Abstract
Edible films were prepared from the gelatin of farmed giant catfish skin (GC) (Pangasianodon gigas), bovine bone gelatin (BB) and their combination. The physical, mechanical, thermal and chemical properties of the resulting films were characterized and compared. The molecular weight distributions of the giant catfish skin gelatin-containing samples had high quantities of α-chains, β-components and high molecular weight cross-links. The bovine bone gelatin-containing samples showed low contents of major bands with high degradation band components. The GC films had higher water activity (0.45) and mechanical properties [tensile strength: TS 41 MPa and elongation at break: EAB 34 (%)] but lower thickness (38 μm) and water vapor permeability than those of BB films. The lowest light transmission (200–800 nm) and film transparency (3.34) were found in the GC films. However, the color attribute (L, a and b) of BB films was closer to the low-density polyethylene commercial film (ΔE 1.2). The highest value of film and protein solubility (100%) was found in BB film, while the lowest value (41–56%) was found in GC film. The GC films showed the most compact, smooth and continuous surface without porous structures, which corresponds to the greater properties of films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deterioration of food during storage and transportation could be retarded or prolonged using better packaging. In general, packaging is needed for the protection of food from the outside environmental air and/or from post-contamination by microorganisms. Traditional non-biodegradable plastic films are extensively used for all industries over the world. The destruction of these plastics by burning is considered to be one of the contributors to global warming. To reduce the problem, edible or biodegradable films/materials are being applied in various kinds of packaging and are partly substituting for plastic containers [1]. In addition, edible or biodegradable films could make mass transfer barriers for moisture, oxygen, carbon dioxide, lipids, flavors and aromas between food products and the atmosphere [2, 3]. Edible films prepared from different types or sources of biomaterials such as proteins, polysaccharide, lipids or their blends exhibit different film properties. Among all materials, proteins are considered to provide desirable mechanical, gas barrier and transparency properties, as well as high nutritional value [4]. In recent years, various kinds of protein have gained the interested researchers to develop edible films. Gelatin is an important protein widely used for producing edible films/packaging. This is due to the uniqueness of gelatin among various hydrocolloids to form thermo-reversible properties with a melting point close to human body temperature. This is particularly significant in edible and pharmaceutical applications [5].
Gelatins are usually obtained by partial degradation or denaturation of collagen from bones and hides of bovine or porcine. However, gelatin from alternative sources has received more attention due to the outbreak of bovine spongiform encephalopathy (BSE) and the foot-and-mouth disease crisis [6]. Additionally, gelatin from bovine and porcine sources cannot be used for some foods due to esthetic and religious objections [7]. Gelatins from alternative sources have been produced from various fish by-products such as skin, bones, fins and scales [8]. Recently, gelatin from the skin of farmed giant catfish, which is one of the commercially important fresh water fish species, was successfully extracted and characterized [8]. One of the possible applications of giant catfish skin gelatin is the production of edible films. Therefore, in this work, the giant catfish skin gelatin was used to form edible film, and the properties of the film were also characterized and compared with the films from bovine bone gelatin (one of the most used gelatins) and their gelatin blends.
Materials and methods
Chemicals
Trichloroacetic acid, Folin-Ciocalteu’s phenol reagent, glycerol and other analytical grade reagents were obtained from Merck (Darmstadt, Germany). Beta-mercaptoethanol (βME), l-tyrosine, bovine serum albumin (BSA) and protein markers were purchased from Sigma Chemical Co. (St. Louis, MO, USA.). Electrophoresis reagents were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Food grade bovine bone gelatin (bloom strength ~245) was obtained from Halagel (Thailand) Co., Ltd. (Bangkok, Thailand).
Fish skin preparation
Farmed giant catfish (Pangasianodon gigas) with a range of total length of 75–80 cm were obtained from a farm in Chiang Rai and transported to the School of Agro-Industry, Mae Fah Luang University, Chiang Rai. The fish was washed with tap water. The skin was then removed and scraped to remove fat and foreign matter, and it was then cut into small pieces (0.5 × 0.5 cm). The prepared skin was kept on ice prior to the gelatin extraction.
