Abstract
Traditional balsamic vinegar (TBV) is a natural product prepared with cooked and concentrated locally grown grape must. It has been demonstrated that TBV contains phenols and shows antioxidant activity. In this study we investigated the antioxidant properties of TBV in relation to its content of phenolic compounds, polymeric tannins and Maillard reaction products (MRPs). Results show that TBV has a high antioxidant activity measured with both FRAP and ABTS assays, which is higher or equal to those obtained in some red wines. About 45% of the antioxidant activity of TBV is due to the total polyphenolic fraction. Among polyphenols, tannins contribute to about 50% of the antioxidant activity of the total polyphenolic fraction. The residual antioxidant activity of TBV is due to the melanoidins (about 45%) synthesized during the boiling of the must and the ageing of TBV and to other compounds such as low molecular weight MRPs. When we investigated the effect of heating on the browning and on the formation of antioxidant MRPs in must model systems, we observed a major formation of antioxidant MRPs for the model system containing both amino acids and sugars with respect to the model system containing only sugars. We also tested the effect of some representative phenolic compounds present in must. Only polyphenols were stable in the model solution; however, at our experimental conditions they did not influence the browning and the formation of MRPs. Independent of their bioavailability, dietary antioxidants play an important role in protecting the gastrointestinal tract from oxidative damage and also possibly against a buildup of peroxides and their assimilitation in the digestive tract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional balsamic vinegar (TBV) from Modena and Reggio Emilia is a natural product with a large national and international consumption, prepared from cooked and concentrated locally grown grape must in wooden barrels containing aged TBV, and with natural yeasts providing alcoholic fermentation of sugars and acetobacters. Trebbiano, or other white or red grapes grown in Modena and Reggio Emilia provinces, supplies the grape juice for the production of traditional balsamic vinegar. This grape juice, also called must, is slowly concentrated to at least three times the starting volume to produce the cooked and concentrated must that represents the raw starting material for making TBV [1]. The alcoholic fermentation and acetic biossidation of cooked and concentrated must is performed in a set of barrels composed of at least five wooden casks of different volumes. Once per year, the transfer of a volume fraction from the barrel containing the younger vinegar to the older one is carried out. Thus, from the barrel containing the vinegar in the oldest ageing stage (barrel 1), a fraction is withdrawn and bottled. Barrel 1 is filled up with the same volume of vinegar from the barrel at an earlier ageing stage (barrel 2). This procedure is carried out until the barrel containing the youngest vinegar (barrel 5) is reached. This barrel will be integrated with the new cooked must [1]. The final result is a dark, thick and aromatic product with high sugars and organic acids content [1, 2]. In addition, TBV contains phenolic acids [3] and flavonoids [4] and shows a high antioxidant activity [4]. This antioxidant activity is partially due to its phenolic content and to other compounds present in TBV, such as compounds synthesized during must cooking, as a result of caramelization or of Maillard reaction.
The Maillard reaction occurring between an amino acid or protein and a reducing sugar is a ubiquitous food reaction that takes place during storage, cooking and heat processing [5]. This reaction may produce a large number of Maillard reaction products (MRPs) such as colorless aroma compounds, ultraviolet absorbing intermediates and dark-brown polymeric compounds called melanoidins [5].
The Maillard reaction plays an important role in the production of some foods such as bakery products, coffee or malt products. In these, the Maillard reaction is related to the development of characteristic aroma, taste and color. In addition, the Maillard reaction in amino acid–sugar model systems [6–8] or protein–sugar model systems [9, 10] as well as in food [11, 12] has been associated with the formation of compounds with strong antioxidant activity.
Several studies have investigated the formation of 5-hydroxymethyl furfural (HMF) during the cooking of must [13–16]. HMF can be formed by heat treatment of food that is rich in sugars and is the main product of glucose and fructose degradation in TBV. HMF is also a major product of the Maillard reaction. However, as demonstrated in our previous work [4], HMF does not show antioxidant properties and, therefore, does not contribute to the high antioxidant activity of TBV. It has recently been demonstrated [15, 16] that the Maillard reaction can occur during boiling and storage of grape juice and its model system and results in the accumulation of HMF and brown pigment formation.
Taking into account these observations, it is reasonable to think that during the cooking of must, MRPs with antioxidant activity are synthesized and could be partially responsible for the high antioxidant activity of TBV.
