Abstract
Supercritical CO2 extracts of the marine diatom Chaetoceros muelleri (gracilis) have been investigated for their potential use as food preservatives, namely, as antimicrobials. A screening of different pressures and temperatures for supercritical CO2 extraction was assayed in order to determine the main factors controlling the yield and antimicrobial activity of the extracts. Since the potential antimicrobial activity of these CO2 extracts is mainly induced by the lipidic fraction, HPLC with evaporative light scattering detection (HPLC-ELSD) and GC with flame ionization detection (GC-FID) were used to identify lipid families and fatty acids, respectively. Antimicrobial activity of the extracts was measured against Staphyloccocus aureus, Escherichia coli and Candida albicans. Possible correlations between antimicrobial activity of extracts and their chemical composition were investigated, concluding that the total triglycerides and the DPA content seem to be the main parameters controlling the antimicrobial activity of the studied extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diatom is a basic component of marine hatchery operations because it serves as alternative natural source of polyunsaturated fatty acids [1]. The diatom Chaetoceros muelleri is considered one of the most popular strains used for feeding shrimp larvae depending its composition mainly on the cultivation method. Triglycerides, polar lipids and free fatty acids are the main components of the lipid fraction when this Chaetoceros muelleri microalgae is cultured in batch [2].
The ability of fatty acids to interfere with bacterial growth and survival has been known for several decades [3]. Structure–function relationship studies on free fatty acids against human pathogenic bacteria indicate that antimicrobial activity can depend on both the chain length and the degree of unsaturation [4]. It has also been demonstrated that compounds, such as cholesterol, can antagonize the antimicrobial properties of fatty acids [5]. Consequently, both composition and concentration of free lipids can influence antimicrobial properties [3].
Supercritical fluid extraction is a well known technique to extract different types of lipids [6, 7], furthermore, extraction of polar lipids and free fatty acids can be improved by adding small quantities of ethanol [8].
The goal of the present investigation was the screening of the potential antimicrobial activity of supercritical extracts of Chaetoceros muelleri obtained under several extraction conditions. The effects of different temperature and pressure on the supercritical CO2 yield were investigated. All extractions were done considering algae at the optimum cultivation conditions and the extracts were analyzed to determine the composition and concentrations of free lipids trying to correlate it with their antimicrobial properties.
Experimental
Cell culture
The microalgae Chaetoceros muelleri (CCMP1316) (CHGRA) was grown in batch cultures in f/2 medium [9] with addition of silicates. The culture was previously synchronized with three periods of 4 days each. Lighting (100 μmol m−2 s−1) was applied by Phillips tubes fluorescent in a 12:12 light–dark cycle, temperature and salinity were maintained at 24±1 °C and 35 PSU (practical salinity units), respectively. A continuous airflow was supplied to the cultures and pH remained between 7.5 and 8.2 by adding CO2. Under these conditions growth curves were previously determined for each microalgae by counting cells with a Neubauer chamber. Cells were harvested at late logarithmic phase of each treatment by centrifugation at 7000 rpm/min for 10 min, freeze-dried and maintained at −20 °C until analyzed.
Supercritical fluid extraction
A Suprex PrepMaster (Suprex, Pittsburgh, PA, USA) supercritical fluid extractor was used for all the experiments. Sample (1 g of Chaetoceros dry weight basis mixed with 0.2 ml of ethanol, 99.5% Panreac, Barcelona, Spain) was placed into a 5 ml stainless-steel extraction cell. The supercritical CO2 (N38 quality, AL, Air liquide España, Madrid, Spain) flow rate was controlled using a needle valve as variable restrictor. Total extraction time was 60 min; during the first 15 min extraction was static followed by 45 min of dynamic extraction. Extracts were collected in a glass vessel cooled by ice.
Different extraction pressures and temperatures were selected as variables to study the effect of the experimental conditions on the extraction yield obtained. Pressure was selected between 200 and 400 atm and temperature between 40 and 80 °C. The different combinations (pressure-temperature of CO2) provided medium-high extraction densities from 0.6 to 0.96 g/ml; this range of conditions has been suggested previously to extract non-polar compounds from different materials [10].