Extraction of fish skin gelatin
Gelatin was extracted from the washed giant catfish skin by the method described in Jongjareonrak et al. [9]. To remove non-collagenous proteins and pigments, the washed skin was soaked in 0.2 M NaOH with a skin to solution ratio of 1:10 (w/v) at 4 ± 1 °C with continuous gentle stirring. The solution was changed 3 times every 30 min. The alkaline-treated skin was then washed with tap water until it was neutral or faintly basic (pH < 7.5). To swell the collagenous material in the fish skin matrix, the alkaline-treated skin was soaked in 0.05 mol/L acetic acid with a skin to solution ratio of 1:10 (w/v) for 3 h at room temperature (25 ± 1 °C) with continuous gentle stirring. Acid-treated skin was washed as previously described. The swollen fish skin was soaked in distilled water with a skin/water ratio of 1:10 (w/v) at 45 ± 1 °C for 12 h with continuous stirring to extract the gelatin. The mixture was then filtered using two layers of cheesecloth. The resultant filtrate was freeze-dried. The dry matter from freeze-dried process was ground and then referred to as “gelatin powder”.
Preparation of gelatin films
Gelatin powder was mixed with distilled water to obtain the film-forming solution (FFS) with a protein concentration of 4% (w/v). Glycerol was used as a plasticizer at a concentration of 25/100 g protein. The solution was incubated at 60 °C for 30 min in a water bath occasionally stirred. De-aerated film-forming solution (4 ± 0.01 g) was cast onto a rimmed silicone resin plate (50 × 50 mm) and dried with a ventilated oven environmental chamber (model H110 K-30DM; Seiwa Riko Co., Tokyo, Japan) at 25 ± 0.5 °C and 50 ± 5% relative humidity (RH) for 24 h. The dried films obtained were manually peeled.
Analyses of gelatin film properties
Film thickness
The film thickness was measured using a micrometer (Gotech, Model GT-313-A, Gotech Testing Machines Inc, Taiwan). Nine random locations around each of the ten film samples were used for thickness determination.
Water activity
The water activity (Aw) of the gelatin film samples was determined with a Novasina (Novasina AG, Pfäffikon, Switzerland) at a constant temperature of 25 ± 1 °C. Three samples in each treatment were used for testing.
Mechanical properties
Prior to testing the mechanical properties, the films were conditioned for 48 h at 50 ± 5% RH at 25 °C. The tensile strength (TS) and elongation at break (EAB) were determined with the modification of ASTM method D882–97 (ASTM., 1998) [10] using a Universal Testing Machine (Lloyd Instrument, Hampshire, UK). Five samples (2 × 5 cm) with an initial grip length of 3 cm were used for testing. The cross-head speed was set at 30 mm/min.
Water vapor permeability
Water vapor permeability (WVP) of the film was measured using the ASTM E96-95 standard method (ASTM., 1999), a modified ASTM method [11]. The films were sealed onto a permeation cup containing silica gel (0% RH) with silicone vacuum grease and an O-ring to hold the film in place. The cups were then placed in a desiccator saturated with water vapor at 30 °C. The cups were weighed at 1-h interval over an 8-h period, and the WVP of films was calculated as follows:
where w is the weight gain of the cup (g), x is the film thickness (m), A is the area of exposed film (m2), t is the time of gain (s), and (P 2 −P 1 ) −1 is the vapor pressure differential across the film (Pa). The WVP was expressed as g m−1 s−1 Pa−1. A total of five samples were determined for each film.
Color, light transmission and film transparency
The color of the film was determined using a CIE colorimeter (Hunter associates laboratory, Inc., VA, USA) and expressed as L, a and b. From the Hunter color values, total color difference (ΔE) was calculated as:
where L′, a′ and b′ are values of standard low-density polyethylene (LDPE) film (L ′ = 90.492, a ′ = −1.014 and b ′ = 0.642).
The ultraviolet (UV) and visible light barrier properties of the films were measured at selected wavelengths between 200 and 800 nm, using UV-16001 spectrophotometer (Shimadzu, Kyoto, Japan). The film transparency was calculated by the following equation [12]:
where T600 is the fractional transmittance at 600 nm and x is the film thickness (mm).
