Abstract
A study on the characterization of Tunisian extra-virgin olive oil varieties produced in their place of origin has been carried out. Due to the influence of the genotype and environmental, agronomic and technological factors on the chemical composition of olive oil and its quality, all the olives studied were collected on the same season, and the oil was obtained under the same processing technique. Several analyses were performed to characterize the different olive oils: free acidity, peroxide value, fatty acid composition, radical scavenging activity, Rancimat assay, pigments content and phenolic compounds by HPLC–MS. In order to evaluate all the results obtained (36 parameters for each variety), different statistical analyses were used to discriminate the extra-virgin olive oil varieties: one-way analysis of variance was performed to check significant differences among cultivars (p ≤ 0.05); PCA was applied to the data showing that variables such as oleic, linoleic, quinic and vanillic acids, apigenin, luteolin, taxifolin, oleuropein aglycon, pinoresinol acetate, elenolic acid and oxidative stability allowed discriminating among the different varieties of extra-virgin olive oil studied. Besides, LDA model was able to classify the samples depending on their geographical origin in Tunisia (North, Centre and South).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The olive tree (Olea europaea L.) is one of the most important crops in Mediterranean countries. Agriculture is the mainstay of Tunisian economy, the olive tree being one of its principal agricultural and economical sectors. Tunisia represents one of the biggest producers of olive oil worldwide; today, it is the fourth largest exporter after Spain, Italy and Greece [1]. Its olive-growing areas are found all around the country, where a wide range of edapho-climatic conditions exists, from lower semi-arid to arid conditions. As it is widely known, the environmental factors, as well as genotype, agronomic and technological factors influence very much the chemical composition of olive oil, having a great impact on its quality [2–4].
Furthermore, virgin olive oil, due to its use without refining, shows very interesting nutritional and sensorial properties, being one of the pillars of the Mediterranean diet. Its fatty acid composition, monounsaturated, and its natural antioxidants provide numerous advantages for health [5–7].
The genetic diversity in the olive sector of Tunisia is very wide; there are many different olive varieties in the various regions of the country. However, Chemlali and Chetoui are the two main olive cultivars in Tunisia. Chetoui represents more than 20% of the national olive oil production [8], while Chemlali is the most abundant olive variety, which represents two-thirds of olive plantation, principally used for oil extraction, and it is the major contributor of the national olive oil production [9]. Despite the fact that these two cultivars are the most important from the economical point of view, there are other cultivars that are grown in restricted geographical localizations and that have a limited diffusion outside these areas. The study of these less-common cultivars appears of particular interest because they may have agronomic characteristics which can influence the quality and oxidative stability of the olive oil obtained.
Many studies have been carried out about olive cultivars, and their olive oils in Tunisia: comparison of different cultivars and growing areas of autochthonous olive oils [10, 11] and comparison of Tunisian and European varieties cultivated in Tunisia [2, 12–14]. However, there is not much information about the characterization and comparison of the Tunisian autochthonous varieties of olive oil grown in their area of origin.
The present work was carried out on six monovarietal extra-virgin olive oils (EVOO) from the two main (Chemlali and Chetoui) and four minor Tunisian cultivars (Oueslati, Jarboui, El Hor and Chemchali) produced in their area of origin. Several analyses were performed to characterize the different olive oils: free acidity, peroxide value, fatty acid composition, radical scavenging activity, Rancimat assay, pigments content and phenolic compounds by HPLC–MS. This is a preliminary study with the aim of finding any variable able to discriminate among the monovarietal extra-virgin olive oils and evaluate the possibility to determine whether the parameters studied were able to classify the cultivars depending on their geographical origin.
The Tukey’s honest-test was used to determine the level of significance of each evaluated parameter characterizing the varieties. Pearson test allowed checking the correlations between different analyses. The classification of EVOO samples according to their geographical origin was performed by linear discriminant analysis (LDA). Finally, principal component analysis and agglomerative hierarchical clustering were performed to check the usefulness of chemical parameters as a tool to discriminate among the monovarietal EVOO.
Materials and methods
Chemicals
All solvents used were analytical or HPLC grade (Panreac, Barcelona, Spain) and used as received. Double-deionized water was obtained with a Milli-Q water purification system (Millipore, Bedford, MA, USA). Standard compounds such as hydroxytyrosol, tyrosol, luteolin, apigenin, o-coumaric acid, ferulic acid and quinic acid were purchased from Sigma–Aldrich (St. Louis, MO, USA), (+)-pinoresinol was acquired from Arbo Nova (Turku, Finland) and oleuropein from Extrasynthèse (Lyon, France). The stock solutions containing these analytes were prepared in methanol/water (50/50, v/v).
