Abstract
This paper reports on the diversity and dynamics of the dominant microbial populations during manufacturing and ripening of Lighvan, a traditional, starter-free Iranian cheese made from raw ewe and goat’s milk as determined by culturing and PCR-DGGE. Similar dominant populations, composed of Lactococcus lactis and Lactobacillus spp. strains, were found by both techniques. However, discrepancies regarding the identity of the Lactobacillus species were encountered. Lactobacillus curvatus and Lactobacillus sakei proved to be dominant by PCR-DGGE; in contrast, Lactobacillus paraplantarum, Lactobacillus paracasei, Lactobacillus brevis and Lactobacillus plantarum were the majority cultivable organisms. RAPD typing of lactobacilli isolates showed wide genetic diversity among the species. Moreover, strain compositions change over time; L. brevis and L. paraplantarum were dominant in milk and were replaced by L. plantarum and L. paracasei strains as ripening progressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lighvan cheese is a semi-hard, starter-free, traditional Iranian cheese made from a mixture of raw ewe and goat’s milk in the mountainous area of Lighvan in the province of Azerbaijan, Iran. The manufacture of Lighvan cheese involves coagulation of evening and morning milk with lamb rennet at 28–32 °C (depending on the season). The coagulum is cut into walnut-size pieces that are then transferred to rectangular-shaped bags and piled up for whey drainage. The resulting curd mass is then cut into 25 × 25 × 25 cm3 cubes and placed in a 22% salt brine flow for 6 h. After removal, curd cubes are kept in a basin for 3–5 days, in which whey drainage continues. During this period cubes are turned upside down between 9 and 15 times. Finally, they are packed in 10–12% salt brine. Ripening takes place in deep-natural or man-made caves for 3–4 months at an average temperature of 10–12 °C.
Despite the popularity of Lighvan cheese and its ever increasing consumption, its chemical composition has been little studied. Even less is known about the microbial communities involved in its manufacture and ripening; only the dominant bacterial genera of mature cheeses have been reported [1, 2]. Knowledge in this area is of particular interest since the typical sensorial properties of traditional cheeses rely on the complex biochemical processes driven by the indigenous microbiota [3] (as well as on the animal breeds used to provide the milk, their nutrition, and the cheese-making practices followed). Further, the Iranian Organization of Standardization now recommends the universal pasteurization of milk for improving the safety of cheeses. Pasteurization denatures milk proteolytic and lipolytic enzymes that might contribute to the characteristics of Lighvan cheese and eliminates key microorganisms involved in cheese acidification and/or ripening [4]. Commercial starter cultures therefore become necessary, but their use could lead to a loss of its ‘genuine’ characteristics [5, 6]. However, starters based on autochthonous microorganisms should be well adapted to the milk produced in the manufacturing area and in equilibrium with endemic phages [7], allowing to establish themselves in the cheese matrix better than commercial cultures [8], while preserving the typical sensorial properties of the cheese [9].
The microbial characterization of food ecosystems is currently performed using an array of culture-independent molecular techniques [10] (these are faster, more reliable and cheaper than conventional culturing techniques), followed by identification of the microorganisms by biochemical and physiological tests and molecular methods. Denaturing gradient gel electrophoresis (DGGE) can be used to track changes in microbial communities via sequence-specific separation of PCR-amplified fragments [11]. This technique has been used to characterize the microbial diversity in many dairy environments [12–16] and follow microbial population dynamics in cheese manufacturing and ripening [17–21]. In some cases, however, a combination of culturing and molecular techniques is preferred since complementary results are obtained [22, 23]. In addition, culturing allows the isolation, identification and selection of starter and adjunct cultures.
This paper reports on the basic chemical characteristics of Lighvan cheese during manufacture and ripening, as well as on a preliminary inspection of the dominant microbial communities as determined by DGGE. In addition, the Lactobacillus species in milk, curd and cheese at different stages of ripening (lactobacilli were the majority species throughout ripening) were identified by phenotypic and molecular methods. The characterization of these Lactobacillus species would therefore be of much help in the design of specific starter cultures for the production of standardized Lighvan cheese.