Fourier transform infrared (FTIR) spectra analysis
Prior to analysis, films were conditioned in a desiccator containing silica gel for 7 days at room temperature to obtain the most dehydrated films possible. The FTIR spectra of the films were recorded using a horizontal ATR Trough plate crystal cell (451ZnSe; 80 mm long, 10 mm wide and 4 mm thick) (PIKE Technology Inc., Madison, WI) equipped with a Bruker Model Equinox 55 FTIR spectrometer (Bruker Co., Ettlingen, Germany) at room temperature. For the spectra analysis, the film sample was placed onto the crystal cell and it was clamped into the mount of the FTIR spectrometer. A spectrum in the range of 400–4,000 cm−1 with automatic signal gain was collected over 16 scans at a resolution of 4 cm−1. They were rationed against a background spectrum recorded from the clean empty cell at 25 °C. The spectra obtained were used to determine possible interactions of functional groups between gelatin molecules.
Differential scanning calorimetry
Prior to analysis, the films were conditioned in a desiccator containing silica gel for 1 week at room temperature to obtain the most dehydrated films possible. The films’ thermal properties were determined using Perkin Elmer Differential Scanning Calorimetry (DSC) (Model DSC-7, Norwalk, CT, USA). Temperature calibration was performed using the Indium thermogram. The film samples (5–10 mg) were accurately weighed into aluminum pans, sealed and scanned over a range of 20–150 °C with a heating rate of 5 °C/min. Ice water was used as a cooling medium, and the system was equilibrated at 20 °C for 5 min prior to the scan. The empty aluminum pan was used as a reference. The transition temperature and the transition enthalpy were estimated from the maximum of endothermic peak and the area under the DSC thermogram.
Film solubility and protein solubility
The film solubility was determined according to the method of Gennadios et al. [13]. The conditioned film samples were weighed and placed in a 50-mL centrifuge tube containing 10 mL of distilled water. The mixture was shaken at a speed of 250 rpm using a shaker (Heidolth Inkubator 10,000, Schwabach, Germany) for 24 h. The undissolved debris was then removed by centrifugation at 3,000 g for 20 min. The pellet was dried at 105 °C for 24 h and weighed. The weight of the solubilized dry matter was calculated by subtracting it from the initial weight of the dry matter. It was then expressed as a percentage of the total weight.
To determine the protein solubility, the protein concentration in the supernatant was determined using the Lowry method [14]. Protein solubility was expressed as the percentage of the total protein in the film, which was solubilized with 0.5 M NaOH for 24 h.
Electrophoretic analysis
The protein patterns of gelatin, film-forming solution and the gelatin films were analyzed by SDS–PAGE according to the method of Laemmli [15]. To solubilize the films, the samples were mixed with a solubilizing solution (1% SDS). The mixtures were heated at 85 °C for 1 h in a water bath to dissolve the proteins. The solubilized samples were then mixed with a sample buffer (0.5 M Tris–HCl, pH 6.8 containing 4% (w/v) SDS, and 20% (v/v) glycerol) and 10% (v/v) βME at a ratio of 1:1 (v/v). The samples (16 μg protein) were loaded into the polyacrylamide gel made of 7.5% running gel and of 4% stacking gel and subjected to electrophoresis at a constant current of 15 mA per gel using a Mini Protean II unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, the gel was stained with 0.05% (w/v) Coomassie blue R-250 in 15% (v/v) methanol and 5% (v/v) acetic acid and then destained with 30% (v/v) methanol and 10% (v/v) acetic acid.
Surface morphology and gelatin film microstructure
The surface morphology of the gelatin films was examined using a Canon Powershot A95 digital camera (Canon Marketing (Thailand) Co., Ltd., Bangkok, Thailand). The gelatin films’ microstructure was observed using a scanning electron microscope (SEM) (JEOL JSM-5800 LV, Tokyo, Japan) at an acceleration voltage of 10 kV and magnification at 10,000×.
Statistical analysis
The data were subjected to analysis of variance (ANOVA). A comparison of means was carried out by Duncan’s multiple range test. The analysis was performed using a SPSS package (SPSS 10.0 for window, SPSS Inc, Chicago, IL).
Results and discussion
Thickness of gelatin film
The thickness of gelatin-based films from giant catfish skin (GC), bovine bone (BB) and their combination (CG) is shown in Table 1. The average thickness of all the films was in a range of 38−40 μm, and the highest film thickness was observed in the CG film. The thickness of all the films was similar to those values of bigeye snapper (35.32 μm) and brown stripe red snapper (37.18 μm) skin gelatin films (3% protein) as found in Jongjareonrak et al. [9]. Thickness generally affects film properties such as mechanical properties, water vapor permeability, light transmission and film transparency.