Lipid composition analysis
Lipid fraction of extracts was analyzed using two chromatographic techniques. Liquid chromatography coupled to evaporative light scattering detector (HPLC-ELSD), to identify the different lipid classes, and gas chromatography coupled to flame ionization detector (GC-FID) to identify the free and esterified fatty acids.
HPLC-ELSD
The analyses were done on a Kromasil silica 60 column (250 mm×4.6 mm, Análisis Vínicos, Tomelloso, Spain) coupled to a CTO 10A VP 2 oven, a LC-10AD VP pump, a gradient module FCV-10AL VP, a DGU-14A degasser, and a evaporative light scattering detector ELSD-LT from Shimadzu (IZASA, Spain). Details of the chromatographic method used to analyze the products of the extraction are described elsewhere [11]. All HPLC solvents were HPLC purity from Labscan (Dublin, Ireland).
GC-FID
To prepare ethyl esters of free and esterified fatty acids, samples were mixed with chloroform/ethanol 2/1 (v/v) and ethylated by addition of 1 ml of a solution of sulfuric acid in ethanol (0.9 M). This mixture was allowed to stand overnight at 50 °C. After addition of 200 μl miliQ water, the resulting mixture was extracted with two 1 ml portions of n-hexane and the final extract was then dried with sodium sulfate.
One microliter of derivatized sample was injected into a Perkin-Elmer autosystem XL (Wellesley, MA, USA) gas chromatograph with a 30 m BTR-Carbowax column (0.25 mm i.d.). Injector and detector temperatures were set at 220 and 230 °C, respectively. The temperature program was as follows: starting at 100 °C and then heating to 180 °C at 20 °C/min; followed by heating from 180 to 220 °C at 15 °C/min. The final temperature (220 °C) was held for 30 min. Identification of the ethyl esters of the various fatty acids was based on a menhaden oil fish standard (#4-7085) obtained from Supelco (Bellefonte, PA).
Antimicrobial activity measurement
Microbial strains
The extracts were individually tested against a panel of microorganisms including two bacteria (Staphyloccocus aureus ATCC 25923 and Escherichia coli ATCC 11775) and one yeast (Candida albicans ATCC 60193). Staphyloccocus aureus and Escherichia coli strains stock cultures were kept on nutrient agar at 4 °C. Candida albicans was kept on Sabouraud dextrose agar at 4 °C.
Determination of minimum inhibitory concentration (MIC) and minimal bactericidal and fungicidal concentration (MBC)
A broth microdilution method was used, as recommended by the National Committee for Clinical Laboratory Standards (NCCLS), for determination of the minimum inhibitory concentration [12]. All tests were performed in Mueller-Hinton broth supplemented with 0.5% Tween 20 (Fluka, Germany), with the exception of yeasts (Sabouraud dextrose broth + 0.5% Tween 20). The inocula of bacterial strains were prepared from overnight Mueller-Hinton broth cultures at 37 °C. Yeasts were cultured overnight at 25 °C in Sabouraud dextrose broth. Test strains were suspended in Muller-Hinton (bacteria) or Sabouraud dextrose (yeasts) broth to give a final density 107 cfu/ml. The Chaetoceros muelleri extract dilutions in DMSO ranged from 10 to 200 mg/ml.
The 96-microwell plates were prepared by dispensing into each well: 165 μl of culture broth, 5 μl of inoculums and 30 μl of different extracts dilutions. The final volume of each well was 200 μl. Plates were incubated for bacteria and yeasts at 37 and 25 °C for 24 and 48 h, respectively. Negative controls were prepared using 30 μl of DMSO (dimethylsulfoxide), the solvent used to dissolve microalgal extracts. Chloramphenicol and amphotericin B (Sigma, Madrid) were used as positive reference standards to determine the sensitivity of the microbial species used. After incubation, the MIC of each extract was determined by visual inspection of the wells bottom (bacterial growth was indicated by the presence of a white “pellet” on the well bottom). The lowest concentration of the extract that inhibited growth of the microorganism, as detected as lack of the white “pellet”, was designated the minimum inhibitory concentration. The minimum bactericidal and fungicidal concentration was determined by making subcultures from the clear wells that did not show any growth. Each test was performed in triplicate and repeated twice.