Materials and methods
Samples and sampling conditions
A series of Lighvan cheese samples composed of curd (3 days) and cheeses of 30, 45, 75 and 90 days of ripening were collected from a single manufacturer and analyzed by the DGGE technique after total microbial DNA extraction and PCR amplification of variable V3 regions of ribosomal DNA genes. For the microbial and chemical analysis, milk and curd (3 days) samples and samples of cheeses at 30, 45, 75 and 90 days of ripening were obtained from independent batches of Lighvan manufactured by three different producers. Samples were all taken according to standard FIL-IDF 50B [24] and transported to the laboratory under refrigerated conditions.
Chemical analysis
FIL-IDF Standards 21 B [25] and 4 A [26] were followed for examining total solids in milk and cheese, respectively. The pH was measured according to FIL-IDF Standard 104 A [27], and the NaCl and protein content according to FIL-IDF Standards 88 A [28] and 20 B [29], respectively.
DGGE analysis
Extraction of DNA from cheese
Cheese samples homogenized in 2% sodium citrate were used for DNA isolation. The DNA extraction was accomplished essentially as described by Ercolini et al. [30], but with the following modification: cheese homogenates were treated with pronase (2.5 mg/ml) (Sigma Chemical Co., St. Louis, MO, USA) for 1 h at 37 °C before lysis of the cells.
PCR amplification
The total DNA from cheese samples was used as a template in PCR-amplifications of the V3 region of the bacterial 16S rRNA gene using universal primer F357 (5′-TACGGGAGGCAGCAG-3′¸ to which a 39 bp GC sequence was linked to give rise to GC-F357) and R518 (5′-ATTACCGCGGCTGCTGG-3′) [11]. The D1 domain of the 26S rRNA fungal gene was amplified with primers GC-NL1 (5′-GCCATATCAATAAGCGGAGGAAAAG-3′) and LS2 (5′-ATTCCCAAACAACTCGACTC-3′) [15].
The PCR was performed in 50 μL volumes containing 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.2 mM of the primers, 1.5 U of Taq-polymerase (Roche Diagnostics, Barcelona, Spain) and 100 ng of extracted DNA. The conditions established for the amplification of the prokaryotic and eukaryotic sequences were those described by Muyzer et al. [11] and Cocolin et al. [15], respectively.
Electrophoresis conditions
DGGE was performed using a DCode apparatus (Bio-Rad, Richmond, CA, USA) at 60 °C and employing 8% polyacrylamide gels with a formamide denaturing range of 40–60% for bacteria and 30–50% for fungi. Electrophoresis was conducted at 75 V for 17 h and at 130 V for 4.5 h for bacterial and fungal amplifications, respectively. Bands were visualized using a UV transilluminator after staining with 0.5 μg/ml ethidium bromide (Sigma).
Identification of DGGE bands
DNA bands in the polyacrylamide gels were assigned to species by either comparison with a control ladder corresponding to known strains [17] or, after isolation, by reamplification with the same primers without the GC-clamps followed by sequencing. The sequences obtained were compared with those in the GenBank database using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/), and with those of the Ribosomal Database Project (http://rdp.cme.msu.edu/index.jsp); a species was allocated when identities of >97% were detected [31, 32].
Microbial counts
Cheese samples were emulsified in sterile 2% (w/v) sodium citrate, serially diluted in sterile saline solution, and plated in duplicate on agar plates of the following media (all from Merck), PCA (for counting total aerobic microorganisms), M17 (for lactococci/enterococci), MRS pH 5.4–5.5 (for lactobacilli) and YGC (for moulds and yeasts). Plates were all incubated in aerobic conditions at 30 °C for 72 h, except those of YGC which were incubated at 25 °C for 72 h.
Identification and typing of lactobacilli
Colonies of all morphologies on the MRS agar plates (MRS is selective for lactobacilli) from the three different samples of milk and cheeses at 30 and 90 days of ripening were chosen at random, purified twice by subculturing, and maintained at −20 °C in MRS to which 15% glycerol (w/v) (a cryoprotectant) had been added until analysis. The isolates were subjected to Gram staining and analyzed for growth in MRS at 10 and 15 °C, growth in 5 and 10% NaCl, and production of gas from glucose using Durham tubes.