Mechanical properties of gelatin film
The mechanical properties of gelatin-based film were expressed in terms of tensile strength (TS) and elongation at break (EAB) (Table 1). The TS of gelatin films varied with sources of gelatin (GC, BB and CG). Among all gelatin, the GC skin gelatin rendered the film with the highest TS (40.74 MPa) and EAB (34.14%) (p < 0.05). The films from BB and CG showed no differences in TS (p > 0.05). The result suggested the difference in film-forming characteristics of gelatin between two sources. Different protein constituents, as well as other components, in different gelatin sources, might govern the film formation. Film formation generally takes place by the development of a three-dimensional network of protein molecules by ionic, hydrophobic and hydrogen bonds [16]. From the results, EAB of GC film was greater than BB film (24.63%). When BB was combined with GC in a ratio of 50:50, the EAB was improved (p < 0.05). The increasing TS and EAB of film is attributed to the occurrence of advanced cross-linking molecules within film structures or by the formation of high molecular weight biopolymers.
Water activity and water vapor permeability of gelatin film (Aw, WVP)
Film cast from GC, BB and CG had water activity in the range of 0.42–0.45 (p > 0.05) (Table 1). Water activity affects the growth and multiplication of microorganisms. When there is Aw < 0.9, growth of most bacteria is inhibited. Microorganisms cease growth at Aw < 0.6. The interactions between water and food components mutually change the properties of food products, especially their stability, texture, taste, and lead to spoilage [17].
The WVP of film from GC, BB and CG was shown in Table 1. The differences in WVP were observed among films prepared from different sources of gelatins (p < 0.05). The CG film showed the highest WVP, while the lowest one was observed in the BB film. Fish gelatins are known to contain lower proline and hydroxyproline; thus, their hydrophobicity increases when compared with gelatin from mammal sources [18]. Constituents with lower polarity could absorb less water from the surrounding atmosphere. Water vapor permeation through a hydrophilic film depends on both diffusivity and solubility of moisture in the film matrix [19]. Additionally, more pronounced protein cross-linking of the film along with more rigid and denser structures might retard water diffusion through the films. The CG film’s water vapor transmissibility rates were higher than GC and BB films, suggesting a decreased value of the film as a barrier to moisture. Film with higher thickness (CG) could adsorb more water from the environment. Water vapor permeability is dependent on the relative polarity of the polymers used. If the films are cationic and strongly hydrophilic, water interacts with the polymer matrix and the water vapor permeability increases.
Light transmission and transparency of gelatin film
Light transmission in UV (200–280 nm) and visible ranges (350–800 nm), as well as the transparency of all films, is shown in Table 2. Generally, all films exhibited lower light transmission in the UV range than in the visible range. The light transmission in the UV range was from 0 to 52.37%, while the transmission in the visible range was from 70 to 90%. The highest transmission in all the selected wavelengths was found in the BB film, while the lowest was found in the GC film. This result suggested that gelatin-based films could prevent UV transmission and therefore retard lipid oxidation induced by UV light in a food system. The result was consistent with Jongjareonrak et al. [9] who observed low transmissions of light in the UV range of fish gelatin-based films. Aromatic amino acids (tyrosine and phenylalanine) are well known to be sensitive chromophores in absorbing light at a wavelength below 300 nm [20]. The increase in light transmission at selected wavelengths of films was in the order of GC, CG and BB. Light transmission values of GC, BB and CG were in a similar range as bigeye snapper and brownstripe red snapper skin gelatin [9].
The highest transparency was obtained in BB film, but no significant difference was observed in CG and GC (p > 0.05) (Table 2). The higher transparency value indicated that the film was less transparent. From the result, it was suggested that GC and CG films were more transparent than BB films. This indicated that the transparency properties of BB films could be improved by combining them with GC. Giant catfish skin gelatin film was transparent and clear enough for use as seen by the packaging.