In this study we investigated the antioxidant properties of TBV, in relation to its content in polyphenols, polymeric tannins and MRPs, and the formation of antioxidant Maillard reaction products during the heating of must model systems with the aim of increasing the knowledge about the formation of antioxidant compounds during the cooking and concentration of grape must.
Materials and methods
Materials
l-arginine, l-glutamic acid, l-glutamine, l-proline, l-threonine, catechin, gallic acid, bovine serum albumin (fraction V), sodium dodecyl sulfate (SDS), triethanolamine, 4-aminoantipyrine (4-AP), horseradish peroxidase (HRP) type II and 2,4,6-tripyridyl-S-triazine (TPTZ) were supplied from Sigma (Milan, Italy). 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was supplied from Calbiochem (La Jolla, CA, USA). All the other chemical reagents were from Carlo Erba (Milan, Italy). Sephadex C-18 columns (quantity of sorbent 500 mg, volume above packing 6 mL, catalogue number 205350) were supplied from Alltech (Deerfield, IL, USA). Traditional balsamic vinegar (TBV) samples were kindly supplied by the “Consorzio fra produttori di Aceto Balsamico Tradizionale di Reggio Emilia” (Reggio Emilia, Italy). The absorbance was read using a Jasco V-550 UV/Vis spectrophotometer.
Extraction and determination of polyphenol
Polyphenolic compounds were extracted from three TBV samples utilizing a previously developed protocol [4]. Briefly, 2 mL of 10 times diluted vinegar (1 g of TBV brought to 10 mL with distilled water) was passed through a preconditionated Sephadex C-18 column. The columns were washed thrice with 2 mL of water and the adsorbed phenols were eluted thrice with 3 mL of methanol (HPLC grade) on the basis of UV monitoring. At the end of the separation, the methanolic fractions (M1, M2 and M3) containing polyphenols were separately analyzed. These methanolic fractions represent the total polyphenolic fraction of TBV. The total phenolic content of the methanolic fractions was determined using an enzymatic method [4, 17]. In a 3 mL spectrophotometric cell, 0.1 mL of appropriately diluted methanolic fractions or catechin standard solutions was added to 3 mL of 0.1 M potassium phosphate-buffered solution, pH 8, containing 3 mM 4-AP, 2 mM H2O2, and 10 U of horseradish peroxidase (HRP). The absorbance value was read at 500 nm at the endpoint of 15 min. Catechin standard solutions were prepared by dissolving catechin in water at a concentration ranging from 1 to 30 mg/L. The total phenolic content was expressed in milligrams of catechin equivalents per kilogram of TBV. The total phenolic content was also determined with the Folin-Ciocalteu reagent [18]. In a 1.5 mL Eppendorf tube, 790 μL of distilled water, 10 μL of appropriately diluted methanolic fractions or catechin standard solutions and 50 μL of Folin-Ciocalteu reagent were added and mixed. A volume of 10 μL of water or methanol was used as blank. After exactly 1 min, 150 μL of 20% aqueous sodium carbonate was added, and the mixture was mixed and left to stand at room temperature in the dark for 120 min. Detection was achieved at 760 nm. Catechin standard solutions were prepared by dissolving catechin in water at a concentration ranging from 50 to 500 mg/L. The total phenolic content was expressed in milligrams of catechin equivalents per kilogram of TBV.
Fractionation of phenolic compounds
Polyphenolic compounds were fractionated from three TBV samples utilizing Sephadex C-18 columns [19]. Briefly, 2 mL of 10 times diluted vinegar (1 g of TBV brought to 10 mL with distilled water), adjusted to pH 7 with 5 N NaOH, was passed through a preconditionated Sephadex C-18 column to adsorb neutral phenolic compounds. Non-adsorbed phenolic acids were washed with 3 mL of water at pH 7. After acidification of this effluent to pH 2 with HCl 0.1 N, phenolic acids were fixed into a second preconditioned acidic Sephadex C-18 column. The columns were washed with 3 mL water at pH 2 to eliminate interfering compounds and the adsorbed phenolic acids (fraction I) were eluted with 2 mL of methanol (HPLC grade). The fraction containing catechins and olygomeric procyanidins (fraction II) was eluted with 2 mL of 16% acetonitrile at pH 2 after acidic preconditioning of the first C-18 column. The flavonols (fraction III) and polymeric procyanidins (fraction IV) were eluted using 2 mL ethyl acetate and 2 mL methanol (HPLC grade), respectively. Polyphenols in all the fractions were determined with both the enzymatic and Folin-Ciocalteu assays. Owing to the different responses of individual phenolic compounds in the enzymatic method [20], we chose different standards for each fraction. Standards were: caffeic acid in methanol for fraction I, catechin in 16% acetonitrile at pH 2 for fraction II, quercetin in ethyl acetate for fraction III and catechin in methanol for fraction IV. Each standard was tested at concentrations ranging from 50 to 500 mg/L. In Folin-Ciocalteu assay, catechin was used as standard compound.