For molecular identification, total genomic DNA from isolates was prepared as described for the total microbial DNA in cheese, but excluding the pronase treatment. The isolates were grouped by random amplification of polymorphic DNA (RAPD) analysis using primer M13, as reported by Fontana et al. [33]. A reproducibility study to determine the minimum percentage of similarity necessary for strain discrimination was performed according to Sánchez et al. [34]. Representative strains with distinct RAPD patterns were identified by amplification (using universal primers) and sequencing of the 16S rRNA gene.
Strains of the Lactobacillus plantarum group (L. plantarum, L. paraplantarum and L. pentosus) were distinguished by multiplex PCR using species-specific recA gene-based primers as described by Torriani et al. [35].
Statistical analysis
Means of the bacterial counts with a significant difference (P < 0.05) were compared by the least squares differences (LSD) test, using the SPSS program (SPSS Inc., Chicago, IL, USA). The similarity of the RAPD patterns was expressed by the Pearson’s product moment correlation coefficient. Clustering of the groups was performed by the unweighted pair group method using arithmetic averages (UPGMA) with the MVSP software (Kovach Computing Services, Anglesey, UK).
Results
Analysis of Lighvan cheese by DGGE
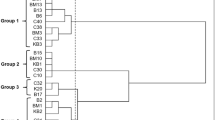
Figure 1 summarizes the DGGE profiles for the V3 region of the 16S rRNA gene (bacteria) and those for the D1 domain of the 26S rDNA gene (fungi). Though the study is limited by analyzing a small number of temporally related cheese samples, we assumed that the particular Lighvan cheese technology allows growth of only certain microbial species. Consequently, they were considered as representative of Lighvan cheese. The variability in microbial composition, assessed by the number of bands and their migration position in the DGGE gels, was very low among the samples. Overall, 12 bacterial bands were recorded, corresponding to 11 species (Fig. 1; Table 1). Each band corresponded to a different species, except for two bands (Fig. 1A, bands a) that belonged to species of the Lactobacillus plantarum group (L. plantarum, L. pentosus or L. paraplantarum). In addition, an overall eight fungal bands were obtained that belonged to five different species (Fig. 1B); three of these bands (bands m) belonged to Debaryomyces hansenii alone. For the bacterial bands, the highest diversity was obtained in the curd sample (seven different bands) (line 1, Fig. 1A). In contrast, the most fungal sequences (six bands) were observed at the end of ripening (lines 4, 5, from the 75-, 90-day-old cheese, respectively) (Fig. 1B).
DGGE profiles of microbial populations from the Iranian Lighvan cheese during manufacturing and ripening. Samples: 1, curd, 2, 3, 4, and 5 cheeses of 30, 45, 75 and 90 days. A DGGE profiles of the V3 variable region of the bacterial 16S rRNA gene. a Species of the Lactobacillus plantarum group, b Enterococcus faecalis/Enterococcus faecium, c Lactobacillus versmoldensis, d Lactobacillus sakei, e Lactobacillus curvatus, f Streptococcus parauberis, g Streptococcus gallolyticus, h Lactococcus lactis, i Escherichia coli, j Serratia spp., k Lactobacillus casei/Lactobacillus paracasei. B DGGE profiles of PCR amplicons of the domain D1 of 26S rDNA representing the fungal biodiversity and evolution. l Candida zeylanoides, m Debaryomyces hansenii, n Kluyveryomyces lactis/Kluyveromyces marxianus, o Saccharomyces servazzi, p Rhodotorula mucilagenosa
The dynamics of the microbial species can be followed in both Fig. 1 and Table 1. Some bands increased and others decreased in intensity during ripening. For example, the band corresponding to Lactococcus lactis (band h, Fig. 1A) was very intense in curd samples, after which it became gradually less intense before disappearing in the 90-day-old cheese. Similarly, the band of Enterococcus faecalis/Enterococcus faecium (band b) was only observed in curd and 30-day-old cheese. The intense band ‘f’ corresponding to Streptococcus parauberis abruptly disappeared in the 75-day sample (line 4, Fig. 1A). In contrast, some bands only appeared late in ripening, e.g., those pertaining to Streptococcus gallolyticus (band g, Fig. 1A) and Lactobacillus casei/Lactobacillus paracasei (band k). This was also observed in the fungal populations. The band corresponding to Candida zeylanoides (band l, Fig. 1B) became less intense over ripening, while those corresponding to D. hansenii increased in intensity (bands m, Fig. 1B).