Samples
Oils used in this study were obtained from six Tunisian olive varieties cultivated in their origin regions: North (Chetoui, Chemlali and Jarboui), Centre (Oueslati and El Hor) and South (Chemchali). Only healthy fruits, without any kind of infection or physical damage, were processed. Olives were harvested in the same season in 2008. The maturity index of all the olives was of 3 and was based on the degree of skin and pulp pigmentation according to the method developed by the Agronomic Station of Jaén [15]. After harvesting, the olives were washed and deleafed. Then the fruits were crushed with a hammer crusher, and using an Abencor analyzer (MC2 Ingenierias y Sistemas, Sevilla, Spain) the paste was mixed at 25 °C for 30 min, centrifuged without addition of warm water and then transferred into dark glass bottles.
Oil yield
The determination of oil content was done as follows: 40 g of fruit samples was dried in an oven at 80 °C to constant weight. Then, the dry olives were extracted with petroleum ether using a Soxhlet apparatus according to Donaire et al. [16]. The results were expressed as percentage of dry matter (%DM).
Analytical indices
Determination of free acidity, peroxide value and specific ultraviolet absorbance were carried out following the analytical methods described in the EC Regulation [17, 18].
Fatty acid composition
The fatty acid composition of oil samples was determined as methyl esters by capillary gas chromatography analysis after alkaline treatment. The gas chromatograph (VARIAN CP-3800 Gas Chromatograph) was equipped with an autosampler (CP-8400), a capillary column HP Innowax (Agilent Technologies, USA) (30 m × 0.53 mm, 1 μm), a split–splitless injector and a flame ionization detector (FID). Alkaline treatment was carried out by mixing 0.1 g of oil dissolved in 3 mL of n-hexane with 0.5 mL of 0.2 N methanolic potassium hydroxide solution according to the method of Reg EC 2568/91 [19]. One microlitre of methyl esters was injected. Seven fatty acids including C16:0, C16:1, C18:0, C18:1, C18:2, C18:3 and C20:0 were identified from their retention times compared to those of standard compounds.
Radical scavenging activity
The olive oil samples were examined for their capacity to scavenge the stable 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) according to Kalantzakis et al. [20]. Olive oil was solved in ethyl acetate (10%, w/v), 1 mL of this solution was added to 4 mL of a freshly prepared DPPH solution 10−4 M in a screw-capped 10-mL test tube. The reaction mixture was then shaken vigorously for 10 s in a Vortex apparatus, and the tube was maintained in the dark for 30 min, after which a steady state was reached. The absorbance of the mixture was measured at 515 nm against a blank solution using a UV–VIS dual beam spectrophotometer (UVS-2700 Labomed, Inc). A control sample was prepared and measured daily. The radical scavenging activity (RSA) towards DPPH was expressed as the % reduction in DPPH concentration by the constituents of the oils: %[DPPH]red = 100*(1-[DPPH]30/[DPPH]0), where [DPPH]0 and [DPPH]30 were the concentrations of DPPH in the control sample (t = 0) and in the test mixture after the 30-min reaction, respectively. The DPPH concentration in the reaction medium was calculated from the following calibration curve, determined by linear regression: A515nm = 12.024[DPPH]-0.0101 (r = 0.999), where [DPPH] was the concentration of DPPH, expressed as mol/L.
Rancimat assay
Oxidative stability was evaluated by Rancimat method [21]. Stability was expressed as the oxidation induction time (h), measured with the Rancimat 743 apparatus (Metrohm, Switzerland), using an oil sample of 3.5 g warmed to 100 °C and an air flow of 10 L h−1.
Pigment contents
Oil (7.5 g) was accurately weighted and dissolved in 25 mL of cyclohexane. Chlorophyll and carotenoid contents were determined from the absorption spectra of the oils. Absorption at 670 nm is usually considered to be related to the chlorophyll fraction (pheophytin ‘a’ as its major component) and 470 nm to the absorption of the carotenoid fraction (lutein). Chlorophyll and carotenoid amounts were calculated using the specific extinction values, by the method of Minguez-Mosquera et al. [22]. The extinction coefficients applied were E 0 = 613 for pheophytin ‘a’ and E 0 = 2,000 for lutein. Thus, pigment contents were calculated as follows:
where A is the absorbance and d is the spectrophotometer cell thickness (1 cm). Chlorophyll and carotenoid contents were expressed as milligrams of pheophytin ‘a’ and lutein per kilogram of oil, respectively.