Extraction and determination of tannin
Tannins were extracted from three TBV samples utilizing a protein precipitation method [21]. Briefly, 1 mL of 10 times diluted TBV (1 g of TBV brought to 10 mL with distilled water) was added to 2 mL of standard protein solution. The solutions were mixed and allowed to stand at room temperature for 15 min and were then centrifuged for 15 min at 5,000g. The standard protein solution consists of bovine serum albumin dissolved at a concentration of 1 mg/mL in 0.2 M acetate buffer, pH 5, containing 0.17 M sodium chloride. After centrifugation, the supernatant was discarded and the surface of the pellet was washed with acetate buffer without disturbing the pellet. The precipitate was then dissolved in 4 mL of sodium dodecyl sulfate (SDS) triethanolamine solution that contained 1% SDS and 5% (v/v) triethanolamine. This solution represents the tannic fraction of TBV. The tannins were determined by mixing 2 mL of tannic fraction with 0.5 mL of ferric chloride reagent (0.01 M ferric chloride in 0.01 N HCl). The absorbance value was read at 510 nm, approximately 15–30 min after the addition of the ferric chloride reagent. Catechin standard solutions were prepared by dissolving catechin in water at a concentration ranging from 5 to 100 mg/L. The tannin content was expressed in milligrams of catechin equivalents per kilogram of TBV.
Extraction of high molecular weight melanoidins
High molecular weight (>10 KDa) melanoidins were extracted from three TBV samples as described by Morales and Babbel [22]. Different TBV samples of 1 g weight were diluted to 10 mL with water and then filtered (Whatman filter papers 40, Whatman, UK). One aliquot (400 μL) of each filtered sample was subjected to ultrafiltration with Microcon YM-10, regenerated cellulose 10 KDa (Millipore, Italy) at 14,000g for 50 min at 4 °C. The retentate, containing high molecular weight melanoidins and the filtrate containing low molecular weight compounds, such as polyphenols and Maillard reaction products, were filled up to 400 μL with distilled water. The filtrate was analyzed for the total polyphenolic content with Folin-Ciocalteu assay as described above.
Preparation of must model systems
Must model systems were prepared in accordance with Bozkurt et al. [16]. To test the influence of sugars and amino acids on the browning and on the production of antioxidant compounds, two different model systems were prepared. Model system 1 was prepared to test the effect of the caramelization reaction and contained only sugars (98 g/L fructose and 106 g/L glucose). Model system 2 contained the same sugars as that of model system 1 and five amino acids (1047 mg/L arginine, 449 mg/L proline, 210 mg/L glutamine, 58 mg/L glutamic acid, 49 mg/L threonine). All the model solutions were dissolved in water (50 mL) and the pH was carefully controlled and adjusted to 3 with HCl. The screw cap was kept loose to expose the must model systems to air while heating in boiling water at 100 °C for 240 min, which is the time necessary to reduce the volume to about 30% of the starting volume, simulating conditions of must cooking. At different times, 0.5 mL of the sample was collected from must model systems and immediately frozen until the analysis was performed. In addition, to test the influence of the phenolic compounds on the browning and on the production of antioxidant compounds, two different model systems were prepared. Model system 3 was prepared to test the stability of the phenolic compounds during heating to 100 °C. The phenolic compounds used were gallic acid (as representative of phenolic acids) and catechin (as representative of flavonoids). We chose gallic acid and catechin because they are easily separable and are the most utilized polyphenol standards in literature. As these belong to different phenolic groups, they may present different behaviors during must cooking and Maillard products formation. The concentrations of gallic acid and catechin were 17 and 35 mg/L, respectively, in accordance with the value of total phenolic acids (hydroxybenzoic and cinnamic acids) and total flavonoids (procyanidins and flavonols) determined in white grape (Thompson seedless) must by Spanos and Wrolstad [23]. Model system 4 contained the same sugars and the same amino acids as that of model system 2 and the same phenolic compounds of model system 3.