Statistical analysis
The statistical methods used were cluster analysis (Average Linkage method from standardized variables) to discover natural groupings of the variables and principal component analysis (PCA) from standardized variables to examine the relationship among them. Statgraphics program for Windows release 5.1 (StatPoint Inc., VA, USA) was used for data processing.
Results and discussion
Supercritical fluid extraction
Different extraction conditions have been studied in the present work to obtain extracts with antimicrobial activity. The experimental conditions using supercritical CO2 and their corresponding extraction yields are shown in Table 1. As can be seen, yields were ranging from 1.8 to 3.9‰ under these conditions. Although different pre-treatments of the microalga using ultrasounds and microwaves were tested to increase these extraction yields, no significant improvements were obtained. A possible explanation for these low yields can be related to the siliceous cell wall of Chaetoceros muelleri [13] that, in fact, can preclude the diffusion of the supercritical extractant inside the cell. Even considering these low yields, an initial trend can be observed; extract 3 (obtained at 200 atm and 80 °C) showed the lowest yield while extract 2 (obtained at 400 atm and 40 °C) gave the highest and these values correlate with the minimum and maximum CO2 density, respectively, as can be seen in Table 1. These results are also in agreement with data obtained by other authors extracting different components from microalgal matrices [14, 15]. In spite of these low yields, the antimicrobial activity of these extracts was studied based on the huge interest that nowadays exists on new natural sources of food preservatives and nutraceuticals [16, 17].
Antimicrobial activity
Different microbial species, including a gram negative bacteria (Escherichia coli), a gram positive bacteria (Staphylococcus aureus) and a yeast (Candida albicans), were used to screen the potential antimicrobial activity of supercritical extracts from Chaetoceros muelleri. Their antimicrobial activity was quantified measuring their minimum inhibitory concentration (MIC) and minimal bactericidal and fungicidal concentration (MBC). Results obtained are given in Table 2, showing that Candida albicans was the most sensitive microorganism to these extracts since the lowest concentration of extract was needed to kill this microorganism (lowest MBC values), compared to Escherichia coli and Staphylococcus aureus which needed a higher concentration (higher MBC values). Comparing the results obtained for the different extracts, the most active ones were 2, 3 and 5, followed by 1 and 4 (see Table 2). These results show that the use of medium values of pressure and temperature provides higher extraction of antimicrobial compounds. Moreover, the use of extreme pressure and temperature values (400 atm and 80 °C) gave less active extracts (experiment 1 in Table 2). This behavior is difficult to explain due to the complexity of the extracts obtained, since depending on the extraction conditions, different compounds can be enriched in the extracts thus providing diverse interactions/synergies among them. Interestingly, the results of Table 2 also show that, in general these extracts possess a good antimicrobial activity and that they could be useful for the food industry.
A liquid–liquid extract of raw microalga using DMSO was obtained for comparing its antimicrobial activity with those obtained using supercritical CO2. The results show (see Table 2) that the antimicrobial activity of the DMSO extract was about threefold lower than the obtained with the supercritical CO2 extracts. This indicates that the use of supercritical CO2 is more suitable to extract compounds with antimicrobial activity from Chaetoceros. Besides, some other additional advantages of using CO2 have to be taken into account; namely, supercritical CO2 extraction is an environment-friendly procedure and the achieved extracts can be directly used by the food industry as no toxic solvents are present (in opposition to classical liquid–liquid extraction).
Lipid composition of extracts
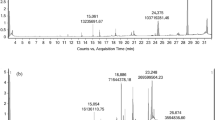
In an attempt to identify compounds responsible for the antimicrobial activity of these Chaetoceros muelleri supercritical extracts and based on the well-known ability of different fatty acids to inactivate microoganisms [3], different families of lipids were analyzed [11]. Figure 1 shows a typical HPLC-ELSD chromatogram of one of the supercritical extracts of Chaetoceros muelleri (experiment 3 in Table 1, 200 atm, 80 °C). As can be seen, a nice separation of the different lipid families found in the Chaetoceros extract is obtained, in which compounds such as triglycerides (TAG), diglycerides (DAG), monoglycerides (MAG), sterols, free fatty acids (FFA) and hydrocarbons were detected.
