Abstract
A recent patent application and some other studies showed that there is a relation between acrylamide formation and cations. In fact, to date, there is no concrete evidence on the formation or elimination of some compounds in foods and current hypotheses are based only on observations in model systems. To find that this is a logical explanation, we conducted a series of experiments, to show (i) the formation and the elimination of acrylamide with the addition of some cations, and (ii) the formation of hydroxymethylfurfural and furfural in a glucose–asparagine model system. The results indicated that the presence of cations reduced acrylamide formation, but increased hydroxymethylfurfural and furfural formation during heating. There was strong evidence that the cations effectively prevented the formation of Schiff base, which is the key intermediate leading to acrylamide, and mainly changed the reaction path toward the dehydration of glucose leading to hydroxymethylfurfural and furfural.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of theoretical mechanisms have been proposed for the formation of acrylamide in heated foods. Most probably, acrylamide in food results largely from the Maillard reaction between amino acids (primarily asparagine) and a reactive carbonyl (e.g., glucose and fructose), proceeding through intermediates that include a Schiff's base [1–4]. A recent study revealed that, besides acrylamide, 3aminopropionamide, which may be a transient intermediate in acrylamide formation, was also formed during heating when asparagine was reacted in the presence of glucose [5]. Several factors, such as the initial concentration of reactants and their ratio, temperature and time of processing, and pH and water activity, have been shown to influence the levels of acrylamide in heat-processed foods [6]. The influence of temperature on the formation of acrylamide has been repeatedly demonstrated [1, 7–10].
Yasuhara et al. (2003) have discussed the oxidation and/or thermal degradation of lipids in fried foods as a possible mechanistic route contributing to the formation of acrylamide via an acrylic acid intermediate [11]. Lindsay and Jang (2005) have tested this hypothesis by accelerating the oxidation during frying through the introduction of ferric chloride to the surface of sliced potatoes before frying [12]. Although transition metal cations like iron are well known for accelerating lipid oxidation via catalysis of hydroperoxide decompositions, the treatment of potato slices with Fe3+ instead reduced acrylamide formation significantly. They noted that the introduction of Ca2+ as a nontransition state cation treatment also reduced acrylamide formation hypothesizing ionic associations involving ions and charged groups on asparagine and related intermediates were likely to be involved. A recent patent application also showed that polyvalent cations are able to reduce acrylamide formation during heating [13]. However, these preliminary findings give no concrete evidence on the formation or elimination of some other compounds during the Maillard reaction.
This study was aimed at investigating the effects of several monovalent, divalent, and trivalent cations on Maillard reaction products such as acrylamide, hydroxymethylfurfural, and furfural in a glucose–asparagine model system, proposing a chemical mechanism for the action of cations.
Materials and methods
Chemicals and consumables
Acrylamide (99+%) and13 C3-labelled acrylamide (99% isotopic purity) were obtained from Sigma (Diesenhofen, Germany) and Cambridge Isotope Laboratories (Andover, MA, USA), respectively. Glucose and asparagine were reagent grade and obtained from Sigma (Diesenhofen, Germany). Hydroxymethylfurfural and furfural were obtained from Acros Organics (Geel, Belgium). NaCl, KI, CaCl2, ZnCl2, MgCl2.6H2O, and FeCl3.6H20 were all reagent grade and obtained from Merck (Darmstadt, Germany).
The analytical column, Zorbax SB-AQ (4.6 mm× 150 mm, 5 μm) was obtained from Agilent (Wilmington, DE, USA). The 0.45 μm microspin centrifuge filter tubes were obtained from Alltech Associates, (Deerfield, IL, USA). Glass vials with septum screw caps were supplied by Agilent Technologies (Wilmington, DE, USA).
Stock solutions of glucose and asparagine were prepared at a concentration of 300 mM by dissolving 270 and 198 mg in 5 ml of water, and, of Na+, K+, Ca2+, Mg2+, Zn2+, and Fe3+ at a concentration of 600 mM by dissolving 176 mg, 498 mg, 333 mg, 610 mg, 408 mg, and 811 mg of NaCl, KI, CaCl2, ZnCl2, MgCl2·6H2O, and FeCl3·6H2O in 5 ml of water, respectively.