Measurements of browning
The browning index of the three TBV samples, total polyphenolic fractions and high molecular weight melanoidins extracted from TBV samples was determinate by measuring the color as absorbance at 420 nm in a 1 cm glass cuvette. The samples were appropriately diluted in water to give absorbance values <1.
Measurements of antioxidant activity
Antioxidant activity of the three TBV samples, total polyphenolic fractions and high molecular weight melanoidins extracted from TBV samples were measured both as reducing ability and radical scavenging activity. For the determination of the reducing ability of samples, a protocol based on the ferric reducing/antioxidant power (FRAP) assay was utilized [24]. Briefly, 0.1 mL of diluted sample was added to 3 mL of FRAP reagent that was freshly prepared by mixing 300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3 at a ratio of 10:1:1. After exactly 6 min, the absorbance was read at 593 nm. For the determination of the ABTS radical scavenging activity of samples, a protocol based on the ABTS assay was utilized [25]. ABTS was dissolved in distilled water to 14 mM concentration. ABTS radical cation (ABTS·+) was produced by reacting ABTS stock solution at the ratio of 1:1 with 4.9 mM potassium persulphate and leaving the mixture to stand in the dark at room temperature for 12–16 h before use. The resulting blue-green ABTS radical solution was diluted in ethanol to an absorbance of 0.700 ± 0.050 at 734 nm. A volume of 40 μL of diluted sample was added to 1960 μL of the resulting blue-green ABTS·+. The mixture, protected from the light, was incubated in the Jasco V-550 spectrophotometer at 37 °C for 10 min; the decrease in absorbance at 734 nm was measured at the endpoint of 10 min.
Vitamin C standard curves that correlate the concentration of vitamin C (ranging from 1 to 150 mg/L) and the amount of absorbance reduction (ABTS scavenging assay) or absorbance increase (FRAP assay), caused by vitamin C, were obtained. The results were calculated as milligrams of vitamin C per kilogram of TBV or milligrams of vitamin C per liter of must model solutions.
Statistical analysis
All data are presented as mean ± SD for at least five replications for each prepared sample. The statistical analysis and regression analyses were performed using GraphPad Instat (GraphPad Software, San Diego, CA, USA). Differences of P < 0.05 were considered significant. Correlation between the antioxidant capacity and the browning index was established using regression analysis at a 99% significance level.
Results and discussion
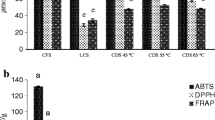
All three samples of TBV show a high antioxidant activity measured with both FRAP and ABTS assays (Table 1) that is higher than or equal to that obtained in red wine [4, 26].
The values obtained with the ABTS assay were higher than those obtained with FRAP assay in each sample analyzed. The difference in the antioxidant activity obtained with FRAP and ABTS assays could be due to the different reaction mechanism involved. FRAP assay detects compounds that act only by the single electron transfer (SET) mechanism, while ABTS assay detects compounds that act either by direct reduction via the electron transfers or by radical quenching via the hydrogen atom transfer (HAT) mechanism [27, 28]. It is known that polyphenols present in fruits, vegetables and related products such as red wine, tea and vinegar are potent antioxidants and are responsible for the main part of the antioxidant activity of these products. As reported in Table 1, TBV also contains polyphenolic compounds and polymeric tannins that contribute significantly to its high antioxidant activity. The concentration of unfractionated polyphenols of TBV, determined with peroxidase assay in the methanolic fractions after Sephadex passage, was 1574.0 ± 47.5 mg catechin/kg TBV. Catechin, which we have chosen as a reference compound, as well as quercetin, apigenin and m-hydroxybenzoic acid, has a middle molar absorbance (ε) value with the peroxidase method. Moreover, catechin is widely used in the Folin-Ciocalteau assay. For these reasons, we have expressed the values of the unfractionated phenols as catechin equivalent, both for peroxidase and Folin-Ciocalteau methods. In the literature [20], the ε of a number of compounds determined by the peroxidase method is reported and it is easy to express concentration with other standards. When polyphenols were determined with the Folin-Ciocalteu assay, the concentration was 2,205.4 ± 11.1 mg catechin/kg TBV, higher than that determined with peroxidase assay. TBV methanolic fractions may contain compounds that interfere with Folin-Ciocalteu assay, mainly melanoidins that have been shown to react in a concentration-dependent manner with the Folin-Ciocalteu reagent [4]. For this reason, we have eliminated from TBV the high molecular weight melanoidins before C18 passage. When melanoidins were eliminated, the total polyphenol concentration obtained with Folin-Ciocalteu method in the methanolic fractions were 1,882.2 ± 53.8 mg catechin/kg TBV closer to that obtained with enzymatic assay.
