Abstract
Acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) from the skin of blacktip shark (Carcharhinus limbatus) were isolated and characterized. The yield of ASC (20.01%) was much higher than that of PSC isolated from the residue of ASC extraction (0.86%). Both collagens had protein as their major constituent with the trace amounts of ash and fat. Based on protein patterns and TOYOPEARL® CM-650M column chromatography, both collagens contained α- and β-chains as their main components and were characterized as type I collagen with the cross-link of α2-chain. Similar peptide maps of both collagens, digested by either V8 protease or lysyl endopeptidase, were observed but they were totally different from those of type I collagen from calf skin hydrolyzed by the same enzyme. Thermal transition temperature (T max) of ASC and PSC were 34.23 and 34.37 °C, respectively. Fourier-transform infrared spectra suggested that both collagens were in triple-helical structure. From zeta potential analysis, isoelectric points (pI) of ASC and PSC were estimated to be 6.78 and 7.02, respectively. Thus, blacktip shark skin may serve as an alternative source of collagen and acid solubilization process could be implemented with ease and high yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Collagen is one of the major structural components of vertebrates and is generally found in skin, bone and other connective tissues [1]. Collagen has been used in leather and film industries, pharmaceutical, cosmetic and biomedical materials and foods [2]. Most commercial collagens are obtained from mammals, mainly from bovine bone, bovine hide and porcine skin [3]. Nevertheless, these collagens have the limitation for applications because of religious constraints and anxiety of consumers about contamination of bovine spongiform encephalopathy (BSE) in products obtained from bovine origin. Consequently, increasing interest has been paid to the alternative collagen sources, especially fish skin and bone from seafood processing by-products due to their abundance and low cost.
Shark is an elasmobranch, which has been exploited for its meat (fillets and fish ball products), fin (dried shark fin), liver (shark liver oil), skin (sun-dried products, leather) and cartilage (shark cartilage powder and shark cartilage chondroitin) [4]. Shark skin can serve as an excellent source of collagen with unique characteristics. Collagens have been isolated and characterized from elasmobranchs, including shark, skate and ray [5–7]. Blacktip shark have been used for shark fin and fillet production in Thailand. Skin and cartilage generated during processing of blacktip sharks have not been fully utilized and are mainly used for fish meal or fertilizer with a low market value. Therefore, these collagenous materials could serve as a potential source of extractable and value-added collagen. Additionally, the cost of waste management or waste disposal could be reduced. However, little information regarding the collagen from elasmobranchs, especially shark from tropical region, is available. Therefore, the objectives of this investigation were to isolate and characterize collagen from blacktip shark skin, a by-product from shark fin processing.
Materials and methods
Chemicals and enzymes
All chemicals used were of analytical grade. Type I collagen from calf skin, pepsin from porcine stomach mucosa (EC 3.4.23.1), (750 units/mg dry matter), V8 protease from Staphylococcus aureus (EC 3.4.21.19) and lysyl endopeptidase from Achromobacter lyticus (EC 3.4.21.50) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Type II collagen from porcine cartilage, high molecular weight markers and TOYOPEARL® CM-650M were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan), GE Healthcare UK Limited (Buckinghamshire, UK) and Tosoh Corporation (Tokyo, Japan), respectively.
Collection and preparation of shark skin
Skin of blacktip shark (Carcharhinus limbatus) with a size of 70–100 cm in length was obtained from Blue Ocean Food Products Co., Ltd. in Samutsakhon province, Thailand. The frozen shark skin (10 kg) packed in a zip-lock bag (1 kg/bag) was placed in ice with the skin to ice ratio of 1:2 (w/w) in a polystyrene box and transported to the Department of Food Technology, Prince of Songkla University by bus within 10 h. Upon arrival, the ice was removed and the skin was then kept at −20 °C until used; the storage time was not longer than 2 months. Prior to collagen extraction, the frozen skin was thawed with running tap water until the core temperature reached 5 °C. Then, it was washed with cold tap water (≤10 °C). The residual meat on the skin was removed by a stainless knife. Thereafter, the clean skin was washed thoroughly with cold tap water and cut into small pieces (1.0 × 1.0 cm2) using a pair of scissors. Prepared skin was washed with cold tap water again until ammonia smell disappeared. Two different lots of shark skin were used; for each lot, the extraction was carried out in duplicate.