Of particular interest was a bacterial band (band i) belonging to Escherichia coli; this was present at all stages of manufacture and ripening at almost the same intensity. A further Enterobacteriaceae-related band was also identified in the 90-day cheese samples (band j; Serratia spp.). Also of note was the presence of several bands of high intensity for some samples that were completely absent for others. The majority of these bands belonged to Lactobacillus species, such as L. plantarum, very intense in the 75-day-old sample (line 4, band a, Fig. 1A). Lactobacillus sakei was present in only the 90-day-old cheese (line 5, band d, Fig. 1A), and Lactobacillus curvatus was present in the 30, 75 and 90-day-old cheese sample S2 (band e).
Basic chemical characteristics of Lighvan cheese
Table 2 shows the gross chemical compositions of the milk, curd and cheese at 30, 45, 75, and 90 days of ripening. Although only small standard deviations were recorded for most of the variables studied, random differences could be attributed to the analysis of samples from distinct producers and batches. Generally, total cheese solids increased over ripening as a result of water desorption (water in the cheese moving out to the brine). Consequently, the NaCl content of the cheese increased; indeed, extremely high salt contents were recorded in early ripening (around 10% in moisture at day 10; data not shown). The pH of the curd was lower than 5.0, recovering slowly over ripening. These two parameters, a high NaCl content and a low pH, determine type and numbers of the microbial populations over ripening.
Microbial counts
The counts of total mesophilic bacteria, lactococci and lactobacilli over manufacturing and ripening were obtained via serial dilution of the samples and plating on appropriate media (Fig. 2). The counts for all were high in the milk (from 2.3 × 106 cfu/ml for lactobacilli to 1.9 × 107 cfu/ml for total mesophilic bacteria). They continued to increase by about two logarithmic (log) units in the curd (up to 8.8 × 108 cfu/g for total viable microorganisms). A sharp reduction (about two log units) was recorded in all counts at 4 weeks, with figures becoming relatively stable after this date. With the exception of the milk sample, lactococci and lactobacilli counts were found at similar levels in most samples. On YGC medium, counts for yeasts and moulds were always below the detection limit (<100 cfu/g) (data not shown in Fig. 2).
Total viable aerobic microorganisms, lactococci and lactobacilli scored on PCA, M17 and MRS, respectively, in milk, curd, and cheeses of 30, 45, 75 and 90 days of ripening. Average results of three samples at each sampling point are being reported. Bars indicate standard deviations. Asterisks denote counts of microbial populations differing significantly (P < 0.05) among the distinct media
Identification, typing and population dynamics of lactobacilli
DGGE and plate enumeration showed lactobacilli to be a typical and distinctive mark of Lighvan cheese; as mentioned, different Lactobacillus species were detected by either these techniques. Therefore, attempts were made to accurately characterize the lactobacilli.
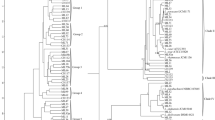
One hundred and sixty-five colonies were randomly selected from the counting MRS plates for milk and 30- and 90-day-old cheeses; of these, 126 proved to be Gram-positive, catalase-negative bacilli. All 126 were subjected to RAPD using primer M13 to group strains before identification; 28 different genotypes were obtained with a minimum similarity level of 87% (the value obtained in the reproducibility study) (Fig. 3). The genotypes were numbered (G1–G28) and marked with a letter to indicate their origin (M for milk, Y for the 30-day-old cheese, R for the 90-day-old cheese). The genotypes separated into two unrelated clusters (C1, C2) which were subsequently split into 11 groups at a similarity level of 58% (Fig. 3). Representative strains of these different groups were subjected to molecular identification by partial amplification of the 16S rRNA gene, sequencing and comparison of the sequences to those in databases. The 11 groups were firstly assigned to three different species: Lactobacillus paracasei, Lactobacillus brevis and species of the L. plantarum group. Each group represented only one species, but the same species was found in different groups, revealing the high genetic diversity among Lighvan Lactobacillus strains. Cluster C1 included all strains of L. brevis, and embraced two groups and seven genotypes (21 isolates). Cluster C2 embraced nine groups and 21 different genotypes, including strains of L. paracasei (three groups, 10 genotypes, 30 isolates) and strains of the L. plantarum group (six groups, 11 genotypes, 75 isolates). The isolates of this last group were all subjected to multiplex PCR for distinguishing among the different species (L. paraplantarum, L. pentosus and L. plantarum). Only L. paraplantarum and L. plantarum strains were found, representing four of the 11 groups (eight genotypes, 64 isolates) and two of the 11 groups (three genotypes, 11 isolates), respectively.