When polyphenols are fractionated into phenolic acids, flavanols, flavonols and polymers, the phenolic concentration was determined in each fraction with enzymatic assay using specific standards. The phenolics most represented were phenolic acids (37.8 ± 1.7% of the total polyphenols), followed by catechins (36.0 ± 1.8%), polymeric procyanidins (18.8 ± 1.3%) and flavonols (7.4 ± 1.5%). The total phenolic content obtained from the sum of the concentrations of each phenolic fraction was 1,398.3 ± 33.9 mg/kg of TBV, which is similar to the value previously obtained in the methanolic fractions. When the phenolic concentration was determined in each fraction with Folin-Ciocalteu assay, the sum of the concentrations was 2,125.4 ± 29.5 mg catechin/kg TBV, which is similar to the value determined with Folin-Ciocalteu assay in unfractionated poyphenols containing melanoidins. The value of tannins obtained with the peroxidase assay after fractionation, differs slightly from the value obtained with the protein precipitation method (262.68 ± 18.2 mg catechin/kg TBV and 307.2 ± 49.9 mg catechin/kg TBV, respectively). This difference could be probably due to an overestimation of the concentration of tannins in the protein precipitation method owing to the non-specific binding of catechin to the protein–tannin complex [21], causing catechin precipitation. On the other hand, when polyphenols are fractionated [19], olygomeric procyanidins are recovered in fraction II probably causing underestimation of the concentration of tannins in fraction IV.
Phenolic compounds are already present in the must. Antonelli et al. [13] found that uncooked must contains about 260 mg/kg of polyphenols. Taking into account that during the cooking the must is concentrated about 3.3 times, the concentration of polyphenols in concentrated and cooked must should be 858 mg/kg. During the ageing, TBV is concentrated about 1.8 times [2]; therefore, the amount of polyphenols in aged TBV should be 1,544 mg/kg. This value is close to the data obtained in our study, suggesting that the increase in the concentration of polyphenols from the uncooked must to the aged vinegar could only be due to the concentration process. However, it is not possible to exclude that other processes such as extraction from wood or precipitation of the largest tannins could be important in determining the concentration of polyphenols in aged TBV.
The cooking of must and the successive ageing of the vinegar favor the Maillard reaction and the formation of antioxidant MRPs. Absorbance at 420 nm represents the browning index and is related to the brown pigment formation due to the Maillard reaction [11]. As can be seen in Table 1, all three samples of TBV show a high browning index that can suggest the presence of brown melanoidins. However, some polyphenols such as flavonols and flavanol olygomers and polymers can themselves contribute to the absorbance at 420 nm of TBV. In particular, the total polyphenolic fraction of TBV accounts for the 42.9 ± 11.1% of the total absorbance at 420 nm (the average value obtained from Table 1 was defined as 100% of browning index). The residual value of absorbance at 420 nm is related to the presence of brown melanoidins. As reported by Antonelli et al. [13], the browning index of cooked must is about 3, while the browning index of aged TBV observed in our samples is 78.4 (Table 1). This increment in the browning index is not explicable with the concentration of the product and indicates that the Maillard reaction continues during the ageing of TBV, being favored by the reduction in water content [29] of the vinegar during the ageing.
To verify the effect of the incidence of phenolic compounds and tannins on the overall antioxidant activity of TBV, we tested the total polyphenolic fraction and tannic fraction extracted from the TBV samples for the antioxidant activity assay. Phenolic compounds contribute to 47.4 ± 8.3 and 43.9 ± 6.2% of the overall antioxidant activity of TBV measured by FRAP and ABTS assays, respectively (Table 2).
Among polyphenols, tannins contribute to 45.3 ± 3.8 and 57.5 ± 2.7% of the antioxidant activity of the total polyphenolic fraction measured by FRAP and ABTS assays, respectively. The residual antioxidant activity of TBV could be due to other compounds present such as Maillard reaction products. To verify the effect of the incidence of melanoidins on the overall antioxidant activity of TBV, we tested the high molecular weight fraction corresponding to melanoidins extracted from the TBV samples for the antioxidant activity assays. Melanoidins contribute to 43.6 ± 2.3 and 46.4 ± 1.1% of the overall antioxidant activity of TBV measured by FRAP and ABTS assays, respectively (Table 2). The residual antioxidant activity (about 10%) is due to other compounds such as low molecular weight MRPs.
