10 mM CaCl2 and 20 mM sodium phosphate buffer (pH 7.8). The mixture was stirred at 22 °C for 30 min, and the pH was maintained at 7.8 with a pH meter. After the removal of insoluble calcium phosphate salts, calcium contents in the supernatant were determined by flame atomic absorption spectrometry (FAAS). The experiments were performed in triplicate, and values were expressed as mean ± standard error (SD).
Isolation and purification of calcium binding peptides
After demineralization by D751 Chelex resin, the protease hydrolysate was fractionated by ultrafiltration system with two different molecular weight cutoffs (1 and 5 kDa). Three fractions (<1 kDa, 1–5 kDa, and >5 kDa) were separated. The calcium binding activities of these three fractions were determined.
After ultrafiltration, the active fractions were concentrated and applied on a SP-Sepharose Fast Flow anionic exchange column (15 mm × 500 mm), which was pre-equilibrated with 20 mM pH 4.0 sodium acetate buffer. A linear gradient of NaCl (0–1 M) in the same buffer was maintained at a flow rate of 1.5 mL/min and monitored at 280 nm. The ultraviolet (UV) absorption peaks were collected. The protein and calcium binding activities of the fractions were determined. The fraction with the highest calcium binding activity was pooled and lyophilized. The active fraction was dissolved in distilled water and isolated on a Sephadex G-25 column (16 mm × 600 mm) equilibrated and eluted with distilled water at a flow rate of 0.8 mL/min. All eluted peaks were monitored at 214 nm, and also collected for calcium binding capacity analysis. The active fraction was collected and lyophilized. The active fraction was dissolved in distilled water and purified by reversed-phase high-performance liquid chromatography (RP-HPLC) on an ODS semi-preparative column (Inertsil ODS-SP, 5 μm, 20 mm × 250 mm) using linear gradient elution from A (0.05% trifluoroacetic acid (TFA) in water) to B (0.05% TFA in 50% acetonitrile) within 50 min at a flow rate of 15 mL/min. The elution was monitored at 214 nm. The active fraction was collected and lyophilized. The active fraction was dissolved in distilled water and further purified by RP-HPLC on an ODS analytical column (Inertsil ODS-SP, 5 μm, 4.6 mm × 250 mm) with the same gradient of acetonitrile at 0.8 mL/min.
Peptide sequence analysis
Molecular weight (MW) and peptide amino acid sequence of the purified calcium binding activity fraction were determined in positive ion mode using electrospray ionization (ESI) and ESI/MS/MS, respectively. ESI mass spectrometry was performed using a triple quadrupole instrument Applied Biosystems API 3000 (PE Sciex, Toronto, Canada). The amino acid sequence of active peptide was obtained over the m/z range 50–1,500 and was identified using the SEQUEST sequencing algorithm.
Results and discussion
Degradation of shrimp processing byproducts protein by various proteases
To produce calcium binding capacity peptides, shrimp processing byproducts protein was separately hydrolyzed using various commercial digestive enzymes. In this experiment, five proteolytic enzymes such as alcalase, trypsin, pepsin, flavourzyme, and protamex were selected to evaluate their effectiveness on degradation of shrimp processing byproducts protein for calcium binding activity. DH after proteolytic digestion was observed to be 15.2 and 18.4% for protamex and trypsin, respectively. However, the other proteolytic enzymes showed DH around 10% (Fig. 1). In the calcium binding activity assay (Fig. 1), the hydrolysate from trypsin or alcalase proteolysis showed higher calcium binding activity (0.294 and 0.217 mmol/g-protein, respectively) among the tested hydrolysates. The trypsin-proteolytic hydrolysate, which showed the highest DH and calcium binding activity, was selected for further study. Alcalase and trypsin have been used for the preparation of bioactive molecules from shrimp protein [15, 18–20, 25] or other marine protein [26–28].