HPLC–MS analysis
Sample preparation
The polar fraction was extracted according to Tsimidou [23]. Of the oil sample, 2.5 g was weighted and dissolved in 5 mL of n-hexane. After that 5 mL of methanol/water (60/40) were added, the mixture was vortexed and then centrifuged at 2,275 g during 10 min. The polar extract was evaporated to dryness in a rotary evaporator under reduced pressure and at a temperature of 35 °C. The residue was dissolved in 0.25 mL of methanol/water (50:50 v/v) and finally filtered through a 0.45-μm filter before the HPLC analysis.
Chromatographic separation
An Agilent 1200-RRLC system (Agilent Technologies, Waldbronn, Germany) equipped with a vacuum degasser, autosampler, a binary pump and a UV–VIS detector was used for the chromatographic determination. Polyphenolic compounds were separated by using a method previously described in bibliography [24]. The compounds separated were monitored with DAD (240 and 280 nm) and with a mass spectrometry detector.
Mass spectrometry
The RRLC system was coupled to a Bruker Daltonik microTOF (time of flight) mass spectrometer (Bruker Daltonik, Bremen, Germany) using an orthogonal electrospray interface (model G1607A from Agilent Technologies, Palo Alto, CA, USA) equipped with an ESI interface. Parameters for analysis were set using negative ion mode with spectra acquired over a mass range from m/z 50–1000. The optimum values of the ESI–MS parameters were as follows: capillary voltage, +4.5 kV; drying gas temperature, 190 °C; drying gas flow, 9 L/min; and nebulizing gas pressure, 2 bar. External calibration was performed using a sodium formiate solution injected at the beginning of the run, and all the spectra were calibrated prior to the polyphenol identification.
The accurate mass data for the molecular ions were processed using the software Data Analysis 3.4 (Bruker Daltonik), which provided a list of possible elemental formulas by using the Generate Molecular Formula TM editor. The Generate Molecular FormulaTM editor uses the sigma FitTM algorithm, a CHNO algorithm, which provides standard functionalities such as minimum/maximum elemental range, electron configuration and ring plus double-bond equivalents, as well as a sophisticated comparison of the theoretical with the measured isotope pattern (SigmaValueTM) for increased confidence in the suggested molecular formula.
Statistical analysis
One-way analysis of variance (ANOVA, Tukey’s honest significant difference multiple comparison) and Pearson’s linear correlations were evaluated using Statistica 6.0 software (2001, StatSoft, Tulsa, OK, USA).
Agglomerative hierarchical clustering (AHC) was performed using XLSTAT 2008.
The standardized variables were used to perform principal component analysis (PCA) and cluster analyses using XLSTAT software (v. 2010.4.01, Addinsoft, NY, USA). To group the accessions based on chemical dissimilarity, agglomerative hierarchical clustering (AHC) was conducted on the Euclidean distance matrix with the Ward method.
LDA statistical data treatment was performed using SPSS (v. 15.0, Statistical Package for the Social Sciences, Chicago, IL, USA).
Results and discussion
Oil yield of olives
As reported in Table 1, all the studied varieties are characterized by a high oil yield according to the classification of Tous et al. [25]. Expressed as percentage of dry matter, the oil yield presented significant differences between the six olive varieties; the content of oil in the samples was in the range of 48–57%, the Chetoui olives being the richest in oil with a mean value of 56.9%, and the Oueslati being the one with the lowest content in oil (47.7%).
VOOs samples included in our study were produced using Abencor system which is suitable for processing small quantities of olives, especially for typical productions such as monovarietal olive oils. However, it is important to mention that the mini and microcondition of olive oil production could lead to obtain an oil different from that produced in industrial conditions as previously reported by Cerretani et al. [26] and Inarejos-García et al. [27].
Free acidity, peroxide value and specific ultraviolet absorbance
For all analysed oils, the mean values of studied quality parameters fell within the range allowed by the regulation EC [17, 18] for the extra-virgin olive oil category (free acidity ≤ 0.8%; peroxide value ≤ 20 Meq O2 kg−1; K270 ≤ 0.22; K232 ≤ 2.5) (Table 1).
Free acidity of the oils studied was in a range from 0.25 to 0.60%. This fact means that, taking into account the acidity of olive samples, all olive oils could be classified as “extra-virgin olive oils”.