Numerous studies have shown the formation of MRPs with antioxidant activity in model systems constituting a sugar and an amino acid [6–8]. Generally, these studies have been done without pH control [30–32] or at neutral [6] or basic [7, 8] pH value. The pH has a great influence on the kinetics of Maillard reaction; in fact, it is favored by high pH values [33, 34]. At the same time, high pH values favor the formation of melanoidins with high antioxidant activity [11, 35]. Since pH has a great influence on the Maillard reaction products with antioxidant activity [11], it is necessary to match the pH value of the model systems to that of the food being modeled.
The pH value of must is normally about 3; therefore, we carried out the analysis to verify the formation of antioxidant MRPs during the heating of must model systems with a controlled pH value of 3. It has been demonstrated [13] that during the cooking of must, the pH value is only slightly affected by the process with a recorded decrease of 0.3 pH units. Also in our model systems, the pH value decreases by 0.3 ± 0.1 pH units. During heating, the volume of the must model solutions is progressively reduced to about 80 (concentration ratio 1.25), 60 (concentration ratio 1.64), 45 (concentration ratio 2.22) and 30% (concentration ratio 3.33) of the starting volume after 60, 120, 180 and 240 min of heating, respectively.
Figure 1 shows the formation of brown pigment in must model systems heated to 100 °C and pH 3.0, reporting the uncorrected data for the concentration ratio and, therefore, simulating the real conditions of cooking must.
Brown pigment formation in must model systems at 100 °C and pH 3.0; uncorrected data. Symbols used are: filled circle, model system 1 (only sugars); filled triangle, model system 2 (sugars and amino acids); filled square, model system 3 (only polyphenols); multi symbol, model system 4 (sugars, amino acids and polyphenols)
As reported in Material and methods, model system 1 contained only sugars to verify the effect of caramelization on the total rate of browning, while model system 2 contained both sugars and amino acids. The presence in the reaction mixture of the amino acids caused an increase in the browning of the model solution that is statistically significant (P < 0.05) with respect to the model system containing only sugars. These data show that the non-enzymatic browning during the heating of must model system occurs through the formation of new compounds synthesized either by caramelization or, in particular, by the Maillard reaction and are in agreement with previously reported data [16].
The models used in our study were selected according to the major components of food responsible for the browning reaction. However, the presence of minor components that are not present in the model systems can influence the browning reaction and presumably the formation of antioxidant compounds. For example, browning tends to be accelerated in the presence of metal ions [36] and inhibited by salts [36, 37] and some organic acids [38]. All these components are present in must and could have an influence on the browning and on the formation of MRPs with antioxidant activity.
In addition, the must contains a great quantity of phenolic compounds that can be involved in the Maillard reaction [11], for example interacting with HMF [39]. This interaction has been demonstrated with catechin and results in the formation of colored compounds [39]. However, the role of phenolic compounds in the development of antioxidant activity in heated sugar–amino acid systems has only recently been considered [40, 41]. For example, it has been demonstrated [41] that the incorporation of ferulic acid in glucose–proline model system results in a significant increase in antioxidant activity as a consequence of the reaction between ferulic acid and Maillard reaction products. Authors supposed that ferulic acid inhibits the free radicals generated during the early stages of the Maillard reaction, leaving MRPs with antioxidant activity to accumulate [41]. To verify the possible role of phenolic compounds present in the must on the browning reaction and on the formation of MRPs with antioxidant activity, we carried out experiments incorporating some polyphenols in the must model systems. As shown in Fig. 1, no detectable browning was observed after 240 min of heating to 100 °C and pH 3.0 of model solution 3 containing only polyphenols. The browning in model system 4 (containing amino acids, sugars and polyphenols) was the same as that of model system 2 (containing amino acids and sugars), suggesting that polyphenols, in our experimental conditions, do not influence the browning rate of the model system. The browning value determined in the model system 4 (3.88 ± 0.3) is higher than the browning value observed during the heating of real samples of must (about 3) [13]. This observation could be explained by the fact that the must contains not only sugars, amino acids and polyphenols, but also all those substances reported above that can influence the browning rate of a real must sample.