Color of gelatin film
The Hunter L (Lightness), a (redness/greenness) and b (yellowness/blueness) color values of gelatin-based films are presented in Table 3. The GC films showed significantly lower values of L and a but highest in b value compared to the others (p < 0.05). When compared to the low-density polyethylene film, GC and BB films showed the highest and the lowest of total color differences, respectively. The total color difference (ΔE) value of BB films (1.20) was lower than CG (1.42) and GC (1.56) films, respectively. This indicated that the BB film had color properties closer to the low-density polyethylene film. Among all films, BB film had a lighter color and more yellowness than other films where the highest redness was observed in GC films.
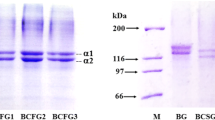
Protein pattern of gelatin film
Protein patterns of gelatin, film-forming solution and gelatin-based film from GC, BB and CG are shown in Fig. 1. The electrophoretic analysis of gelatin in all film-forming solutions showed similar molecular weight distribution to the GC. The predominant bands including α-1, α-2 chains and their dimers (β-components) were observed. Marked differences in protein patterns were observed between BB and GC. The major components of gelatin (α-1, α-2 and β) were clearly observed in giant catfish skin gelatin, while these components were not found in BB. In addition, a high intensity of high molecular weight (HMW) bands (β- and γ-components) was observed in all GC-containing samples, especially in film-forming solutions and films. From the results, no β- and γ-component and slight α1- and α2-chains band intensity was observed in BB, BB film-forming solutions and BB films. The concomitant degradation bands (MW < 100 kDa) were also found in all BB-containing samples. The lower content of high MW cross-links and α-chains might result in the lowered mechanical properties of the BB films, unlike the GC and CG films that have higher mechanical properties.
Thermal properties
The transition temperature (Tt) and transition enthalpy of GC, BB and CG films are shown in Fig. 2. Generally, the transition temperature and transition enthalpy of gelatin films from all samples showed no significant differences, as observed from the maximum of the endothermic transition temperature. Figure 2 presents a typical curve for gelatin showing a glass transition (first small peak: Tg), melting (big endothermic peak: Tm) and deterioration peaks. The glass transition, melting and deterioration peaks of the films prepared from GC, BB and CG were around 55–60 °C, 83–89 °C and 134–140 °C, respectively.
In addition, there is a relatively small endothermic peak (between Tm and deterioration peaks) in GC film, while it is not found in the others. In gelatin, the Tg reported has been explained by the block copolymer model for amino acid content of gelatin. Tg of gelatin occurs at ~60 °C and is associated with the glass transition of an amino acid blocks in the peptide chain. Native fish gelatin film had Tm of 76.5 °C, which is higher than those films made from bovine skin gelatin that had the Tm of 65.06 °C as found by de Carvalho & Grosso [21]. On the other hand, the dry film made from pigskin gelatin had Tm of 91 °C [22]. This difference of the thermal property is reasonable due to the different specification of gelatin source used. However, Bigi et al. [23] reported that the first transition is more likely related to the volatilization of absorbed water in the gelatin sample, while the higher temperature transition is related to the relative amount of triple helix in gelatin.
The transition temperature indicated the precise temperature that caused a disruption of the protein interaction formed during film preparation. Tt from GC (89.50 °C) was higher than that of BB (88.42 °C) and CG (83.36 °C). The higher Tt might be due to the greater imino acid amount (proline and hydroxyproline) of GC (211 residues/1,000 residues) [8]. The amount of imino acids showed the direct positive correlation with the thermal stability of protein via a hydrogen bond [24]. In addition, the integrity and molecular weight of protein chains might contribute to the network structure of the films obtained [25]. The film from BB comprised of proteins with lower molecular weight and greater content of degradation peptides, compared with those of GC (Fig. 1). This might be associated with the weaker network interaction of BB films. As a consequence, melting at lower temperature was found as indicated by the lower Tt in the films from BB (88.42 °C) and CG (83.36 °C). However, CG films had the lowest Tt value (83.36 °C). According to Jongjareonrak et al. [9], the transition peak observed in the DSC curves of gelatin films indicated the presence of the triple-helical structure. Thus, thermal properties of gelatin films were affected by the gelatin source.
The typical DSC thermograms of the gelatin film are shown in Fig. 2. During the heating scan, two endothermic peaks were observed. First, a step change in heat flow was assigned to a glass-to-rubber transition relating to the amorphous regions of a biopolymer. They were in a glassy state at the water contents of interest at room temperature [26, 27]. The glass transition was followed by an endotherm associated with the melting and dissociation of the ordered regions. The resulting thermograms indicated that before the heating scan was taken, gelatin films from BB, GC and their combination (CG) had a partial crystalline structure. These results are also consistent with previous reports [28, 29].