Proximate analysis
Shark skin, acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) were subjected to proximate analysis. Moisture, ash, fat and protein contents were determined according to the AOAC [8] methods 950.46, 928.08, 960.39 and 920.153, respectively. The converting factor of 6.25 was used for calculation of protein content.
Assay for proteolytic activity of pepsin
Proteolytic activity of porcine pepsin was determined using hemoglobin as a substrate at pH 2.0 and 50 °C for 20 min according to the method of Nalinanon et al. [9]. After termination of enzymatic reaction, the oligopeptide content in the supernatant was determined by the method of Lowry [10] using tyrosine as a standard. One unit of activity was defined as that releasing 1 μmole of tyrosine per min (μmol Tyr/min).
Isolation of collagen from shark skin
The collagens, both ASC and PSC, were extracted according to the method of Kittiphattanabawon et al. [11] and Nalinanon et al. [12] with a slight modification. All procedures were carried out at 4 °C. To remove non-collagenous proteins, the prepared shark skin was mixed with 0.1 M NaOH at a solid/alkali solution ratio of 1:10 (w/v). The mixture was stirred for 6 h continuously using an overhead stirrer (W20.n, IKA®-Werke GmbH & CO. KG, Staufen, Germany) at a speed of 300 rpm and the alkali solution was changed every 2 h. Then, the pretreated skin was washed with cold tap water until pH of wash water became neutral or faintly basic.
To extract collagen, the pretreated skin was soaked in 0.5 M acetic acid with a solid to solvent ratio of 1:15 (w/v) for 48 h with a continuous stirring, followed by filtration with two layers of cheesecloth. The collagen in filtrate was precipitated by adding NaCl to a final concentration of 2.6 M in the presence of 0.05 M Tris(hydroxymethyl) aminomethane, pH 7.5. The resultant precipitate was collected by centrifugation at 20,000g at 4 °C for 60 min using a refrigerated centrifuge (Avanti® J-E, Beckman Coulter, Palo Alto, CA, USA). The pellet was dissolved in a minimum volume of 0.5 M acetic acid. The solution was then dialyzed against 25 volumes of 0.1 M acetic acid for 12 h, followed by the same volume of distilled water for 48 h. Then, the resulting dialysate was freeze-dried using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). The obtained collagen from acid solubilization process was referred to as “acid-soluble collagen, ASC”. The undissolved residue obtained after acid extraction was used for PSC extraction. The residue was soaked in 0.5 M acetic acid with a solid to solvent ratio of 1:15 (w/v) and porcine pepsin (20 unit/g of residue) was added. The mixtures were continuously stirred at 4 °C for 48 h, followed by filtration using two layers of cheesecloth. The filtrate was collected and subjected to precipitation and dialysis in the same manner that was used for ASC as previously described. The obtained collagen from pepsin solubilization process was referred to as “pepsin-soluble collagen, PSC”. Both collagens were subjected to analyses.
Characterization of collagen from shark skin
UV–Vis measurement
Collagen was dissolved in 0.5 M acetic acid to obtain a concentration of 1 mg/mL. The solution was then subjected to UV–Vis measurement using a double-beam spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan). Prior to measurement, the baseline was set with 0.5 M acetic acid. The spectrum was obtained by scanning the wavelength in the range of 220–600 nm with a scan speed of 50 nm/min at room temperature.
