Abstract
The % content and the % fatty acid (FA) composition of the three isomeric classes of diacylglycerol (DAG) fraction (sn-1,2-, sn-2,3-, sn-1,3-DAG) were valued in four groups of extra virgin olive oils, stored in different conditions of temperature and time, with the objective to study the influence of the cited parameters on olive oil DAG classes. The separation and % quantitation of all isomeric DAG classes were carried out by normal phase-high performance liquid chromatography (NP-HPLC) on total DAG fraction previously isolated by TLC and subjected to derivatization step with (S)-(+)-(1-naphtyl)-ethylisocyanate; moreover the % FA composition of each DAG class was determined by HRGC analysis. The results showed notable differences on the % content of each DAG class as well as the ratios between the classes. The samples analysed as soon as they were produced showed the highest % contents for sn-1,2-DAG and the lowest for sn-1,3- and sn-2,3-DAG. The samples stored at 30 °C generally showed the highest % contents of sn-1,3-DAG, because of the isomerization processes of sn-1,2-DAG to the more stable sn-1,3-DAG. The results of structural analysis of the sn-1,2-DAG class besides confirmed that isomerization processes occurred and that the storage temperature had a predominant role; this research emphasizes the importance of the valuation of % contents together with the structural analysis of the isomeric DAG classes as useful analytical parameters to evaluate the storage conditions and the conservation status of olive oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many authors have recently emphasized the importance of diacylglycerol (DAG) fraction to evaluate the quality of the fatty foods [1–11].
As regards the virgin olive oil, the knowledge of the quantitative/qualitative analysis of DAG fraction can give significant information about the characteristics of product, the extraction technology, the occurrence of physical and chemical treatments, the time and conditions of storage [9]. In particular, the relationships between the isomeric DAG groups, named 1,2-DAG and 1,3-DAG, were proposed as new analytical parameters to value the virgin olive oil quality [4]. However, these studies have generally considered the two isomeric groups 1,2-DAG and 1,3-DAG, without distinguish, in the 1,2-DAG group, the sn-1,2-DAG and sn-2,3-DAG enantiomeric classes. This occurrence is relevant because only the sn-1,2-DAG is the biosynthetic intermediate, because the microsomial enzymes of many species of oil seeds catalyse the triacylglycerol (TAG) formation, essentially through the reaction of the so-called Kennedy pathway [12]. The other two isomeric DAG classes present in virgin olive oil, the sn-2,3-DAG and sn-1,3-DAG, respectively rise above all from chemical and/or enzymatic hydrolysis of TAG and from isomerization processes [13].
These observations show that the DAG fraction can assume the significance of a valuable “fingerprint” of the virgin olive oil, because the ratios among DAG isomeric classes are influenced by the initial quality and by the storage modality of product [11].
Thus, an analytical approach able to achieve the separation and the successive analysis not only of the racemic mixture of sn-1,2-(2,3)-DAG from sn-1,3-DAG but of all the three isomeric classes seemed to be of great interest in order to obtain parameters useful in the evaluation of the alteration status of a virgin olive oil sample.
A significant part of this work is related to the stereospecific analysis of the free sn-1,2-DAG class and the comparison of its total and sn-2 position FA % compositions with the data obtained from the fraction sn-1,2-TAG [15] (sn-1,2-DAG obtained from TAG by partial hydrolysis with Grignard reagent).
The objective of this work is the evaluation of the modifications of the DAG classes % contents as a result of different storage conditions and the evaluation of the total and positional FA compositions of sn-1,2-DAG to point out the occurrence of isomerization processes.
Materials and methods
Material
The following four groups of extra virgin olive oil (EVOO) samples were considered:
-
1.
group 1 (EVOO-1), five samples of EVOO from differently ripened olives, obtained from “Dolce Agogia” cultivar by cold pressing in a pilot plant; the drupes were collected from the end of September to middle December, every 20 days;
-
2.
group 2 (EVOO-2), an EVOO sample obtained as soon as produced by an industrial system in Umbria; this sample was analysed immediately (zero time) and aliquots were stored in the dark at 15 °C or at 30 °C for 1, 3, 6, 9 and 12 months;
-
3.
group 3 (EVOO-3), three series of three aliquots of a commercial EVOO sample: the first was stored in the dark at 4 °C for 7 months, the second at 30 °C for 7 months and the third at 30 °C for 9 months;
-
4.
group 4 (EVOO-4), three commercial samples of EVOO, stored in the dark for 7 months at 15 °C or at 25 °C and for 19 months at 4 °C.
All the solvents and reagents were of analytic grade or high performance liquid chromatography (HPLC) grade.
All the samples were analysed immediately after extraction from the fruit or after the cited storage time.
The oil samples were analysed, at least twice or thrice when necessary, according to the following procedures.