Purification of the calcium binding peptide
To purify the calcium binding activity peptides, hydrolysate with 18.4% DH by trypsin, which exhibited the highest calcium binding activity, was initially ultrafiltrated into three fractions (Table 1), Fraction I (<1 kDa), Fraction II (1–5 kDa) and Fraction III (>5 kDa), using ultrafiltration membrane. The three MW groups were lyophilized and investigated by calcium binding activity assay. Among the all MW groups, shown in Table 1, Fraction I exhibited highest calcium binding activity (0.445 mmol/g-protein). Bioactive peptides usually contain 3–20 amino acid residues, and low MW peptides are more potent as bioactive peptides than high MW peptides [29]. Some calcium binding peptides from other marine protein hydrolysates had been identified to be 1–3.5 kDa [6–8]. But in our experiment, the fraction of less than 1 kDa has more potential calcium binding activity. Some antioxidant peptides, as well as to be having metal chelating activity, with MW less than 1 kDa had been identified [30, 31]. Oligo-peptides of carnosine (MW = 226) and anserine (MW = 240) also had been identified to be having high metal chelating activity [32]. Therefore, we selected Fraction I for purification of the calcium binding activity peptide.
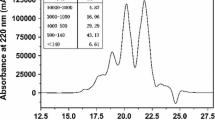
The Fraction I with MW <1 kDa was shown to exhibit the highest calcium binding activity and then was separated into seven fractions (F1-F7) by anionic exchange chromatography on SP-Sepharose Fast Flow column with a linear gradient of NaCl (0–1 M) at a flow rate of 1.5 mL/min (Fig. 2). Each fraction was pooled, lyophilized and its calcium binding activity was assayed. As shown in Fig. 2, fraction F5 possessed the highest calcium binding activity (0.77 mmol/g-protein) among all fractions. This fraction was further separated by size exclusion chromatography on Sephadex G-25 column. Each fraction was pooled, lyophilized and its calcium binding activity was assayed. The elution profile of the peptides and the calcium binding capacity were shown in Fig. 3. The fraction F53 showed the highest calcium binding activity of 1.49 mmol/g-protein among the four fractions. The fraction F53 was further purified by semi-preparative reversed-phase HPLC and fractionated into four major fractions. The elution profile of peptides is shown in Fig. 4. Fraction F531 showed the highest calcium binding activity (2.70 mmol/g-protein). The fraction F531 was pooled, lyophilized and separated through repeated chromatography using analytical RP-HPLC column. The repeated chromatography (Fig. 5) showed that there was only one major fraction. Therefore, we selected fraction F531 for the amino acid sequence identification of the calcium binding activity peptide.
Elution profile of trypsin hydrolysate (DH of 18.3%) by SP-Sepharose Fast Flow anionic exchange chromatography. The column (15 mm × 500 mm) was equilibrated and eluted by 20 mM pH 4.0 sodium acetate buffer with a linear gradient of NaCl (0–1 M) at a flow rate of 1.5 mL/min and the elution was monitored at 280 nm. All data were expressed as mean values (mean ± SD, n = 3)
Elution profile of the active fraction from ion-exchange chromatography was further purified by Sephadex G-25 size exclusion chromatography. The column (16 mm × 600 mm) was equilibrated and eluted with distilled water at a flow rate of 0.8 mL/min, and the elution was monitored at 214 nm. All data were expressed as mean values (mean ± SD, n = 3)
RP-HPLC elution profile pattern on a semi-preparative Inertsil ODS-SP column (20 mm × 250 mm) of the fraction F53. Elution was achieved with a linear gradient of acetonitrile (0–50%) at a flow rate of 15 mL/min. The elution was monitored at 214 nm. All data were expressed as mean values (mean ± SD, n = 3)
Identification of the calcium binding activity peptide
The fraction F531, shown the highest calcium binding activity, was analyzed by ESI–MS for molecular mass determination and ESI–MS/MS for the characterization of the peptide. The peptide (fraction F531) was identified to be Thr-Cys-His (Fig. 6). In fact, the antioxidant activity of histidine (His)-containing peptides has been reported to be attributed to the chelating and lipid radical-trapping ability of the imidazole ring [33–36]. Scogin [37] indicated that divalent metal ions such as zinc ion were bound to bacitracin (histidine-containing decapeptide) through the δ-N of the imidazole ring of His. Graham [38] also reported that histidine-containing peptides had high affinity to divalent ions. The calcium binding activity of this peptide (2.70 mmol/g-protein) was approximately equal with that of CPP-II [6], but slightly higher than that of hoki frame peptide (2.06 mmol/g-protein) [6] or oligo-phosphopeptide from hen egg yolk phosvitin (2.19 mmol/g-protein) [10]. The Thr-Cys-His peptide from shrimp process byproducts gives about 1:1 stoichiometry (0.95 mol calcium per mol peptide) and which may contribute to the imidazole ring of His. From this view, the calcium binding activity of this peptide was lower than that of hoki frame peptide (3.21 mol calcium per mol peptide) [6].