Amino acid analysis
ASC and PSC were hydrolyzed under reduced pressure in 4.0 M methanesulphonic acid containing 0.2% (v/v) 3-2(2-aminoethyl) indole at 115 °C for 24 h. The hydrolysates were neutralized with 3.5 M NaOH and diluted with 0.2 M citrate buffer (pH 2.2). An aliquot of 0.4 mL was applied to an amino acid analyzer (MLC-703; Atto Co., Tokyo, Japan).
Electrophoretic analysis
Protein pattern was analyzed by the method of Laemmli [13]. The collagens were dissolved in 5% SDS solution. The mixtures were then heated at 85 °C for 1 h using a temperature-controlled water bath model W350 (Memmert, Schwabach, Germany), followed by centrifugation at 8,500g for 5 min to remove undissolved debris. Solubilized samples were mixed with the sample buffer (0.5 M Tris–HCl, pH 6.8 containing 4% SDS and 20% glycerol in the presence or absence of 10% (v/v) βME) at a 1:1 (v/v) ratio. The mixtures were loaded onto polyacrylamide gel made of 7.5% separating gel and 4% stacking gel and subjected to electrophoresis at a constant current of 20 mA/gel. After electrophoresis, gels were fixed with a mixture of 50% (v/v) methanol and 10% (v/v) acetic acid for 30 min, followed by staining with 0.05% (w/v) Coomassie blue R-250 in 15% (v/v) methanol and 5% (v/v) acetic acid for 1 h. Finally, the gels were destained with a mixture of 30% (v/v) methanol and 10% (v/v) acetic acid for 1 h and destained again with the same solution for 30 min. High molecular weight markers were used to estimate the molecular weight of proteins. Type I and type II collagens from calf skin and porcine cartilage, respectively, were used as standard collagens.
TOYOPEARL® CM-650M column chromatography
Collagen components were fractionated using TOYOPEARL® CM-650M column chromatography according to the method of Nagai et al. [14] with a slight modification. Collagen samples (20 mg) were dissolved in 5 mL of starting buffer (20 mM sodium acetate buffer, pH 4.8) at room temperature (25–26 °C) and were placed in boiling water for 1 min. The mixtures were centrifuged at 20,000g at room temperature for 30 min to remove undissolved debris. The supernatants were loaded onto the TOYOPEARL® CM-650 M column (1.0 × 5.0 cm) previously equilibrated with the starting buffer, approximately 10 volumes of column, at a flow rate of 60 mL/h. After loading, the unbound proteins were washed with the starting buffer until A230 was less than 0.05. The column was eluted with a linear gradient of 0–0.2 M NaCl in the same buffer at a flow rate of 40 mL/h with a total volume of 200 mL. The eluent was monitored at 230 nm and the 2-mL fractions were collected. The selected fractions were subjected to SDS-PAGE using 7.5% separating gel and 4% stacking gel as previously described in section of electrophoretic analysis.
Peptide mapping
Peptide mappings of collagen samples were performed according to the method of Kittiphattanabawon et al. [11] with a slight modification. The samples (6 mg) were dissolved in 1 mL of 0.1 M sodium phosphate, pH 7.2 containing 0.5% (w/v) SDS. The mixtures were then preheated at 37 °C for 3 h and 300 μL transferred to test tubes for digestion. To initiate the digestion, 20 μL of each enzyme solution, including Staphylococcus aureus V8-protease or lysyl endopeptidase from Achromobacter lyticus with concentrations of 5 and 50 μL/mL, respectively, were added to the mixtures. The reaction mixtures were then incubated at 37 °C for 1 h. The reactions were terminated by subjecting the reaction mixture to boiling water for 3 min. Peptides generated by the protease digestion were determined by SDS-PAGE using 7.5% separating gel and 4% stacking gel. Peptide mapping of type I collagen from calf skin was conducted in the same manner and the peptide patterns were compared.