A comparison of the lipid composition of the five CO2 extracts of Table 1 and a raw microalgae extract obtained using classical liquid–liquid extraction is shown in Fig. 2. As can be observed, free fatty acids (FFA) were, in general, the main components accounting, in some extracts, for more than 75% of the total lipids. In general terms, free fatty acids were the most abundant family after sterols followed by DAGs, TAGs and MAGs. Analysis of sterol fraction allowed identifying cholesterol as the main product; the presence of cholesterol had been previously suggested by other authors in Chaetoceros [2, 18]. As can be seen in Fig. 2, the relative contribution of the different families to the total lipid fraction mainly depends on the extraction conditions, corroborating the specificity that the use of supercritical CO2 as extractant provides (e.g., compare in Fig. 2 extracts 4 and 5 in terms of MAG, FFA and TAG contents).
Lipid class composition of the five supercritical fluid extracts of Table 1 and a raw Chaetoceros muelleri extract using liquid–liquid extraction. (TAG: tryglicerides; FFA: free fatty acids; 1,3-DAGs: 1,3-diacylglicerides; 1,2-DAGs: 1,2-diacylglicerides; MAGs: monoglycerides)
A detailed FFA content was performed by using GC-FID analysis. This was required on the basis of the fact that antimicrobial activity has already been linked to fatty acids content [19, 20]. Figure 3 shows the GC chromatogram of the extract 3 (obtained with CO2 at 200 atm and 80 °C), the one that has provided the best antimicrobial activity. The chromatogram is divided in zones including the fatty acids with the same carbon chain length. As can be seen, C16 fatty acids (C16:0, C16:2, C16:3) are the most abundant family of fatty acids in this extract, being also remarkable the presence of long chain polyunsaturated fatty acids (EPA, DPA and DHA) whose antimicrobial activity has already been suggested [3]. Figure 4 shows the distribution of the different fatty acids detected in the five supercritical extracts compared to the raw extract. As can be seen, big differences can be found among the extracts which also could be associated to the extraction conditions used, corroborating the specificity of the extraction procedure used in this work.
Fatty acid composition of the five supercritical fluid extracts of Table 1 and a raw Chaetoceros muelleri extract using liquid–liquid extraction
Cluster analysis has been employed to establish some correlation between antimicrobial activity and the lipid composition of the extracts, including TAG, DAG, MAG, FFA, sterols, hydrocarbons content and the relative composition of fatty acids (as mol%). Figure 5 shows the dendrogram of standardized variable data obtained using the Pearson's correlation coefficient (absolute value) as measure of similarity among two variables and the average linkage as linkage rule between groups. In this dendrogram, a significant relationship can be observed between the antimicrobial activities against all the microorganisms tested and the triglycerides (TAGs) and DPA content. There exists a negative correlation between the antimicrobial activity and the TAGs (with Pearson correlation coefficients ranging from −0.745 to −0.796) and a positive correlation with DPA (Pearson correlation coefficient for antimicrobial activity against Staphylococcus aureus equal to 0.839), meaning that an increase of DPA implies an increase in the value of Minimal Bactericidal Concentration (MBC) and, therefore, a decrease in the effective antimicrobial activity. The opposite is observed with TAGs, meaning that an increase in its relative contribution to the composition of the sample implies a higher antimicrobial activity (or lower MBC concentration). Although the other variables grouped together in the cluster, no relationship can be observed with the antimicrobial activity. Even though FFA have been strongly associated to the antimicrobial activity of different extracts [4, 21, 22], in this particular case, no correlation was observed either individually or as a sum of fatty acids. Moreover, the total content on fatty acids of recognized antimicrobial activity (such as C16:1, C18:2 and C18:3) [3, 23] did not give any correlation with the observed antimicrobial activities suggesting a more complex behavior, a strong contribution of other lipids and/or an inhibition of the fatty acids antimicrobial effects due to the cholesterol concentration in the extracts [5].
Principal component analysis was also applied to establish relationships among antimicrobial activity and lipid composition. Five principal components were obtained that explained 97.6% of the total variance of data. Rotation of the five principal components (through Varimax method) provided the following results: the first principal component, which explained 30% of the total variance, was negatively correlated with C18 (−0.95), C18:1 (−0.95), C16 (−0.91) and C12 (−0.85) while the second component (which explained 25.2% of the variance) was strongly correlated positively with the antimicrobial activity against Staphylococcus aureus (0.978), Escherichia coli (0.939), Candida albicans (0.939) and DPA (0.822) and negatively correlated with TAGs (−0.821) what really confirms the results obtained by cluster analysis.