Concerning the peroxide values, samples ranged from 2.03 meqO2/kg of Oueslati to 4.0 meqO2/kg of Chetoui and Chemchali. These low values are a measure of the high freshness of the oils analysed [28, 29].
The specific ultraviolet absorbance K232 varied from 1.70 to 2.17 having the highest values in Oueslati, Chetoui and El Hor varieties, while K270 ranged from 0.20 of Oueslati to 0.13 of Chemlali.
Fatty acid composition
Methyl ester fatty acid composition and their levels in the analysed oils are shown in Table 1. As it can be observed, oleic (C18:1), linoleic (C18:2) and palmitic (C16:0) acids are the major fatty acids present in the studied samples. The fatty acid composition of olive oils varies widely depending on the cultivar. These findings are in good agreement with those of other authors working on Tunisian olive oil varieties [12, 30].
Among studied samples, the Oueslati olive oil showed the highest percentage of oleic acid (C18:1) and the lowest percentage of linoleic acid (C18:2) (69.9 and 12.1%, respectively), and the Chetoui olive oil showed the lowest percentage of palmitic acid which did not exceed 11.5%. Concerning palmitoleic (C16:1), stearic (C18:0), linolenic (C18:3) and arachidic (C20:0) acids, the studied olive oil varieties presented low amounts of all of them. The highest percentage of palmitoleic acid was found in Chemlali cultivar, stearic acid presented the highest amount in Chetoui variety, while Jarboui olive oil was the richest in linolenic acid.
Other interesting points for the chemical characterization of studied oils are the proportions of some classes of free fatty acids. The monounsaturated fatty acids have great importance because of their nutritional implication and effect on oxidative stability of oils [5]. Table 1 shows that the proportion of monounsaturated fatty acids also changed according to the cultivar. It reached a maximum value of 71.3% for the Oueslati olive oil, which was characterized, among the studied oils, by the highest MUFAs/PUFAs and C18:1/C18:2 ratios (5.6 and 5.8, respectively). The C18:1/C18:2 ratio has the most marked relationship with stability, and it is said that an oil presents a good stability index if this value is over 7. Nevertheless, Tunisian olive oils are described in bibliography to present lower C18:1/C18:2 ratios compared to most of the European ones [12, 31].
Pigment contents
The olive oil colour is directly related to the chlorophyll and carotenoid contents, and it has been proposed as a characterizing factor and as a quality index related to the oil extraction method and to the olive variety [22]. Besides, the colour is the first attribute of virgin olive oil evaluated by consumers. In analysed oils and according to the cultivar, chlorophyll and carotenoid contents ranged from 2.17 and 0.87 mg/kg to 5.42 and 2.41 mg/kg, respectively (Table 1), Jarboui olive oil being the richest in terms of both of them.
Identification and quantification of phenolic compounds
The identification of phenolic compounds was carried out comparing their migration times, UV data and mass spectra provided by TOF–MS with those of authentic standards when available. Remaining compounds were identified by the interpretation of their mass spectra obtained by the TOF–MS using Generate Molecular Formula Editor. In this way, 24 phenolic compounds could be identified. Table 2 includes the identified compounds, migration time, molecular formula, calculated and experimental m/z, sigma value and tolerance (ppm) in generated molecular formula. Major phenolic compounds previously observed in bibliography [32, 33] were also detected in the present study.
To build the calibration curves and carry out the quantification of the identified phenolic compounds, eleven standards were used: hydroxytyrosol (HYTY), tyrosol (TY), oleuropein (Ol), pinoresinol (Pin), luteolin (Lut), apigenin (Apig), taxifolin, vanillic acid, o-coumaric acid, quinic acid and ferulic acid. All of them presented good linearity between different concentrations, and regression coefficients were higher than 0.990 in most cases.
HYTY, TY, Pin, Lut, Apig, taxifolin, vanillic acid, ferulic acid, quinic acid, and o-coumaric acid were quantified using their own commercial standards. HYTY-acetate (HYTY-Ac) was quantified using HYTY calibration curve; elenolic acid (EA), H-elenolic acid (H-EA), Ol aglycon (Ol Agl), decarboxylated Ol Agl (D-Ol Agl), 10-H-OL Agl, ligtroside aglycon (Lig Agl), decarboxylated Lig Agl (D-Lig Agl), H-D-Ol Agl, methyl D-Ol Agl were quantified with oleuropein calibration curve; acetoxy Pin (Ac-Pin), H-Pin and syringaresinol with Pin calibration curve and sinapinic acid using Ferulic acid calibration curve.