Thermal transition analysis
The collagens were rehydrated by adding deionized water to dried samples at a solid to solution ratio of 1:40 (w/v). The mixtures were allowed to stand for 2 days at 4 °C prior to analysis. Thermal transition analysis was performed using a differential scanning calorimeter (DSC7, Perkin Elmer, Norwalk, CT, USA). Temperature calibration was run using the Indium thermogram. The sample (5–10 mg) was accurately weighed into aluminum pans and sealed. The sample was scanned at 1 °C/min over the range of 20–50 °C using iced water as the cooling medium. An empty pan was used as the reference. The maximum transition temperature (T max) was determined from the thermogram. Total denaturation enthalpy (ΔH) was estimated by measuring the area of DSC thermogram.
Zeta potential analysis
Collagens were dissolved in 0.5 M acetic acid to obtain a final concentration of 0.05% (w/v). The mixtures were continuously stirred at 4 °C using a magnetic stirrer model BIG SQUID (IKA®-Werke GmbH & CO.KG, Staufen, Germany) until the samples were completely solubilized. Zeta (ζ) potential of collagen solutions was measured by zeta potential analyzer model ZetaPALs (Brookhaven Instruments Co., Holtsville, NY, USA). Solutions (20 mL) were transferred to autotitrator model BI-ZTU (Brookhaven Instruments Co., Holtsville, NY, USA), in which the pH of solutions was adjusted to 2–12 using either 1.0 M nitric acid or 1.0 M KOH. The zeta potential of the solution obtained at all pHs determined was recorded.
Fourier-transform infrared (FTIR) spectroscopy
FTIR spectra of collagens were obtained using a Bruker Model EQUINOX 55 FTIR spectrometer (Bruker, Ettlingen, Germany) equipped with a deuterated l-alanine tri-glycine sulphate (DLATGS) detector. The horizontal attenuated total reflectance accessory (HATR) was mounted into the sample compartment. The internal reflection crystal (Pike Technologies, Madison, WI, USA), made of zinc selenide, had a 45° angle of incidence to the IR beam. Spectra were acquired at a resolution of 4 cm−1 and the measurement range was 4,000–650 cm−1 (mid-IR region) at room temperature. Automatic signals were collected in 32 scans at a resolution of 4 cm−1 and were ratioed against a background spectrum recorded from the clean empty cell at 25 °C. Analysis of spectral data was carried out using a OPUS 3.0 data collection software program (Bruker, Ettlingen, Germany).
Results and discussion
Proximate composition of blacktip shark skin, ASC and PSC
Shark skin contained moisture (67.62%) as its major component. Its protein and ash contents were 27.40 and 4.15%, respectively, along with trace amounts of fat (0.17%). Bigeye snapper (Priacanthus tayenus) skin contained moisture, protein, ash and fat contents of 64.08, 31.99, 3.23 and 0.98%, respectively [11]. Yields of ASC and PSC were 20.01 and 0.86% (wet weight basis), respectively. Based on the extractable collagen obtained, ASC and PSC constituted 95.88 and 4.12%, respectively. It was noted that PSC from shark skin was much lower than that of brownstripe red snapper (Lutjanus vitta) skin collagen (34.30% based on extractable collagen weight) [15]. In general, intermolecular cross-links occurring at telopeptide region were cleaved by pepsin, leading to increased solubilization [1]. Collagen from blacktip shark skin might have lower intermolecular cross-links compared with that from brownstripe red snapper skin. Thus, most of collagens were extracted during acid solubilization and only a small portion of cross-links was retained for further pepsin solubilization. ASC showed a similar chemical composition to PSC. Both collagens had high protein content (89.84–90.29%) with a low moisture content (6.92–8.31%). Additionally, both ASC and PSC contained a small amount of ash (0.58–0.84%) and fat (0.17–0.20%). From UV–Vis spectra of both collagens, an absorbance at 230 was observed with high intensity, while the absorbance at 280 nm was of low intensity (data not shown). The results indicated high efficacy of non-collagenous protein removal. Collagen commonly has a low amount of tyrosine, which could absorb UV-light at 280 nm [16].