In this study, one calcium binding peptide was identified to have His residue. Further, Thr-Cys-His peptide that displayed highest calcium binding activity may be due to the presence of the sequence of His residue. This His-containing peptide is a novel peptide with calcium binding activity that had never been reported.
Conclusion
Shrimp processing byproducts have the potential to be used as the starting material for the production of protein hydrolysates with calcium binding activity. In this research, we studied the purification of a His-containing peptide exhibiting calcium binding activity from shrimp processing byproducts trypsin hydrolysate. A tri-peptide of His-containing peptide (Thr-Cys-His) was purified and identified to posses high calcium binding activity. This peptide with high calcium binding ability may be due to the presence of the sequence of His residue. Next, we will study its bioavailability through in vitro and in vivo assays. Through these attempts, we may be able to develop a high calcium binding peptide as natural functional additive in food industry.
References
Kitts DD, Yuan YV (1992) Caseinophosphopeptides and calcium bioavailability. Trends Food Sci Technol 3:31–35
Kumagai H, Koizumi A, Sato N, Ishikawa Y, Suda A, Sakurai H, Kumagai H (2004) Effect of phytate-removal and deamidation of soybean proteins on calcium absorption in the in situ rats. Biofactors 22:21–24
Wang Y, Zeng T, Wang S, Wang W, Wang Q, Yu HX (2010) Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition 26:305–311
Larsen T, Thilsted SH, Kongsbak K, Hansen M (2000) Whole small fish as a rich calcium source. Br J Nutr 83:191–196
Jung WK, Lee BJ, Kim SK (2006) Fish-bone peptide increases calcium solubility and bioavailability in ovariectomised rats. Brit J Nutr 95:124–128
Jung WK, Kim SK (2007) Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur Food Res Technol 224:763–767
Jung WK, Park PJ, Byun HG, Moon SH, Kim SK (2005) Preparation of hoki (Johnius belengerii) bone oligophosphopeptide with a high affinity to calcium by carnivorous intestine crude proteinase. Food Chem 91:333–340
Jung WK, Karawita R, Heo SJ, Lee BJ, Kim SK, Jeon YJ (2006) Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem 41:2097–2100
Rui XU (2009) Calcium binding of peptides derived from enzymatic hydrolysates of whey protein concentrate. Int J Dairy Technol 62:170–173
Jiang B, Mine Y (2001) Phosphopeptides derived from hen egg yolk phosvitin: effect of molecular size on the calcium-binding properties. Biosci Biotechnol Biochem 65:1187–1190
Bao XL, Zhang J, Chen Y, Guo ST (2007) Calcium-binding ability of soy protein hydrolysate. Chinese Chem Lett 18:1115–1118
Gillett R (2008) Global study of shrimp fisheries. FAO, Rome
Sachindra NM, Mahendrakar NS (2005) Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour Technol 96:1195–1200
Gildberg A, Stenberg E (2001) A new process for advanced utilization of shrimp waste. Process Biochem 36:809–812
Synowiecki J, Al-Khatee N (2000) The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem 68:147–152
Coward-kelly G, Agbogbo FK, Holtzapple MT (2006) Lime treatment of shrimp head waste for the generation of highly digestible animal feed. Bioresour Technol 97:1515–1520
Mizani M, Aminlari M, Khodabandeh M (2005) An effective method for producing a nutritive protein extract powder from shrimp-head waste. Food Sci Technol Int 11:49–54
Babu CM, Chakrabarti R, Sambasivarao KR (2008) Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT–Food Sci Technol 41:227–235