Table 3 summarizes the concentrations of the phenolic compounds identified in the different olive oil varieties expressed as mg/kg of oil. It could be observed that the distribution of phenolic compounds varied significantly in the different cultivars (p ≤ 0.05). Among major secoiridoids, Ol Agl, Lig Agl and D-Ol Agl were found in higher concentrations in Chetoui/El Hor (1839.9/1915.9 mg/kg), Chemlali (303.15 mg/kg) and Chemchali (2455.3 mg/kg), respectively. Meanwhile, Oueslati and Jarboui presented the lowest content in Ol Agl (222.6 mg/kg) and D-Ol Agl (146.1 mg/kg), respectively.
As regards simple phenols, El Hor cultivar was the richest in terms of HYTY and TY (10.9 mg/kg).
The rest of minor simple phenols identified were present in small amounts for all the samples. Five phenolic acids occurred in the studied samples. Quinic acid presented the highest levels (0.5–5.1 mg/kg), o-coumaric acid ranged from 0.1 up to 0.7 mg/kg, ferulic acid 0.0–0.1 mg/kg, sinapinic acid 0.1–0.6 mg/kg and vanillic acid which was present as traces in Oueslati, Chemlali, El Hor and Jarboui EVOOs was only quantified in Chetoui and Chemchali samples (0.9 and 1.5 mg/kg, respectively).
(+)-Ac-Pin was the lignan which presented higher amounts ranging from 0.46 to 4.45 mg/kg, followed by syringaresinol (0.5–2.3 mg/kg), (+)-Pin (0.3–1.8 mg/kg) and (+)-H-Pin (0.3–0.7 mg/kg), Oueslati olive oil being the richest in terms of lignans.
Concerning the flavonoid composition, luteolin was the most abundant flavonoid in studied samples, and the Jarboui EVOO was characterized by the highest amounts of both flavones Lut and Apig (10.20 and 2.86 mg/kg, respectively) and by the presence of the flavonol (+)-taxifolin which was not detected for the rest of studied oils.
It is known that the oxidative stability variations are affected by some minor compounds such as phenols and tocopherols [34] and as reported by other authors, oxidative stability was positively correlated to secoiridoids amounts (r = 0.940, p < 0.001) [35]. In general, our results agree with those previously reported. In fact, positive correlations were found between major secoiridoids and Ol Agl and DPPH radical scavenging capacity (r = 0.640 and r = 0.715; p ≤ 0.05, respectively). The correlations were even higher between oxidative stability and D-Lig Agl and major secoiridoids (r = 0.822 and r = 0.814; p ≤ 0.05, respectively).
Oxidative stability and radical scavenging activity
Oxidation stability is an important property of olive oil quality and is affected by lipid composition and different antioxidant compounds whose levels may be influenced by cultivar, year and place of production [3].
The oxidative stability of the extra-virgin olive oils was measured as the induction time determined using the Rancimat method. The highest oxidative stability was presented by Chemchali oil with a mean value of 58.8 h. This characteristic may be partially attributed to the low level of rainfall in the south region where it was cultivated as demonstrated by Tovar et al. [36], since oxidative stability is affected significantly by water regime that determines the phenol content of the olive fruit. Furthermore, the stability is the result of the oxidant–antioxidant balance. The weight of the antioxidants as phenolic compounds could be greater than this of fatty acid profile. In fact, Chemchali olive oil did not present the best MUFA/PUFA ratio (4.7 ± 0.0) however presented the highest content in phenolic compounds (3564.1 ± 176.3 mg/kg), and as the result it is the oil with the highest oxidative stability value. Oueslati was the oil with the highest MUFA/PUFA ratio (5.5 ± 0.3); however, a lower concentration of phenolic compounds (2752.3 ± 79.5 mg/kg) provides a lower oxidative stability value (48.5 h).
It is known that especially antioxidant properties are very important due to the scavenging activity of free radicals in foods and in biological systems [37]. When an antioxidant reacts with a free radical, it yields an electron, is oxidized, and becomes a weak, non-toxic free radical that is stable and unable to propagate the reaction. An exogenous supply of antioxidants through the diet is necessary because of the incomplete efficiency of endogenous human antioxidant defences and the occurrence of situations in which excessive free radicals are produced [38].