Amino acid composition of ASC and PSC
The amino acid composition of both ASC and PSC from blacktip shark skin expressed as residues per 1,000 total residues is shown in Table 1. Amino acid composition of ASC and PSC was quite similar. Both collagens had glycine as their major amino acid (317–323 residues/1,000 residues) and were rich in alanine (118–120 residues/1,000 residues), proline (109–111 residues/1,000 residues) and hydroxyproline (88–92 residues/1,000 residues). Cysteine (1 residue/1,000 residues), tyrosine (1–3 residues/1,000 residues), hydroxylysine (5–6 residues/1,000 residues) and histidine (7–8 residues/1,000 residues) were found in very low amounts. Generally, glycine is about one-third of the total amino acid residues. Proline and hydroxyproline constitute about one-fifth and alanine is for about one-ninth [1]. Imino acid content (proline and hydroxyproline) of ASC and PSC was 197 and 203 residues/1,000 residues, respectively, which is much higher than that of Atlantic cod (Gadus morhua) skin (154 residues/1,000 residues) [16] but slightly lower than that of calf skin collagen (215 residues/1,000 residues). The imino acid of fish collagens has been known to correlate with the water temperature of their normal habitat [17]. Hydroxyproline plays an important role in stabilization of the helix structure by preventing rotation of the N–C bond [17].
Protein patterns of ASC and PSC
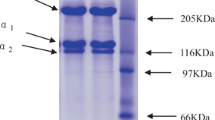
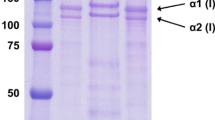
The protein patterns of ASC and PSC from blacktip shark skin determined under non-reducing and reducing conditions are shown in Fig. 1. Similar protein patterns of both collagens tested under both conditions were observed, suggesting that no disulfide bond was found in both collagens. This was concomitant with the negligible content of cysteine (Table 1). Coincidentally, collagen from brownstripe red snapper skin [15], Nile perch skin (Lates niloticus) [18] and bigeye snapper skin and bone [11] contained no disulfide bonds. Both ASC and PSC comprised α1- and β-chains as their major components with the low band intensity of α2-chain. γ-chain was found at very low content. PSC contained a lower amount of γ-chain, compared with ASC. Furthermore, it was noted that the molecular weights of α1-, α2- and β-chains of PSC were slightly lower than those of ASC. This was probably due to the cleavage of telopeptide region of tropocollagen by pepsin used for solubilization [9]. As a result, slightly lower molecular weight was noticeable in PSC. No degradation of PSC was observed, reconfirming that pepsin specifically cleaved at telopeptide region.
Subunit compositions of ASC and PSC
The chromatograms of ASC and PSC dissociated by heat treatment and subjected to TOYOPEARL® CM-650M column are shown in Fig. 2. Two major peaks were obtained. The differences in chromatograms of both collagens were rarely observed, in which the peaks were found at different retention times. The chromatographic fractions indicated by numbers were subjected to SDS-PAGE. For the first peak, the α1-chain was observed for the fraction no. 31–47 and 43–58 of ASC and PSC, respectively. For the second peak, α2- and β- chain were observed for the fraction no. 61–88 and 68–89 of ASC and PSC, respectively. Nevertheless, α1-chain was also found to small extent in the second peak. The results suggested that both collagens might be type I collagen, even though the band intensity of α1-chain was not two-fold higher than that of α2-chain (Fig. 1). It is most likely that α2-chain in shark skin collagen did not exist as a monomer but exclusively as a β12-dimer combined with α1-chain [19]. This was in agreement with the large band intensity of β-chain that appeared in collagen and the fraction no. 75 and 80 for ASC and PSC, respectively. As a result, the low intensity of α2-chain was detected on SDS-PAGE. This result was in accordance with type I collagen from other elasmobranchs [5, 6].