Methods
Isolation of free DAG fraction from olive oil
The oil samples were submitted to two TLC on H3BO3-impregnated silica pre-coated plates (20 cm×20 cm, 250 μm, Sigma, St. Louis, MO) in order to isolate the total DAG fraction (Rf≅0.3) and to minimize the free DAG isomerization (eluent mixture: petroleum ether 40–60 °C/diethylether/formic acid, 70:30:1, v/v/v). The free DAG fraction was obtained by extraction with anhydrous diethyl ether [15].
Separation of DAG classes
The DAG fraction separated by TLC was submitted to derivatization with (S)-(+)-(1-naphtyl)-ethylisocyanate, according to a previously described procedure [14].
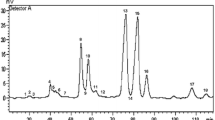
In brief, the DAG mixture (3–4 mg) was dissolved in dry toluene (1 ml), then (S)-(+)-(1-naphtyl)ethyl isocyanate (12.5 μl) and anhydrous 4-pyrrolidinopyridine (4 mg) were added. The mixture was then heated at 50 °C overnight under magnetic stirring and the solvent was removed in a stream of nitrogen. Methanol/water (95:5, v/v; 6 ml) was added and the mixture was warmed to dissolve the residue. A Baker-Bond SPE ODS solid-phase extraction column (500 mg, Baker Analysed – Deventer, Holland) was washed with 10 ml of the cited solvent. The reaction mixture was filtered through a small cotton-wool plug onto the column and washed with further 15 ml of solvent. The required products were then eluted with acetone (10 ml). HPLC separation of the diastereomeric derivatives of free DAG classes was carried out with a Varian 9010 isocratic pump (Varian, Walnut Creek, CA). Two columns of silica gel (Hypersil 3 μm, 25 cm×4.6 mm i.d.; HiChrom, Reading, U.K.) in series and a mobile phase of 0.4% (v/v) n-propanol (containing 2% water) in hexane at a flow rate of 1 ml/min were used [14]. Each sample was dissolved in 50 μl of mobile phase and 10 μl of this solution was injected; detection was at 280 nm.
Analysis of % FA composition of DAG classes
The methyl ester derivatives of fatty acids (FAME), obtained by sodium methoxide-catalysed transesterificaton [14], were analysed by high resolution gas chromatography (HRGC). A Chrompack 9001 capillary gas chromatograph (Chrompack, Middelburg, The Netherlands) was equipped with a split/splitless injection system and with a CP-WAX-58CB (30 m×0.22 mm i.d., film thickness 0.2 μm, Chrompack) capillary column. At first, the oven temperature was maintained 3 min at 150 °C; then, the temperature was programmed (5 °C/min) up to 240 °C and finally held at the final temperature for 10 min. The carrier gas was helium at 1 ml/min. The component FAME were quantified by electronic integration (MOSAIC Software, Chrompack).
Structural analysis of sn-1,2-DAG
The selective hydrolysis of sn-1,2-DAG urethanes was performed under the following conditions: The urethane derivatives of each free DAG class (∼5 mg, obtained as described in section ‘Separation of DAG classes’) were added to 2.5 ml of isoctane, sodium bis-(2-ethylexyl)-sulfosuccinate (25 mg), buffer (1 ml of 0.1 M borate–HCl solution with 1 mM CaCl2, pH 7.5) and 30 mg of porcine pancreatic lipase (EC 3.1.1.1; carboxylic-ester hydrolase), 21 U/mg; this reaction mixture was stirred magnetically at 30 °C for 24 h. After the addition of CHCl3/MeOH (2:1, v/v, 3 ml) and water (enough to permit the subsequent phases separation), the mixture was centrifuged. The chloroformic layer was separated, treated with anhydrous sodium sulphate, concentrated, submitted to Silica TLC plate, and eluted with CHCl3/MeOH/H2O (65:25:2, v/v/v). The band at Rf≅0.8, containing the reaction product, was scraped and submitted to another Silica TLC; the elution was carried out with petroleum ether 40–60 °C/ethyl ether/formic acid (70:30:1, v/v/v). The band containing sn-2-MAG-urethanes (Rf≅0.1) was scraped from silica and submitted to sodium methoxide-catalysed transesterification [14].
Preparation of biosynthetic sn-1,2-DAG -named sn-1,2-TAG- and analysis of total and sn-2 % FA composition
The biosynthetic sn-1,2-DAG of the oil samples were obtained from the respective TAG fractions by partial hydrolysis with Grignard reagent [15]. The HRGC analysis of FAME was carried out, in the conditions described in section ‘Analysis of % FA composition of DAG classes’, in order to obtain the total % FA composition while the sn-2 % FA composition was obtained by the pancreatic lipase method [16].
Results
In Table 1 the results of the % content of DAG classes for the samples of EVOO-1 group have been reported; they show that the ripening involves modifications on the percentages of the three DAG classes since the sn-1,2-DAG decreasing and the sn-1,3-DAG increasing were observed.