Olive oils were examined for their radical scavenging activity towards the stable DPPH free radical. As reported in Table 1, the Chetoui, El Horr and Chemchali were the cultivars which showed higher percentage of DPPH reduced and thus presented higher antioxidant capacity to scavenge the free DPPH radical.
A positive correlation was found between the oxidative stability and the radical scavenging capacity (r = 0.718; p ≤ 0.05).
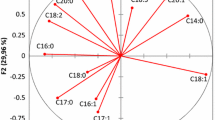
Principal component analysis and cluster analysis
PCA was applied to all VOO samples. Except C20:0, which showed no significant variation among studied VOOs (p = 0.117, α = 0.05), all variables were submitted to a PCA.
The first three PCs explained 73.9% of variance. The first PC accounted for 30.4% of total variance and was highly correlated with luteolin (r = 0.919 at α = 0.05), apigenin (r = 0.911 at α = 0.05), taxifolin (r = 0.938 at α = 0.05), oleic (r = −0.883 at α = 0.05) and linoleic (r = 0.873 at α = 0.05) acids. The second PC (22.4% of variance) was correlated to OL Agl (r = 0.888 at α = 0.05), quinic acid (r = 0.844 at α = 0.05) and Ac Pin (r = −0.814 at α = 0.05), while the third PC was correlated to oxidative stability (r = 0.925 at α = 0.05), EA (r = −0.898 at α = 0.05) and vanillic acid (r = 0.850 at α = 0.05).
Figure 1 shows a projection of the six cultivars in the space defined by the first three principal components. We can distinguish the presence of four principal groups. Group 1 consists of Chemlali and Oueslati varieties which produce oils rich in EA and with fewer amounts of Ol Agl and Lut. Group 2 composed of the variety Chemchali, which is the one that presented the highest oxidative stability, was richer in D-Ol Agl, H-D-Ol Agl and presented low amounts of EA. Group 3 consists of the Chetoui and El Hor olive oil varieties that are characterized by higher amounts of Ol Agl and quinic acid and low amount of Ac-Pin. The last group was the Jarboui variety characterized by the presence of taxifolin, higher amounts of linoleic acid, Lut and Apig and lower amounts of oleic acid.
The results obtained with HCA are analogous to those from PCA: ‘Jarboui’ is the most dissimilar of the varieties, and low similarity exists between ‘Chétoui’, and ‘El Hor’ and the rest of varieties (Fig. 2).
LDA analysis
Linear discriminant analysis (LDA) is one of the most commonly used classification techniques. LDA is a supervised classificatory technique widely recognized as an excellent tool to obtain vectors showing the maximal resolution between a set of previously defined categories. In LDA, vectors minimizing the Wilks’ lambda, λw, are obtained [39]. This parameter is calculated as the sum of squares of the distances between points belonging to the same category divided by the total sum of squares.
Using the normalized variables, an LDA model capable of classifying the EVOO samples according to their respective geographical origin was constructed (North, Centre and South).
When the LDA model was constructed, a good resolution between all the category pairs was achieved (λw = 0.001) as it can be seen in Fig. 3. For this model, and using leave-one-out validation, all the points of the training set were correctly classified. The corresponding evaluation set was then used to check the prediction capability of the model. Using a 95% probability, all the objects were correctly assigned.
Table 4 shows the variables selected by the SPSS stepwise algorithm (the predictors with large discriminant capabilities) and the corresponding standardized coefficients of this model. Variables such as oleic acid and EA were also among the variables that explained the largest portion of the variance in PCA. Besides, oleic acid is the most abundant fatty acid present in olive oil, and the rest of the variables constitute some of the most representative phenolic compounds of olive oil.
Conclusion
After the study of the phenolic profile and other chemical parameters for each of the six Tunisian extra-virgin olive oils and the use of specific statistical analysis, it could be observed that there were significant differences among studied varieties. Specifically, the statistical analysis allowed distinguishing the monovarietal olive oils depending on their concentrations of oleic and linoleic acids, apigenin, luteolin, quinic acid, vanillic acid, taxifolin, oleuropein aglycone, pinoresinol acetate, elenolic acid and oxidative stability. Furthermore, it was possible to classify the varieties of olive oil depending on their geographical origin by using LDA analysis as long as each variety had been grown in its own place of origin. In the case of LDA analysis also variables as oleic acid and elenolic acid play an important role as discriminating functions in the differentiation of the geographical origin.