In order to verify if the increment in the brown pigment were a consequence of the concentration process or of the formation of new compounds, we reported the data corrected for the concentration ratio (Fig. 2). As can be seen in Fig. 2, the browning rate exhibits the same trend as reported in Fig. 1 for all the model systems considered.
Brown pigment formation in must model systems at 100 °C and pH 3.0. Data were corrected for the concentration ratio as reported in the text. Symbols used are: filled circle, model system 1 (only sugars); filled triangle, model system 2 (sugars and amino acids); filled square, model system 3 (only polyphenols); multi symbol, model system 4 (sugars, amino acids and polyphenols)
Figure 3 shows the formation of antioxidant compounds assayed by ABTS assay in must model systems heated to 100 °C and pH 3.0, reporting the data uncorrected for the concentration ratio, and, therefore, simulating the real condition of cooking must.
Antioxidant activity determined with ABTS assay in must model systems at 100 °C and pH 3.0; uncorrected data. Symbols used are: white bar, model system 1 (only sugars); light gray bar, model system 2 (sugars and amino acids); dark gray bar, model system 3 (only polyphenols); black bar, model system 4 (sugars, amino acids and polyphenols)
As can be seen, the heating of each model system caused an increment in the antioxidant activity. The increment in the antioxidant activity is higher for model system 2 than model system 1 indicating that the Maillard reaction is the most important cause that determines the formation of new antioxidants in must model systems. Polyphenols tested in our study have no effect on the formation of antioxidant compounds and the increase in the antioxidant activity observed for the model system 3 was only a consequence of the concentration process. The same trend was obtained when the antioxidant activity was tested with the FRAP assay (data not shown).
Figure 4 shows the formation of antioxidant compounds in must model systems heated to 100 °C, and of pH 3.0, assayed by ABTS assay, reporting the data corrected for the concentration ratio, in order to verify if the increment in the antioxidant activity were a consequence of the concentration process or of the formation of new compounds.
Antioxidant activity determined with ABTS assay in must model systems at 100 °C and pH 3.0. Data were corrected for the concentration ratio as reported in the text. Symbols used are: filled circle, model system 1 (only sugars); filled triangle, model system 2 (sugars and amino acids); filled square, model system 3 (only polyphenols); multi symbol, model system 4 (sugars, amino acids and polyphenols)
Heating of model system 1 containing only sugars caused an increase of antioxidant activity that is related to the browning (r = 0.9440). The presence of the amino acids in model system 2 increased the antioxidant activity with respect to the model system 1 (P < 0.05). The increase in the antioxidant activity of the model system 2 with the heating time is related to the browning (r = 0.9801) suggesting that the brown pigments synthesized either by caramelization or, in particular, by the Maillard reaction are responsible for the increment in the antioxidant activity observed in must model systems. When the antioxidant properties of the model system 3 were investigated, no variations were observed as the heating time increased, suggesting that there was no formation of new antioxidant compounds during the heating of this model system. In addition, the data reported for the model system 3 show that only polyphenols are stable in the model system. A comparison of the antioxidant activity of the model system 4 with the sum of the antioxidant activity of model system 2 and model system 3 shows that the values of antioxidant activity are identical. This fact suggests that catechin and gallic acid at the concentrations considered in our study and under our experimental conditions do not influence the formation of MRPs with antioxidant activity. These results cannot exclude that at higher concentrations or at different pH value, catechin and gallic acid influence the browning reaction and the antioxidant activity of Maillard reaction products. The same trend was obtained when the antioxidant activity was been tested with the FRAP assay (data not shown).
From our results, it is clear that during the boiling of must model systems, MRPs with antioxidant activity can form. The brown pigment formation can continue during the ageing in the vinegar barrel and is favored by the dehydration of the product, so that at the end of the ageing the concentration of brown melanoidins formed during cooking of the must increased, explaining the high antioxidant activity of TBV. Our results show that TBV has a high antioxidant activity due to polyphenols and tannins and MRPs. Independent of their bioavailability, dietary antioxidants play an important role in protecting the gastrointestinal tract from oxidative damage and also possibly against a buildup of peroxides and their assimilitation in the digestive tract [42]. In this contest, TBV, which is rich in antioxidants, is a seasoning that can contribute, along with vegetables and wine, to an increase in the total amount of antioxidants ingested during a meal.