FTIR spectroscopy
FTIR spectra of GC, BB and CG films are depicted in Fig. 3. The spectrum of all gelatin films showed a similar pattern, which indicates that there were no major changes in the functional groups of the gelatin samples used. Gelatin films (GC and CG) revealed absorption band at 3,273 cm−1, (NH-stretching), while BB film showed the major absorption band at 3,293 cm−1. An increased absorption at ~3,293 of BB film compared with others was probably due to the stretching bands of OH-absorbed water molecules. In addition, the exact shape of the difference bands may be influenced by changes in the amide A band of the protein, whose maximum was found at 3,340 cm−1. Absorption bands in the spectra of all films were situated in the amide band region. The amide I and amide II bands of all films were observed at 1,630 and 1,536 cm−1, respectively. These were similar to the amide I (1,630 cm−1) and amide II (1,545 cm−1) of the gelatin films from both bigeye snapper and brownstripe red snapper skin gelatin [9]. Muyonga et al. [30] also reported the amide I and amide II to be at 1,700–1,600 and 1,560–1,500 cm−1, respectively. The amide I vibration mode is primarily a C = O stretching vibration coupled with contributions from the CN stretch, CCN deformation and in-plane NH bending modes. The amide II vibration mode is attributed to an out-of-phase combination of CN stretch and in-plane NH deformation modes of the peptide group [31]. The amide II peak is considered to be much more sensitive to hydration than to secondary structure change [32]. In this study, FTIR spectra analysis was determined in a dry state, and the changes in the amide II band might be related to possible alterations in protein secondary structure. The infrared spectroscopic analysis technique is generally used to determine the secondary structure of protein via the absorption in the amide I and amide II region. From the result, the peaks at amide I and amide II were more intense when GC and BB were combined (CG films). The change in amide I and amide II band intensity for the CG film compared with the GC film suggested that the combination of BB and GC might affect the helix structure and/or alteration in the protein secondary structure of their resulting film (CG). From the result, the change in the films’ FTIR spectra when BB was combined with GC revealed that there might be some interaction between both gelatins. These interactions and/or structural alterations might be associated with the mechanical properties of the resulting films.
Film solubility and protein solubility
The film solubility and protein solubility of GC, BB and CG films are shown in Table 4. From the results, 100% film solubility and protein solubility were observed in the BB films, where only 41.40% film solubility and 56.20% protein solubility were observed in GC. However, when BB was combined with GC, the resulting films (CG) had an intermediate solubility. The lower film and protein solubility observed in the film from GC suggested that the polypeptides in GC underwent more aggregation, leading to more cross-linking and higher molecular weights resulting in lower solubility. The combination of BB and GC might inhibit some inter-chain interactions between GC protein molecules and therefore increase the film and protein solubility of CG film.
The SDS–PAGE results of insoluble and soluble fractions of all films are depicted in Fig. 4. The protein patterns of the insoluble fractions of both GC and CG films were similar to their gelatin patterns. For soluble fractions, all films contained only low molecular weight peptides. These results suggested that high molecular weight peptides including α1-, α2-, ß- and γ-components were the major compositions involved in the formation of film network structures, which underwent cross-linking during film formation, so the films became less soluble.
Surface morphology and gelatin film microstructure
The surface morphology of gelatin film from bovine bone, giant catfish skin and their combination was similar to each other with uniform transparency (Fig. 5). When the film sheet covered the black background, the color of the background remained clearly observable. However, there was more turbidity of the gelatin film present in the dark background compared to the green one, especially for the film containing giant catfish skin gelatins (data not shown). These results were similar to the light transmission and transparency of the gelatin film as mentioned above. Based on the film surface morphology property, applications of edible gelatin film were interesting, especially in food products. When the customers see the product packed inside the package, it is easy decide to buy that product.
SEM micrographs of gelatin films from GC, BB and CG are also observed (data not shown). The GC film had a smooth and continuous surface without grainy and porous structures. This indicated that a film with ordered matrix was formed. For BB films, porous surface with cracks was observed throughout the film surface. However, when GC was combined, the surface of the film became smoother with less cracks and pores. These results suggested that the molecular weight or chain length of polypeptide in gelatin samples might be responsible for the surface structure and properties, especially in the WVP of the resulting films.