These preliminary findings would be hopefully confirmed by the analysis of a larger number of samples that take into consideration the variation of phenolic profile and chemical composition of EVOOs, which may also arise from agronomical practices, maturation index, harvesting years and processing technologies.
References
Haddada FM, Manai H, Oueslati I, Daoud D, Sánchez J, Osario E, Zarrouk M (2007) Fatty acid, triacylglycerol, and phytosterol composition in six Tunisian olive varieties. J Agric Food Chem 55:10941–10946
Aparicio R, Luna G (2002) Characterisation of monovarietal virgin olive oils. Eur J Lipid Sci Technol 104(9–10):614–627
Tura D, Gigliotti C, Pedo S, Failla O, Bassi D, Serraiocco A (2007) Influence of cultivar and site of cultivation on levels of lipophilic and hydrophilic antioxidants in virgin olive oils (Olea Europea L.) and correlations with oxidative stability. Sci Hortic 112:108–119
Gómez-Caravaca AM, Cerretani L, Bendini A, Segura-Carretero A, Fernández-Gutiérrez A, Del Carlo M, Compagnone D, Cichelli A (2008) Effect of fly attack (Bactrocera oleae) on phenolic profile and selected chemical parameters of olive oil. J Agric Food Chem 56:4577–4583
Martínez de Victoria E, Mañas M (2001) In: Barranco D, Fernández-Escobar R, Rallo L (eds) El cultivo del olivo. MundiPrensa, Madrid
Visioli F, Galli C (1994) Oleuropein protects low density lipoproteins from oxidation. Life Sci 55:1965–1971
Visioli F, Galli C (1998) The effect of minor constituents of olive oil on cardiovascular disease: New findings. Nutr Rev 56:142–147
Ben Temine S, Abaza L, Ben Youssef N, Taamalli W, Msallem M, Daoud D, Zarrouk M (2004) Chemical and thermal characterization of Tunisian extra virgin olive oil from Chetoui and Chemlali cultivars and different geographical origin. Riv Ital Sostanze Grasse 81:277–283
Guerfel M, Ouni Y, Taamalli A, Boujnah D, Stefanoudaki E, Zarrouk M (2009) Effect of location on virgin olive oils of the two main Tunisian olive cultivars. Eur J Lipid Sci Technol 111:926–932
Guerfel M, Baccouri O, Boujnah D, Chaibi W, Zarrouk M (2009) Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci Hortic 119:257–263
Kotti F, Chiavaro E, Cerretani L, Barnaba C, Gargouri M, Bendini A (2009) Chemical and thermal characterization of Tunisian extra virgin olive oil from Chetoui and Chemlali cultivars and different geographical origin. Eur Food Res Technol 228:735–742
Baccouri O, Cerretani L, Bendini A, Caboni MF, Zarrouk M, Pirrone L, Daoud D (2007) Preliminary chemical characterization of Tunisian monovarietal virgin olive oils and comparison with Sicilian ones. Eur J Lipid Sci Technol 109:1208–1217
Dabbou S, Issaoui M, Servili M, Taticchi A, Sifi S, Montedoro GF, Hammami M (2009) Characterisation of virgin olive oils from European olive cultivars introduced in Tunisia. Eur J Lipid Sci Technol 111:392–401
Dabbou S, Issaoui M, Esposto S, Sifi S, Taticchi A, Servili M, Montedoro GF, Hammami M (2009) Cultivar and growing area effects on minor compounds of olive oil from autochthonous and European introduced cultivars in Tunisia. J Sci Food Agric 89:1314–1325
Uceda M, Hermoso M (1998) La calidad del aceite de oliva. In: Barranco D, Fernandez-Escobar R, Rallo L (eds) El Cultivo del Olivo. Junta de Andalucıa Ediciones Mundi-Prensa, Madrid (Spain), pp 547–572
Donaire JP, Sanchez-Raya AJ, Lopez-George JL, Recalde L (1977) Etudes physiologiques et biochimiques de l’olive: I. Variations de la concentration de divers metabolites pendant son cycle evolutif. Agrochimica 21:311–321
European Union Commission Regulation 2568/91 (1991) Characteristics of olive and olive pomace oils and their analytical methods. Off J Eur Commun L248:1–82
European Union Commission Regulation (EC) No 2527/95 of 27 October 1995 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off J Eur Commun L248
European Union Commission Regulation 2568/1.7.91 (1991) On the characteristics of olive oils and kernel olive oils and on their methods of analysis. Off J Eur Commun L248