Dendogram obtained after RAPD-PCR analysis of mesophilic lactobacilli isolated through fermentation process of Lighvan cheese. Strains are coded by their origin as, M from milk, Y from 30-day-old cheese, and R from 90-day-old cheese. RAPD patterns obtained after amplification with M13 primer were grouped by means of the Pearson product moment correlation coefficient and UPGMA cluster analysis with MVSP software (Kovach Computing Services, Anglesey, UK)
The change in the species and genotypes present over manufacturing and ripening was followed by tabulating the frequencies of isolation (Table 3). Most genotypes were detected only once during ripening. A few (such as G18, G21, G26) were detected twice. Lactobacillus brevis and L. paraplantarum were the majority species in the milk, accounting for 46 and 54% of the isolates, respectively. After 30 days of ripening L. paraplantarum became the dominant species (86% of the isolates), while in the mature cheeses (90 days) L. paracasei was the majority (61%) followed by L. plantarum (14.3%) and L. paraplantarum (14%).
Discussion
DGGE has been successfully used to identify and track microbial communities in dairy environments [12–16], including those involved in the manufacture and ripening of traditional cheese made from raw milk [17–21]. The length and species-specific heterogeneity of the V3 region of the bacterial 16S rRNA gene, and of the D1 region of the fungal 26S rRNA gene, render them among the best choices for distinguishing bacterial [13, 16, 19, 21] and fungal [15, 17] species, respectively. However, limiting factors, such as heterogeneous copies of rDNA operons, annealing mismatches and preferential amplification have been reported [36, 37].
The bacterial and fungal species detected by DGGE in Lighvan cheese have all been reported before to be present in dairy-related environments. The detection of intense bands in some samples but not in others can be attributed to the analysis of cheese samples from different batches. This phenomenon may indicate that Lactobacillus species commonly grow to high densities during the ripening of Lighvan cheese (see enumeration results), but the dominant species may be different from batch to batch. The production of two bands by L. plantarum strains has been reported before [17]. Further, different copies of the rRNA genes producing distinctive bands by DGGE have been described in fungi [15, 17], as this work shows for D. hansenii. It is worth noting that fungal species might be in such low numbers (<100 cfu/g) that enumeration by dilution and plating may not always be possible. However, they can still be tracked by DGGE using specific primers. Thus, PCR-DGGE was considered a powerful and convenient technique for a rapid inspection of the diversity and relative abundance of the microbial populations throughout manufacturing and ripening of Lighvan cheese.
Other traditional cheeses have bacterial and fungal population dynamics similar to those found for Lighvan [17, 18]: the bacterial diversity is greater in milk and curd samples, becoming reduced as ripening progresses; in contrast, fungal populations increase moderately over ripening [15, 17]. This is probably related to the unfavorable conditions for bacterial growth in cheese (low pH, high salt concentration, presence of organic acids, etc.) as compared to the conditions reigning in milk. Even so, these harsh conditions still allow growth of some bacterial populations, mainly of the lactic acid bacteria (LAB) group. LAB species account for the majority cultivable populations at all stages of manufacturing and ripening, and both lactoccci and lactobacilli seem to be at a comparable level in all samples examined, except in milk where numbers of cocci are higher than those of bacilli (Fig. 2).
The high bacterial counts in the milk may be a consequence of storing evening milk at room temperature; this is then mixed with fresh morning milk in the manufacturing process. The microbial differences between samples, which followed no single trend, were related to the distinct batches analyzed. Uncontrolled environmental conditions affecting the different batches may also account for some differences, as reported for many other cheese varieties [38–40]. The sharp reduction in the bacterial populations between the curd and 30-day-old cheese samples might be ascribed to the high salt concentration of the brines (22% w/v for the initial brine and 12% for the ripening brine). Enterobacteriaceae species and coliforms could survive the harsh conditions imposed by this salt. Thus, bands corresponding to E. coli and Serratia spp. were detected even in the 90-day-old cheese, and, although they were not further identified, Gram negative organisms were observed among the 165 isolates obtained in MRS. Coliform populations are considered an indicator of faecal contamination, thus the presence of these microorganisms in fully mature Lighvan cheese stresses the need to improve the hygiene associated with its manufacture.