Elution profile of ASC (a) and PSC (b) from the skin of blacktip shark on the TOYOPEARL® CM-650M ion-exchange chromatography. The fractions indicated by numbers were examined by SDS-PAGE using 7.5% separating gel. M, C and HMC denote high molecular weight protein markers, collagen and high MW cross-linked components, respectively
Based on the previous reports, collagen from the skin of elasmobranchs were found as (α1)2α2 with cross-linking of α2 into α1α2-dimer (β12) [5, 6]. Additionally, α2-chain was eluted as the second peak, following the first peak of α1-chain (Fig. 2). Nevertheless, it was possible that homotrimer might be included in the collagen from shark skin.
Peptide mapping of ASC and PSC
Peptide maps of ASC and PSC from blacktip shark skin digested by lysyl endopeptidase or V8-protease in comparison with those of type I collagen from calf skin are illustrated in Fig. 3. Marked differences in peptide maps of ASC and PSC from shark skin was observed when compared with that of calf skin collagen. However, similar peptide maps were observed between ASC and PSC when V8-protease was used (lanes 9, 10). On the other hand, some differences in peptide maps of ASC and PSC digested with lysyl endopeptidase were found (lanes 6, 7). It was noted that peptides with MW ranging from 59.2 to 73.2 kDa were different between ASC and PSC. For peptide maps of collagens digested by lysyl endopeptidase (lanes 5–7), α-, β-, γ-chains and high MW cross-linked components of collagen from calf skin and shark skin were hydrolyzed into the degradation peptides with MW ranging from 53 to 220 kDa (10 major peptide fragments for calf skin collagen) and 52.9–277.3 kDa (11 and 9 major peptide fragments for ASC and PSC, respectively), respectively. Thus, calf skin collagen was more susceptible to hydrolysis by lysyl endopeptidase than shark skin collagens. Calf skin collagen might contain a higher amount of lysine than shark skin collagen. As a consequence, lysyl endopeptidase, specific to hydrolyze peptide bonds at the carboxyl side of lysyl residues [20], was able to cleave the peptides in calf skin collagen more effectively.
For peptide maps of collagens digested by V8-protease, the major components, including α-, β-, γ-chain and high MW cross-linked components, of both ASC and PSC were degraded into 13 major peptide fragments with MW of 173.5, 160.1, 147.7, 133.6, 97.6, 92.5, 84.7, 73.2, 70, 63.5, 58.4, 54.5 and 48.8 kDa. Generally, peptide maps of ASC and PSC were similar. In contrast, no degradation band was found in peptide maps of calf skin collagen. The result suggested that calf skin collagen was more tolerant to digestion by V8-protease than shark skin collagens. V8-protease exhibits a high degree of specificity for glutamic acid and aspartic acid residues of peptides and proteins [21]. ASC and PSC contained a high amount of these amino acids (Table 1). Therefore, both ASC and PSC were more likely cleaved by V8-protease. Based on peptide mapping, collagen from calf skin and shark skin were totally different in terms of amino acid sequence and composition.