Conclusions
The present study has demonstrated the interest of using supercritical CO2 to obtain extracts of Chaetoceros muelleri with antimicrobial activity. The strong influence of the supercritical extraction conditions in both, the lipid composition of the extracts and, consequently, in the antimicrobial activity has also been shown. Statistical analysis of all the data, considering lipid composition and relative concentration of fatty acids in the different extracts suggested an important relationship among antimicrobial activity and triglyceride content (TAG) and DPA. This study is presented as a first step to optimize the green extraction of antimicrobials from Chaetoceros muelleri that could be used as food preservatives.
References
Chiou SY, Su WW, Su YC (2001) J Biotechnol 85:247–257
Pernet F, Tremblay R, Demers E, Roussy M (2003) Aquaculture 221:393–406
Benkendorff K, Davis AR, Rogers CN, Bremner JB (2005) J. Exp Mar Biol Ecol 316:29–44
Kabara JJ, Vrable R, Lie Ken Jie MSF (1977) Lipids 12:753–759
Galbraith H, Miller TB, Paton AM, Thompson JK (1971) J Appl Bacteriol 34:803–813
Birtigh A, Johannsen M, Brunner G, Nair N (1995) J Supercrit Fluids 8:46–50
Hurtado-Benavides AM, Senorans FJ, Ibanez E, Reglero G (2004) J Supercrit Fluid 28:29–35
Rodrigues CEC, Reipert ECD, de Souza AF, Filho PAP, Meirelles AJA (2005) Fluid Phase Equilibr 238:193–203
Guillard RR, Ryther JH (1962) Can J Microbiol 8:229–239
Brunner G (2005) J Food Eng 67:21–33
Torres CF, Vazquez L, Senorans FJ, Reglero G (2005) J Chromatogr A 1078:28–34
NCCLS (1999) Performance standards for antimicrobial susceptibility testing National Committee for Clinical Laboratory Standards (NCCLS) M100-S9, vol 19. Wayne, PA
Sze P (1986) A Biology of the Algae, vol 1. WCB/McGraw-Hill Publishers, London, UK
Andrich G, Zinnai A, Nesti U, Venturi F, Fiorentini R (2005) Proceedings of ICheaP-7 (The Seventh Italian Conference on Chemical & Process Engineering), Taormina, Italy, Chemical Engineering Transactions
Lim GB, Lee SY, Lee EK, Haam SJ, Kim WS (2002) Biochem Eng J 11:181–187
Herrero M, Cifuentes A, Ibanez E (2006) Food Chem 98:136–148
Leistner L (2000) Int J Food Microbiol 55:181–186
Parrish CC, Wells JS, Yang Z, Dabinett P (1999) Mar Biol 133:461–471
Borowitzka MA (1999) In: Cohen Z (ed) Pharmaceuticals and agrochemicals from microalgae in “Chemicals from microalgae”. Taylor & Francis, London, UK, pp 313–352
Skrivanova E, Marounek M, Dlouha G, Kanka J (2005) Lett Appl Microbiol 41:77–81
Vairappan CS (2003) Indian J Exp Biol 41:837–845
Yajima M, Nozaki K, Takayanagi T, Yokotsuka K (1997) J Antibact Antifung Agent 25:131–137
Ouattara B, Simard RE, Holley RA, Piette GJP, Begin A (1997) Int J Food Microbiol 37:155–162
Acknowledgements
This work has been financed by Spanish Ministry of Education (Project AGL2005-06726-C04-01 and 02) and by CSIC/CONACYT (2004MX0008). JAM and AT would like to thank Spanish Ministry of Education their grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendiola, J.A., Torres, C.F., Toré, A. et al. Use of supercritical CO2 to obtain extracts with antimicrobial activity from Chaetoceros muelleri microalga. A correlation with their lipidic content. Eur Food Res Technol 224, 505–510 (2007). https://doi.org/10.1007/s00217-006-0353-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0353-6