Four Lactobacillus species were found among the isolates from the milk and cheese samples: L. paraplantarum, L. paracasei, L. brevis, and L. plantarum. These have been reported among the dominant lactobacilli in non-cooked traditional cheeses [34, 38–41]. Further, wide genetic diversity has been reported for cheese-associated lactobacilli [34, 41]. In Lighvan cheese, numerous genotypes were detected in just one sample, and a few were detected twice. This suggests that there is rapid succession of species and strains over manufacturing and ripening. Though variations due to the analysis of independent batches may lead to variations in results, L. brevis and L. paraplantarum strains seem to dominate in milk, but these strains are replaced by L. paracasei and L. plantarum strains as ripening progresses.
It was not surprising to find enterococci-related amplicons, i.e., bands matching E. faecalis/E. faecium sequences, or those corresponding to S. parauberis and S. gallolyticus. In fact, DGGE bands corresponding to enterococcal or related species have been identified in many cheeses [14, 21, 30]. Enterococci-like bacteria develop and survive well in dairy products, and have repeatedly been reported in high numbers in products made of raw milk [42]. The role of these organisms in dairy is at the cross-road of technology and safety; they possess enzymatic systems that may contribute to the final organoleptical profiles of fermented products, but some species produce harmful substances such as biogenic amines and others are opportunistic pathogens in animals and humans. Thought some authors propose the use of enterococci as starter and adjunct cultures [42], the presence of enterococci in cheese is at present a matter of controversy.
The culturing results did not agree well with the DGGE results. With DGGE, a prominent band, identified as belonging to L. curvatus, was observed in 4-week-old cheese sample (band e, line 2, Fig. 1A). Similarly, intense bands identified as belonging to L. curvatus and L. sakei species were observed in 13-week-old cheese sample, plus a faint band corresponding to L. casei/L. paracasei. However, L. paracasei strains were dominant among the isolates while L. curvatus and L. sakei strains were never identified by culturing. Discrepancies between culturing and DGGE results have been reported before [19–21]. In general, DGGE and other molecular techniques do not require organisms to be alive for their sequences to be amplified; DGGE therefore detects the presence of non-viable as well as viable organisms. Moreover, differential resistance of microbial species to the lytic enzymes used in DNA preparation might also account for these differences. The fact that some species are only identified by one of the two methods stresses the importance of combined approaches for fully describing the microbiota of naturally fermented cheeses.
In conclusion, this work provides a first microbiological study of Lighvan cheese by means of culturing and PCR-DGGE analysis, and a detailed identification and typing of the lactobacilli involved in its manufacturing and ripening. The technological characterization of some of these isolates, which is currently in progress, should allow the selection of appropriate strains to be used as adjunct cultures for the standardization and improvement of the overall cheese quality and safety.