Thermal transition of ASC and PSC
Differential scanning calorimetry (DSC) thermograms of ASC and PSC are shown in Fig. 4. The maximum transition temperature (T max) of ASC and PSC were 34.23 and 34.37 °C, respectively. Owning to similarity in T max, pepsin digestion at telopeptide region during PSC extraction might not affect collagen structure, especially triple-helical structure. Similar T max of ASC and PSC was also reported for collagen from brownstripe red snapper skin [15]. However, a difference in enthalpy (ΔH) between ASC (0.91 J/g) and PSC (0.64 J/g) was found. The cleavage of telopeptide region by pepsin or some removal of those peptides might facilitate the denaturation of PSC induced by heat. When comparing T max of collagen from shark skin, both ASC and PSC, with those of collagen from other fish, shark skin collagen had a higher T max than those from cold-water fish skin, such as Atlantic cod (15 °C) [16] and deep-sea redfish (Sebastes mentella) (16.1 °C) [22]. Furthermore, T max of ASC and PSC from blacktip shark skin was also slightly higher than that of other tropical fish, such as bigeye snapper skin (31 °C) [11]. Nevertheless, it was slightly lower than that of mammalian collagen, including calf skin collagen (40.8 °C) [16] and porcine skin collagen (37 °C) [14]. The variation in T max of collagens among these species is related to the habitat temperature and the content of imino acids (proline and hydroxyproline). Shark skin collagen showed a higher imino acid content (197–203 residues/1,000 residues) than did collagen from cold-water fish (154–165 residues/1,000 residues) [16, 22], and tropical fish (190–193 residues/1,000 residues) [11, 16] but contained lower imino acids than that from mammals (215 residues/1,000 residues) [16]. In general, collagen containing a low content of imino acids undergoes denaturation at lower temperatures than do those with higher contents [17].
Zeta potentials of ASC and PSC at different pHs
The zeta potentials of ASC and PSC at different pHs are shown in Fig. 5. The zeta potentials of ASC and PSC sharply decreased as pH increased from 2 to 5. A slight decrease was noticeable at pH above 5 for PSC, while a higher rate of decrease in pH was obtained in ASC in pH range of 5–10. Net charge of zero was observed at pH 6.78 and 7.02, for ASC and PSC, respectively. At pH values below and above their isoelectric point (pI), proteins carry a net positive or a net negative charge, respectively [23]. As a consequence, pI of ASC and PSC were estimated to be 6.78 and 7.02, respectively. The difference in pI and the rate of changes in zeta potential in pH range of 5–10 between ASC and PSC might be caused by the different amino acids mediated by partial removal of telopeptides by pepsin used.
Fourier-transform infrared (FTIR) spectra of ASC and PSC
FTIR spectra of ASC and PSC from the skin of blacktip shark are shown in Fig. 6. The patterns of ASC and PSC spectra were similar to those of collagen from other fish species reported in the literatures [14, 18, 22]. FTIR spectra of ASC and PSC were quite similar, indicating similar secondary structures of both collagens [24]. The amide I band and amide II band of both ASC and PSC were observed at the wavenumber of 1,631–1,634 cm−1 and 1,538–1,541 cm−1, respectively. The amide I band is associated with C=O stretching vibration or hydrogen bond coupled with COO− [25]. Moreover, it is a sensitive marker for the secondary structure of proteins in FTIR analysis [26]. Payne et al. [25] reported that a shift of amide I and II peaks to lower wavenumber is associated with a decrease in the molecular order. No shift in wavenumber of amide I (1,631–1,634 cm−1) and II peaks (1,538–1,541 cm−1) was noticeable for both collagens, suggesting the similarity in intra-molecular alignment of triple helix between both samples. Furthermore, it was implied that pepsin did not affect the triple-helical structure of collagen as indicated by a ratio of approximately 1 between amide III and 1,454 cm−1 band of both ASC (1.04) and PSC (0.99) [27].
The amide A band of ASC and PSC was found at 3,294 and 3,295 cm−1, respectively, suggesting the N–H stretching vibration and the existence of hydrogen bonds. Doyle et al. [28] reported that a free N–H stretching vibration commonly occurs in the range of 3,400–3,440 cm−1, and when the NH group of a peptide is involved in a hydrogen bond, the position is shifted to lower frequencies. Amide B band of both collagens was observed at the wavenumber ranging from 2,922 to 2,925 cm−1, which is related to asymmetrical stretch of CH2 stretching vibration. The result is in accordance with other reports [14, 16, 18] and suggests that the triple-helical structure was not affected by pepsin digestion during collagen extraction and that pepsin specifically cleaved only at telopeptide regions of tropocollagen.