Conclusion
The results demonstrated significant differences in both physical and chemical properties between GC and BB films. GC films had lower light transmission and film transparency, but they were higher in WVP and mechanical properties compared to BB film. The combination of BB and GC could improve the properties of BB film. Specific characteristic of GC-based films can benefit some food packaging applications.
References
Cao N, Yang X, Fu Y (2009) Food Hydrocoll 23:729–735
McHugh TH (2000) Nahrung 44:148–151
Kim SJ, Ustunol Z (2001) J Agric Food Chem 49:4388–4391
Ou S, Kwok KC, Kang Y (2004) J Food Eng 64:301–305
Achet D, He XW (1995) Polymer 36:787–791
Helcke T (2000) Int Food Ingred 1:6–8
Sadowska M, Kolodziejska I, Niecikowska C (2003) Food Chem 81:257–262
Jongjareonrak A, Rawdkuen S, Chaijan M, Benjakul S, Osako K, Tanaka M (2010) LWT- Food Sci Tech 43:161–165
Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M (2006) Food Hydrocoll 20:492–501
ASTM (1998) Standard test method for tensile properties of thin plastic sheeting. D882–97. Philadelphia, PA
ASTM (1999) Standard test method for water vapor transmission of materials. E96–95. Philadelphia, PA
Han JH, Floros JD (1997) J Plast Film Sheet 13:287–298
Gennadios A, Handa A, Froning GW, Weller CL, Hanna MA (1998) J Agric Food Chem 46:1297–1302
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:256–275
Laemmli UK (1970) Nature 227:680–685
Hamaguchi PY, Wu Y, Tanaka M (2007) Food Chem 100:914–920
Yang L, Paulson AT (2000) Food Res Int 33:571–578
Gomez-Estaca J, Montero P, Fernandez-Martin F, Gomez-Guillen MC (2009) Food Hydrocoll 23:1334–1341
Bourtoom T (2008) Songkla Nakarin J Sci Tech 30:149–165
Li H, Liu BL, Gao LZ, Chen HL (2004) Food Chem 84:65–69
De Carvalho RA, Grosso CRF (2004) Food Hydrocolloid 18:717–726
Bigi A, Cojazzi G, Panzavolta S, Roveri N, Rubini K, Roveri N (2001) Biomaterial 22:763–768
Bigi A, Panzavolta S, Rubini K (2004) Biomaterial 25:5675–5680
Sikorski ZE, Scott DN, Buisson DH (1984) Crt Rev Food Sci Nutr 20:301–343
Shiku Y, Hamaguchi PY, Benjakul S, Visessanguan W, Tanaka M (2004) Food Chem 86:493–499
Mousia Z, Farhat IA, Blachot JF, Mitchell JR (2000) Polymer 41:1841–1848
Pinhas MF, Blanshard JMV, Derbyshire W, Mitchell JR (1996) J Ther Anal 47:1499–1511
Sobral PJA, Menegalli FC, Guilbert S (1999) In: Colonna P, Guilbert S (eds) Biopolymer science food and non food applications. Editions INRA, Paris
Sobral PJA, Habitante AMQB (2001) Food Hydrocoll 15:377–382
Muyonga JH, Cole CGB, Duodu KG (2004) Food Hydrocoll 18:581–592
Bandekar J (1992) Biochim Biophys Act 1120:123–143
Wellner N, Belton PS, Tathum AS (1996) Biochem J 319:741–747
Acknowledgments
This research was supported by Mae Fah Luang University and the Thailand Research Fund under the TRF-Master Research Grants (Project No. MRG-WII 525 S 088) to Samart Sai-Ut and also under The Senior Research Scholar program. We also thank Prof. Matthew Robert Ferguson, School of Liberal Arts, Mae Fah Luang University for kindly providing suggestions and corrections for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rawdkuen, S., Sai-Ut, S. & Benjakul, S. Properties of gelatin films from giant catfish skin and bovine bone: a comparative study. Eur Food Res Technol 231, 907–916 (2010). https://doi.org/10.1007/s00217-010-1340-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1340-5