Pyrolysis conditions
In order to determine the effects of monovalent, divalent, and trivalent cations on the formation of acrylamide, a model system composed of glucose and asparagine was used. Ten micromoles of glucose and asparagine were combined in a 25 ml test tube (Pyrex, 25 ml volume, closed system). Varying amounts of each of the cations (0, 1, 5, 10, and 20 μmoles) were included in the reaction mixture. The pH value of the reaction mixture was measured as 4.81 before the addition of cations. Adding up to 20 μmoles of cations into the reaction mixture did not result in a significant change in the pH value. Total reaction volume was adjusted to 100 μl with water to promote the physical interaction of reactants in each case. Energy required to evaporate water was limited by limiting the amount of water present in the medium. Carefully closed test tubes containing the reactants were placed in an oil bath. At least 75% of the test tube by height was dipped into hot oil to initiate the Maillard reaction. The reactions were performed at 150 °C for 20 min where the maximum amount of acrylamide was attained. The water in the medium rapidly evaporated and was held in the upper space of test tube below the stopper. So, evaporative loss of acrylamide was prevented by this arrangement. Increase of temperature in the reaction medium was monitored by measuring outside temperature of the tube wall by means of an infrared thermometer. Outside temperature of the tube wall reached to that of the oil within 45–50 s. It was assumed that outside temperature of the tube wall is identical to inside temperature where the chemical reaction occurs by neglecting the conductive resistance to heat transfer. After heating, the tubes were immediately placed in cold water.
Analysis of pyrolysates by LC-MS
The pyrolysates were suspended in 1.0 ml of water and the aqueous extract was obtained by vortexing for 1 min. The mixture was transferred into an eppendorf centrifuge filter (0.45 μm) and centrifuged for 5 min at 5000 rpm. Twenty microliters of the clear extract were analyzed by using a liquid chromatography system coupled to a single quadrupole mass spectrometer for acrylamide, hydroxymethylfurfural, and furfural, as well as for some key intermediates formed during the reaction. Remaining precursors, glucose and asparagine were also analyzed in the pyrolysates.
An Agilent 1100 HPLC system (Waldbronn, Germany) consisting of a binary pump, an autosampler, and a temperature-controlled column oven, coupled to an Agilent 1100 MS detector equipped with atmospheric pressure chemical ionization (APCI) interface was used. Analytical separations were performed on a Zorbax SB-AQ column using the isocratic mixture of 0.01 mM acetic acid in 0.2% aqueous solution of formic acid at a flow rate of 0.4 ml/min at 40 °C. Data acquisition was performed either in scan mode or in selected ion monitoring (SIM) mode using the interface parameters: drying gas (N2, 100 psig) flow of 4 l/min, nebulizer pressure of 60 psig, drying gas temperatures 325 °C, vaporizer temperature of 425 °C, capillary voltage of 4 kV, corona current of 4 μA, fragmentor voltage of 55 eV. Quantifications were performed based on the signal responses of the ions having m/z of 72, 127, 97, 133, and 181 for acrylamide, hydroxymethylfurfural, furfural, asparagine, and glucose. In order to confirm acrylamide by comparing its signal response to that of13C3-labelled acrylamide, ions having m/z of 72, 55, 75, and 58 were also monitored.
Results and discussion
Effect of cations on acrylamide, hydroxymethylfurfural, and furfural formation
Amino acid asparagine in combination with reducing sugars generates significant amounts of acrylamide when pyrolyzed at temperatures greater than 120 °C [1, 2]. Our results confirmed this finding when equimolar amounts of asparagine and glucose were heated at 150 °C in a sealed glass tube. The formation of acrylamide followed typical kinetic patterns during pyrolysis. The amount of acrylamide generated in the reaction mixture reached to an apparent maximum after 20 min of heating at 150 °C, then, it decreased slowly afterward. Obviously, the content of acrylamide detected in the reaction mixture at 150 °C was the net result of process leading to the formation and degradation of this compound. Similar results have been reported for a model mixture composed of glucose–asparagine at various molar ratios in model and food systems [14–16]. The decline of the curves is most likely due to polymerization as recently reported [17].
When equimolar amounts of asparagine and glucose (10 μmoles each) were pyrolyzed without cations, a maximum of 0.013 μmoles of acrylamide was formed at 150 °C. Meanwhile the signal responses of [M+1] ions for asparagine (m/z 133) and glucose (m/z 181) decreased rapidly. Certain amounts of hydroxymethylfurfural and furfural were also formed during heating. Interestingly, addition of cations significantly influenced the formation of acrylamide, hydroxymethylfurfural, and furfural. In general, the presence of cations in the reaction mixture decreased acrylamide formation while the formation of hydroxymethylfurfural and furfural increased. It was noted that glucose decomposed more rapidly in the presence of cations during heating. However, the rate of asparagine decomposition decreased as the concentration of cation increased in the reaction mixture. We found that pyrolyzing the equimolar mixture of asparagine and glucose with equimolar amounts of monovalent, divalent, and trivalent cations such as K+, Ca2+, Mg2+, Zn2+, and Fe3+ led to a 97% or more reduction in the amounts of acrylamide formed during heating at 150 °C for 20 min (Fig. 1a). In general, the cations decreased the amount of acrylamide formed in the reaction mixture as their concentration increased from 0 to 20 μmoles. This was also true for Na+ to a certain extent. Increasing the amount of Na+ ion from 0 to 5 μmoles decreased acrylamide formation by 59%. Further increase in the amount of Na+ ion did not bring any improvement for the reduction, but increased the amount of acrylamide formed.