Conclusions
ASC and PSC could be extracted from the skin of blacktip shark. A much higher yield was obtained for ASC, in comparison with PSC. Both ASC and PSC were most likely type I collagen, in which α2-chain was covalently cross-linked to β-chain. This might be associated with a higher thermal stability of collagen from blacktip shark skin.
References
Balian G, Bowes JH (1977) In: Ward AG, Courts A (eds) The science and technology of gelatin, Academic Press, London
Sadowska M, Kolodziejska I, Niecikowska C (2003) Food Chem 81:257–262
Regenstein JM, Zhou P (2007) In: Shahidi F (ed) Maximising the value of marine by-products, 1st edn. Woodhead Publishing Limited, Cambridge
Musick JA (2005) In: Musick JA, Bonfil R (eds) Management techniques for elasmobranch fisheries. FAO fisheries technical paper no. 474, FAO, Rome
Bae I, Osatomi K, Yoshida A, Osako K, Yamaguchi A, Hara K (2008) Food Chem 108:49–54
Hwang JH, Mizuta S, Yokoyama Y, Yoshinaka R (2007) Food Chem 100:921–925
Nomura Y (2004) Dev Food Sci 42:147–158
AOAC (2000) Official methods of analysis. Association of Official Analytical Chemists Inc, Arlington
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2008) J Food Sci 73:C413–C419
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005) Food Chem 89:363–372
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2007) Food Chem 104:593–601
Laemmli UK (1970) Nature 227:680–685
Nagai T, Suzuki N, Nagashima T (2008) Food Chem 111:296–301
Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005) Food Chem 93:475–484
Duan R, Zhang J, Du X, Yao X, Konno K (2009) Food Chem 112:702–706
Foegeding EA, Lanier TC, Hultin HO (1996) In: Fennema OR (ed) Food chemistry. Marcel Dekker, New York
Muyonga JH, Cole CGB, Duodu KG (2004) Food Chem 85:81–89
Kimura S, Kamimura T, Takema Y, Kubota M (1981) BBA Protein Struct 669:251–257
Jekel PA, Weijer WJ, Beintema JJ (1983) Anal Biochem 134:347–354
Vercaigne-Marko D, Kosciarz E, Nedjar-Arroume N, Guillochon D (2000) Biotechnol Appl Biochem 31:127–134
Wang L, An X, Xin Z, Zhao L, Hu Q (2007) J Food Sci 72:E450–E455
Damodaran S (1996) In: Fennema OR (ed) Food chemistry, Marcel Dekker, New York
Muyonga JH, Cole CGB, Duodu KG (2004) Food Chem 86:325–332
Payne KJ, Veis A (1988) Biopolymers 27:1749–1760
Surewicz WK, Mantsh HH (1988) Biochim Biophys Acta 952:115–130
Plepis AMDG, Goissis G, Das-Gupta DK (1996) Polym Eng Sci 36:2932–2938
Doyle BB, Blout ER, Bendit EG (1975) Biopolymers 14:937–957
Acknowledgments
This research was supported by grant from under the program Strategic Scholarships for Frontier Research Network for the Joint PhD Program Thai Doctoral degree from the Office of the Higher Education Commission, Thailand. Authors would like to thank the Prince of Songkla University for the financial support, Professor Dr. Hideki Kishimura of laboratory of Marine Products and Food Science, Research Faculty of Fisheries Sciences, Hokkaido University, Japan, for his assistance in amino acid analysis and Mr. Veera Prateeptintong, a managing director of Blue Ocean Food Products, Co., Ltd., Samutsakhon Province, Thailand, for kindly providing blacktip shark skin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kittiphattanabawon, P., Benjakul, S., Visessanguan, W. et al. Isolation and properties of acid- and pepsin-soluble collagen from the skin of blacktip shark (Carcharhinus limbatus). Eur Food Res Technol 230, 475–483 (2010). https://doi.org/10.1007/s00217-009-1191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1191-0