Kalantzakis G, Blekas G, Pegklidou K, Boskou D (2006) Stability and radical-scavenging activity of heated olive oil and other vegetable oils. Eur J Lipid Sci Technol 108:329–335
Gutiérrez F (1989) Determinacion de la estabilidade oxidativa de aceites de oliva virgenes: comparacion entre del método A.O.M. y el método Rancimat. Grasas y Aceites 40:1–5
Minguez-Mosquera MI, Rejano L, Gandul B, Sanchez AH, Garrido J (1991) Color-pigment correlation in virgin olive oil. J Am Oil Chem Soc 68:332–336
Tsimidou M (1998) Polyphenols and quality of virgin olive oil in retrospect. Ital J Food Sci 10:99–116
García-Villalba R, Carrasco-Pancorbo A, Oliveras-Ferraros C, Vázquez-Martín A, Menéndez JA, Segura-Carretero A, Fernández-Gutiérrez A (2010) Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J Pharm Biomed Anal 51:416–429
Tous J, Romero A (1993) Variedades de olivo. Con especial referencia a Cataluña. Fundación “La Caixa”, Barcelona
Cerretani L, Bendini A, Rotondi A, Lercker G, Gallina-Toschi T (2005) Analytical comparison of monovarietal virgin olive oils obtained by both a continuous industrial plant and a low-scale mill. Eur J Lipid Sci Technol 107(2):93–100
Inarejos-García AM, Gómez-Rico A, Salvador MD, Fregapane G (2009) Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur Food Res Technol 228:671–677
Cerretani L, Bendini A, Del Caro A, Piga A, Vacca V, Caboni MF, Gallina-Toschi T (2006) Preliminary characterisation of virgin olive oils obtained from different cultivars in Sardinia. Eur Food Res Technol 222:354–361
Rotondi A, Bendini A, Cerretani L, Mari M, Lercker G, Gallina-Toschi T (2004) Effect of olive ripening degree on the oxidative stability and organoleptic properties of Cv. Nostrana di Brisighella extra virgin olive oil. J Agric Food Chem 52:3649–3654
Haddada FM, Manai H, Daoud D, Fernandez X, Lizzani-Cuvelier L, Zarrouk M (2007) Profiles of volatiles compounds from some monovarietal Tunisian virgin olive oils. Comparison with French PDO. Food Chem 103:467–476
Zarrouk W, Baccouri B, Taamalli W, Trigui A, Daoud D, Zarrouk M (2009) Oil fatty acid composition of eighteen Mediterranean olive varieties cultivated under the arid conditions of Boughrara (southern Tunisia). Grasas y Aceites 60:498–506
Carrasco-Pancorbo A, Neusüß C, Pelzing M, Segura-Carretero A, Fernández-Gutiérrez A (2007) CE- and HPLC-TOF-MS for the characterization of phenolic compounds in olive oil. Electrophoresis 28:806–821
Garcia A, Brenes M, Romero C, Garcia P, Garrido A (2002) Study of phenolic compounds in virgin olive oils of the Picual variety. Eur Food Res Technol 215:407–412
Velasco J, Dobarganes C (2002) Oxidative stability of virgin olive oil. Eur J Lipid Sci Technol 104:661–676
Baccouri O, Guerfel M, Baccouri B, Cerretani L, Bendini A, Lercker G, Zarrouk M, Ben Miled DD (2008) Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem 109:743–754
Tovar MJ, Romero MP, Alegre S, Girona J, Motilva MJ (2002) Composition and organoleptic characteristic of oil from Arbequina olive (Olea europaea L.) trees under deficit irrigation. J Sci Food Agric 82:1755–1763
Gulcin I (2006) Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803–811
Samaniego-Sanchez C, Troncoso González AM, Garcıa-Parrilla MC, Quesada Granados JJ, García López, de la Serrán H, López Martínez MC (2007) Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal Chim Acta 593:103–107
Vandeginste BGM, Massart DL, Buydens LMC, De Jong S, Lewi PJ, Smeyers-Verbeke J (1998) Data handling in science and technology–Part B. Elsevier Science BV, Amsterdam
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taamalli, A., Gómez-Caravaca, A.M., Zarrouk, M. et al. Determination of apolar and minor polar compounds and other chemical parameters for the discrimination of six different varieties of Tunisian extra-virgin olive oil cultivated in their traditional growing area. Eur Food Res Technol 231, 965–975 (2010). https://doi.org/10.1007/s00217-010-1350-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1350-3