It was noted that certain amounts of hydroxymethylfurfural were formed after heating the mixture of asparagine and glucose at 150 °C for 20 min without including any cation in the reaction mixture. However, the amounts of hydroxymethylfurfural formed during the reaction significantly increased as the amount of Ca2+, Mg2+, and Fe3+ included in the reaction mixture increased (Fig. 1b). However, addition of Na+, K+, and Zn2+ had only limited effect on hydroxymethylfurfural formation. These cations almost halved the amount of hydroxymethylfurfural formed at lower amounts (1, 5 μmoles) while they almost doubled it at higher amounts (10, 20 μmoles). No formation was noted for furfural after heating the mixture of asparagine and glucose at 150 °C for 20 min. However, adding certain amounts of K+, Ca2+, Mg2+, Zn2+, and Fe3+ caused furfural formation during heating the reaction mixture. The effect of K+, Zn2+, and Fe3+ was more pronounced for furfural formation (Fig. 1c).
Effects of cations on the reaction path
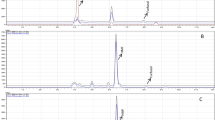
The presence of cations in the reaction mixture not only influenced the type and concentration of characteristic reaction products during heating, but also influenced the rate of decomposition of reaction precursors significantly. The percentage of unreacted glucose and asparagine were approximately 75 and 20%, respectively, after heating the reaction mixture at 150 °C for 20 min. As mentioned earlier, adding cations into the reaction mixture increased the rate of glucose decomposition while most of asparagine remained unreacted, as shown in Fig. 2. These results clearly pointed out that the reaction path was subject to change when certain types of cations were present in the reaction medium. When the concentration of certain cations was increased, reaction proceeded mainly toward the dehydration of glucose leading to hydroxymethylfurfural as one of the characteristic end products. Formation of hydroxymethylfurfural due to dehydration of hexoses was first reported by Haworth and Jones [18]. Antal et al. (1990) experimentally proved that the mechanism of hydroxymethylfurfural formation went through cyclic intermediates [19]. It has been shown that dehydration of hexoses is catalyzed by organic acids, inorganic acids, salts, and Lewis acids [20]. Here, we could successfully show the triple dehydration process of glucose in the presence of Lewis acids by means of scan LC-MS analysis of pyrolyzates. [M+1] ions for single (m/z 163), double (m/z 145), and triple dehydration (m/z 127) of glucose formed during heating the mixture of asparagine and glucose (10 μmoles each) at 150 °C in the presence of cations.
Mechanistic studies have proposed that glycoconjugates, such as N-glycosides and related compounds formed in the early phase of Maillard reaction are the key intermediates leading to acrylamide [2–4]. Based on these studies, the first step in acrylamide production is the formation of Schiff base between the carbonyl and α-amino group of asparagine by means of the dehydration of Nglycosyl compound. Our results showed a clear impact of cations on acrylamide formation. The effect of cations was toward the hindrance of the Schiff base of asparagine, which has a molecular weight of 294. The [M+1] ion of the Schiff base of asparagine (m/z 295) was detectable in the pyrolysate after heating the mixture of glucose–asparagine. However, presence of cations partially or completely eliminated the formation of Schiff base depending on the type and concentration of the cation (Table 1).
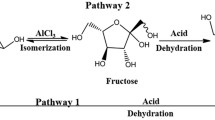
As discussed earlier in detail, there were net effects of cations on the nature and progress of reaction during heating the mixture of glucose and asparagine. According to the proposed reaction mechanism shown in Fig. 3, the reaction path mainly changed from the pyrolysis of asparagine with glucose (path I) to the dehydration of glucose (path II) in the presence of certain cations.