References
Cocchi M, Lambertini P, Manzini D, Marchetti A, Ulrici A (2002) J Agric Food Chem 50:5255–5261
Cocchi M, Durante C, Grandi M, Lambertini P, Manzini D, Marchetti A (2006) Talanta 69:1166–1175
Plessi M, Bertelli D, Miglietta F (2006) J Food Comp Anal 19:49–54
Verzelloni E, Tagliazucchi D, Conte A (2007) Food Chem 105:564–571
Martins SIFS, Jongen WMF, van Boekel MAJS (2001) Trends Food Sci Technol 11:364–373
Yoshimura Y, Iijima T, Watanabe T, Nakazawa H (1997) J Agric Food Chem 45:4106–4109
Jing H, Kitts DD (2004) Arch Biochem Biophys 429:154–163
Osada Y, Shibamoto T (2006) Food Chem 98:522–528
Okamoto G, Hayase F, Kato H (1992) Biosci Biotechnol Biochem 566:928–931
Jing H, Kitts DD (2003) Food Chem Toxicol 40:1007–1015
Manzocco L, Calligaris S, Mastrocola D, Nicoli M, Lerici CR (2001) Trends Food Sci Technol 11:340–346
Morales FJ, Jiménez-Pérez S (2004) Eur Food Res Technol 218:515–520
Antonelli A, Chinnici F, Masino F (2004) Food Chem 88:63–68
Muratore G, Licciardello F, Restuccia C, Puglisi ML, Giudici P (2006) J Agric Food Chem 54:860–863
Göğüş F, Bozkurt H, Eren S (1998) Lebensm Wiss Technol 31:196–200
Bozkurt H, Göğüş F, Eren S (1999) Food Chem 64:89–93
Stevanato R, Fabris S, Momo F (2004) J Agric Food Chem 52:6287–6293
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Meth Enzymol 99:152–178
Oszmianski J, Ramos T, Bourzeix M (1988) Am J Enol Vitic 39:259–262
Ma YT, Cheung PCK (2007) J Agric Food Chem 55:4222–4228
Hagerman AE, Butler LG (1978) J Agric Food Chem 26:809–812
Morales FJ, Babbel MJ (2002) J Agric Food Chem 50:4657–4661
Spanos GA, Wrolstad RE (1990) J Agric Food Chem 38:1565–1571
Benzie IFF, Strain JJ (1999) Meth Enzymol 299:15–27
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans CA (1999) Free Rad Biol Med 26:1231–1237
Lee KW, Kim YJ, Lee HJ, Lee CY (2003) J Agric Food Chem 51:7292–7295
Prior RL, Wu X, Schaich K (2005) J Agric Food Chem 53:4290–4302
Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K (2002) Analyst 127:183–198
Eichner K, Karel M (1972) J Agric Food Chem 20:218–223
Yilmaz Y, Toledo R (2005) Food Chem 93:273–278
Borrelli RC, Fogliano V, Monti SM, Ames JM (2003) Eur Food Res Technol 215:210–215
Morales FJ, Jiménez-Pérez S (2001) Food Chem 72:119–125
Ajandouz EH, Puigserver A (1999) J Agric Food Chem 47:1786–1793
Ajandouz EH, Tchiapke LS, Dalle Ore F, Benajiba A, Puigserver A (2001) Food Chem Toxicol 66:926–931
Lertittikul W, Benjakul S, Tanaka M (2007) Food Chem 100:669–677
Kwak EJ, Lim SI (2004) Amino Acids 27:85–90
Pham CB, Cheftel JC (1990) Food Chem 37:251–260
Kwak EJ, Lim SI (2005) J Sci Food Agr 85:1337–1342
Liu SC, Chang HM, Wu JSB (2003) Food Res Int 36:579–585
Charurin P, Ames JM, Del Castillo MD (2002) J Agric Food Chem 50:3751–3756
Samaras TS, Gordon MH, Ames JM (2005) J Agric Food Chem 53:4938–4945
Halliwell B, Zhao K, Whiteman M (2000) Free Rad Res 33:819–830
Acknowledgments
The authors thank Professor Paolo Giudici and “Consorzio fra produttori di Aceto Balsamico Tradizionale di Reggio Emilia” (Reggio Emilia, Italy) for the supply of traditional balsamic vinegar samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tagliazucchi, D., Verzelloni, E. & Conte, A. Antioxidant properties of traditional balsamic vinegar and boiled must model systems. Eur Food Res Technol 227, 835–843 (2008). https://doi.org/10.1007/s00217-007-0794-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0794-6