Guerard F, Sumaya-Martinez MT, Laroque D, Chabeaud A, Dufosse L (2007) Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem 42:1486–1491
Cheung IW, Li-Chan EC (2010) Angiotensin-I-converting enzyme inhibitory activity and bitterness of enzymatically-produced hydrolysates of shrimp (Pandalopsis dispar) processing byproducts investigated by Taguchi design. Food Chem 122:1003–1012
Destoumieux-Garzon D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bache`re E (2001) Antifungal peptides are generated from the C-terminus of shrimp hemocyanin in response to microbial challenge. J Biol Chem 276:47070–47077
Huang GR, Ren ZY, Jiang JX (2010) Separation of iron-binding peptides from shrimp processing by-products hydrolysates. Food Bioprocess Technol. doi:10.1007/s11947-009-0198-7
Elkund A (1976) On the determination of available lysine in casein and rapeseed protein concentration using 2,4,6-trinitrobenzensulfonic acid (TNBS) as a reagent for free ε-amino group of lysine. Anal Chem 70:434–439
Lowry OH, Rosebrough AL, Farr AL, Randall RJ (1951) Protein measurement with the folin–phenol reagent. J Biochem 193:165–175
Simpson BK, Nayeri G, Yaylayan V, Ashie INA (1998) Enzymatic hydrolysis of shrimp meat. Food Chem 61:131–138
Jun SY, Park PJ, Jung WK, Kim SK (2004) Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur Food Res Technol 219:20–26
Je JY, Qian ZJ, Byun HG, Kim SK (2007) Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem 42:840–846
Lee JK, Hong S, Jeon JK, Kim SK, Byun HG (2009) Purification and characterization of angiotensin I converting enzyme inhibitory peptides from the rotifer, Brachionus rotundiformis. Bioresour Technol 100:5255–5259
Pihlanto-Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H (2000) Angiotensin I-converting enzyme inhibitory properties of whey protein digests: concentration and characterization of active peptides. J Dairy Res 67:53–64
Guo H, Kouzuma Y, Yonekur M (2009) Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem 113:238–245
Gu JL, Lee E (2007) Mineral collagen chelates and methods of making and using same. WIPO Patent, WO/2007/044945
Arihara K, Nakashima Y, Mukai T, Ishikawa S, Itoh M (2001) Peptide inhibitors for angiotensin I-converting enzyme from enzymatic hydrolysates of porcine skeletal muscle proteins. Meat Sci 57:319–324
Murase H, Nagao A, Terao J (1993) Antioxidant and emulsifying activity of N-(long-chain-acyl) histidine and N-(long-chain-acyl) carnosine. J Agri Food Chem 41:1601–1604
Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK (2001) Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J Am Oil Chem Soc 78:651–656
Uchida K, Kawakishi S (1992) Sequence-dependant reactivity of histidine-containing peptides with copper (II)/ascorbate. J Agri Food Chem 40:13–16
Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M (2010) Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem 118:559–565
Scogin DA, Baldwin TO, Gennis RB (1983) Studies on the complex formed between bacitracin A and divalent cations. Biochim Biophys Acta 742:184–188
Graham B, Comba P, Hearn MTW, Spiccia L (2007) An examination of the binding behavior of histidine-containing peptides with immobilized metal complexes derived from the macrocyclic ligand, 1,4,7-triazacyclononane. J Biol Inorg Chem 12:11–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, G., Ren, L. & Jiang, J. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur Food Res Technol 232, 281–287 (2011). https://doi.org/10.1007/s00217-010-1388-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1388-2