Conclusion
Minimization of acrylamide formed during thermal processing of foods is of great importance from the viewpoint of food safety. Since the thermal processing of foods is rather complex due to a wealthy composition of the reaction pool, the results of a single treatment would be diverse. So, any approach putting forward to prevent any adverse effect like acrylamide formation during thermal processing should also consider other possible changes to be made on the resulting food product. The preliminary studies have shown that the addition of polyvalent cations such as Ca2+ prevented acrylamide formation in some model systems suggesting a mitigation strategy regardless of its any possible adverse effects. The addition of cations may be simply considered beneficial in certain foods prior to thermal processing, not only to mitigate acrylamide formation, but also to enrich calcium or zinc in the finished product. The results obtained here revealed that the realization of such a strategy may be problematic in terms of overall food quality, because cations significantly increase the formation of other process contaminants such as hydroxymethylfurfural and furfural while decreasing the formation of acrylamide. In the last decade, the presence of hydroxymethylfurfural in foods has raised toxicological concerns since the compound and its derivatives have been shown to have cytotoxic, genotoxic, and tumoral effects [21–24]. Although recent studies suggest that hydroxymethylfurfural does not pose a serious health risk [25], the subject is still a matter of debate.
References
Mottram DS, Wedzicha BL, Dodson AT (2002) Nature 419:448–449
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert M-C, Riediker S (2002) Nature 419:449–450
Yaylayan V-A, Wnorowski A, Perez-Locas C (2003) J Agric Food Chem 51:1753–1757
Zyzak DV, Sanders RA, Stojanovic M, Tallmadge DH, Eberhart BL, Ewald DK, Gruber DC, Morsch TR, Strothers MA, Rizzi GP, Villagran MD (2003) J Agric Food Chem 51:4782–4787
Granvogl M, Scieberle P (2006) J Agric Food Chem 54:5933–5938
Friedman M (2003) J Agric Food Chem 51:4504–4526
Becalski A, Lau BP-Y, Lewis D, Seaman S (2003) J Agric Food Chem 51:802–808
Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M (2002) J Agric Food Chem 50:4998–5006
Biedermann M, Grob K (2003) Mitt Geb Lebensm Unters Hyg 94:406–422
Rydberg P, Eriksson S, Tareke E, Karlsson P, Ehrenberg L, Törnqvist M (2003) J Agric Food Chem 51:7012–7018
Yasuhara A, Tanaka Y, Hengel M, Shibamoto T (2003) J Agric Food Chem 51:3999–4003
Lindsay RC, Jang S (2005) Model systems for evaluating factors affecting acrylamide formation in deep fried foods. In: Friedman M, Mottram DS (eds) Chemistry and safety of acrylamide in food. Springer, Berlin, pp 329–341
Tomoda Y, Hanaoka A, Yasuda T, Takayama T, Hiwatashi A (2004) US Patent Application (20040126469)
Ehling S, Shibamoto T (2003) J Agric Food Chem 53:4813–4819
Elmore JS, Koutsidis G, Dodson AT, Mottram DS, Wedzicha BL (2005) J Agric Food Chem 53:1286–1293
Gökmen V, Şenyuva HZ (2006) Food Additiv Contam 23(4):348–354
Stadler RH, Robert F, Riediker S, Davidek T, Blank I (2004) J Agric Food Chem 52:5550–5558
Haworth WN, Jones WGM (1944) J Chem Soc 667–670
Antal MJ, Mok WSL, Richards GN (1990) Carbohydr Res 199:91–109
Tyrlik SK, Szerszen D, Olejnik M, Danikiewicz W (1999) Carbohydr Res 315:268–272
Nassberger L (1990) Hum Exp Toxicol 9:211–213
Bruce WR, Archer MC, Corpet DE, Medline A, Minkin S, Stamp D, Yin Y, Zhang XM (1993) Mutat Res 290:111–118
Zhang XM, Chan CC, Stamp D, Minkin S, Archer MC, Bruce WR (1993) Carcinogenesis 14:773–775
Surh YJ, Liem A, Miller JA, Tannenbaum SR (1994) Carcinogenesis 15:2375–2377
Janzowski C, Glaab V, Samimi E, Schlatter J, Eisenbrand G (2000) Food Chem Toxicol 38:801–809
Acknowledgement
We thank the Turkish Academy of Sciences (GEBIP Study Grant) and Scientific and Technical Research Council of Turkey (Project TOVAG COST 927–2) for financial support, Ankara Test and Analysis Laboratory for LC-MS analyses, and Agilent Technologies for supplying some consumables.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökmen, V., Şenyuva, H.Z. Effects of some cations on the formation of acrylamide and furfurals in glucose–asparagine model system. Eur Food Res Technol 225, 815–820 (2007). https://doi.org/10.1007/s00217-006-0486-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0486-7