References
Barouei J, Karbassi A, Ghoddusi HB, Mortazavi A (2008) Int J Food Prop 11:407
Abdi R, Shikh-Zeinoddin M, Soleimanian-Zad S (2006) Pak J Biol Sci 9:99
Duthoit F, Callon C, Tessier L, Montel MC (2005) Int J Food Microbiol 103:259
Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM (2001) Int Dairy J 11:259
Hickey DK, Kilcawley KN, Beresford TP, Wilkinson MG (2007) J Dairy Sci 90:47
Gaya P, Sánchez C, Núñez M, Fernández-García E (2005) J Dairy Res 72:287
Madera C, Monjardín C, Suárez JE (2004) Appl Environ Microbiol 70:7365
Goerges S, Mounier J, Rea MC et al (2008) Appl Environ Microbiol 74:2210
Smit G, Smit BA, Engels WJ (2005) FEMS Microbiol Rev 29:591
Giraffa G, Neviani E (2001) Int J Food Microbiol 67:19
Muyzer G, de Waal EC, Uitterlinden AG (1993) Appl Environ Microbiol 59:695
Cocolin L, Innocente N, Biasutti M, Comi G (2004) Int J Food Microbiol 90:93
Lafarge V, Ogier J-C, Girard V et al (2004) Appl Environ Microbiol 70:5644
Ogier J-C, Lafarge V, Girard V et al (2004) Appl Environ Microbiol 70:5628
Cocolin L, Aggio D, Manzano M, Cantoni C, Comi G (2002) Int Dairy J 12:407
Ercolini D, Moschetti G, Blaiotta G, Coppola S (2001) Syst Appl Microbiol 24:610
Flórez AB, Mayo B (2006) Int J Food Microbiol 110:165
Randazzo CL, Vaughan EE, Caggia C (2006) Int J Food Microbiol 109:1
Ercolini D, Mauriello G, Blaiotta G, Moschetti G, Coppola S (2004) J Appl Microbiol 96:263
Randazzo CL, Torriani S, Akkermans ALD, de Vos WM, Vaughan EE (2002) Appl Environ Microbiol 68:1882
Coppola S, Blaiotta G, Ercolini E, Moschetti G (2001) J Appl Microbiol 90:414
El-Baradei G, Delacroix-Buchet A, Ogier JC (2007) Appl Environ Microbiol 73:1248
Feurer C, Irlinger F, Spinnler HE, Glaser P, Vallaeys T (2004) J Appl Microbiol 97:546
FIL-IDF Standard 50 B (1985) Milk and milk products. Methods of sampling. International Dairy Federation
FIL-IDF Standard 21 B (1987) Milk, cream and evaporated milk. Determination of total solids content (reference method). International Dairy Federation
FIL-IDF Standard 4 A (1982) Cheese and processed cheese. Determination of the total solids content (reference method). International Dairy Federation
FIL-IDF Standard 104 A (1984) Determination of the pH of the serum. Potentiometric method. International Dairy Federation
FIL-IDF Standard 88 A (1988) Cheese and processed cheese products. Determination of the chloride content. Potentiometric titration method. International Dairy Federation
FIL-IDF Standard 20 A (1993) Milk. Determination of nitrogen content, protein-nitrogen content, and non-protein-nitrogen content. Kjeldahl method. International Dairy Federation
Ercolini D, Hill PJ, Dood CER (2003) Appl Environ Microbiol 69:3540
Palys T, Nakamura LK, Cohan FM (1997) Int J Syst Bacteriol 47:1145
Stackebrandt E, Goebel BM (1994) Int J Syst Bacteriol 44:846
Fontana C, Cocconcelli PS, Vignolo G (2004) Int J Food Microbiol 103:131
Sánchez I, Seseña S, Póveda JM, Cabezas L, Palop L (2005) Int J Food Microbiol 102:355
Torriani S, Felis GE, Dellaglio F (2001) Appl Environ Microbiol 67:3450
Becker S, Böger P, Oehlmann R, Ernst A (2000) Appl Environ Microbiol 66:4945
von Wintzingerode F, Göbel UB, Stackebrandt E (1997) FEMS Microbiol Rev 21:213
Flórez AB, Álvarez-Martín P, López-Díaz TM, Mayo B (2006) Eur Food Res Technol 223:503
Arenas R, González L, Bernardo A, Fresno JM, Tornadijo ME (2004) Food Control 15:271
Gobbetti M, Burzigotti R, Smacchi E, Corsetti A, Angelis M (1997) Int Dairy J 7:519
Callon C, Millet L, Montel MC (2004) J Dairy Res 71:231
Giraffa G (2003) Int J Food Microbiol 88:215
Acknowledgments
This research was supported by projects from the Vice Chancellor for Research, University of Tehran, Iran (Grant no. 6122-TNS2007), and the Spanish Ministry of Science and Innovation (Reference, AGL2007-61869-ALI). The authors wish to express their sincere gratitude to Pegah Co. (Azerbaijan, Iran) for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kafili, T., Razavi, S.H., Djomeh, Z.E. et al. Microbial characterization of Iranian traditional Lighvan cheese over manufacturing and ripening via culturing and PCR-DGGE analysis: identification and typing of dominant lactobacilli. Eur Food Res Technol 229, 83–92 (2009). https://doi.org/10.1007/s00217-009-1028-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1028-x