With regard to the samples of the EVOO-2 group (Table 2) the aim was to evaluate the modifications of DAG classes percentage contents in an oil sample, analysed as soon as produced and stored for different times at two different temperatures. In the aliquots stored at 15 °C, the % amount of the sn-1,3-DAG increased from 6.4%, value at zero time, to 19.5% at 9 months of storage, while the sn-1,2-DAG decreased from 84.7% to 65.9%. The same trend was observed also in the samples stored at 30 °C, but higher differences were observed: in particular, the sn-1,3-DAG increased from 6.4 to 46.0% and the sn-1,2-DAG diminished from 84.7 to 37.9%. These results show that both time and temperature are important factors involved in DAG isomerization processes.
Concerning the EVOO-3 group samples (Table 3) oil samples acquired from stores were considered to verify if the DAG classes analysis was still useful in highlighting the “alteration condition” of the oil samples, independently from cultivar, fruit ripeness and extraction system; the results show an increase of sn-1,3-DAG class content and a correspondent reduction of sn-1,2-DAG class. The differences were high when the temperature, from 4 to 30 °C, and/or the time of storage, from 7 to 9 months, increased. The same trend was observed for the % contents of the sn-1,3- and sn-1,2-DAG classes of the group EVOO-4 (Table 4), with changes even higher than those observed for EVOO-2 and EVOO-3 groups, probably due to the initial storage conditions of these commercial samples.
In any case, the worsening of the storage conditions, and particularly the high temperature, caused an increase of sn-1,3-DAG class and a decrease of sn-1,2-DAG, while the sn-2,3-DAG never depart much from the initial quantity; the obtained results show that the storage time was a less important factor; in fact, the samples stored for a long time (19 months) but at low temperatures (4 °C) presented smaller changes in the % contents of DAG classes than those observed for the previous considered samples.
These results have shown that the analysis of isomeric DAG class is able to evidence the “conservation status” of an oil sample and that the modifications of sn-1,2-DAG and sn-1,3-DAG classes are because of isomerization processes catalysed from temperature and time of storage.
Another objective of this work was to study the molecular structure of sn-1,2-DAG class, the only one of biosynthetic origin, to verify the occurrence of isomerization processes. The results of the stereospecific analysis of olive oil TAG showed that the % acidic composition of the sn- positions of the glycerolic backbone are not equivalent [17, 18]; in particular, the sn-2- position is esterified mainly by the unsaturated FA, while the saturated FA are above all located in the sn-1- and in sn-3- positions.
On the basis of these observations, it is possible to deduce that during the isomerization processes the characteristic % FA composition of the sn-1,2-DAG class should exhibit notable change. In Table 5, the % FA compositions of the sn-1,2-DAG of two samples EVOO-3, stored for 7 months at 4 °C and at 30 °C, are compared with the biosynthetic sn-1,2-DAG (sn-1,2-TAG) of the same oil sample, obtained by treatment of the TAG with Grignard reagent and analysed before the storage. The comparison showed a very similar % acidic composition, and this is the expected result in case of an oil sample of recent production or not altered as the sn-1,2-DAG present in the oil samples derived from incomplete biosynthesis of the TAG. However, the obtained results were in contrast with the results reported in Table 3, which showed the occurrence of isomerization processes. To resolve this apparent contradiction, the sn-1,2-DAG class, obtained from the HPLC separation, was subjected to structural analysis, by direct enzymatic hydrolysis with a 1,3-specific lipase; in Table 6, the results of the structural analysis of two samples of EVOO-3 group showed, in position sn-2-, an increase of the % content of the saturated fatty acid and a decrease of the % contents of the unsaturated ones; this occurrence is in accordance with the isomeric process discussed previously.
A similar behaviour is shown also in Table 7, where the results of the % FA composition of the sn-2-position for one sample of EVOO-2 group have been reported; with the increase of storage time and temperature higher percentage of saturated FA and lower of unsaturated ones occurred in sn-1,2-DAG class sn-2-position.
The valuation of the % content of DAG classes can be used as new analytical parameter in the valuation of the alteration or the adulteration state of the extra virgin olive oil; the relative values can assume the significance of alteration indexes, because it can undergo also some substantial modification when other analytical parameters remain unchanged. Finally, the results of the structural analysis of sn-1,2-DAG is a further confirmation of the fact that the modification of the % content of the DAG classes is principally because of isomerization of the DAG classes.
References
Amelotti G, Daghetta A, Ferrario A (1989) Riv Ital Sostanze Grasse LXVI:681–692
Mariani C, Fedeli E, Grob K, Artho A (1991) Riv Ital Sostanze Grasse LXVIII:179–187
Frega N, Bocci F, Lerker G (1993) Riv Ital Sostanze Grasse LXVIII:153–155
Catalano M, De Felice M, Caponio F, De Leonardis T (1995) In: Proceedings 2° Congresso Nazionale di Chimica degli Alimenti, Giardini Naxos, (Italy) 24–27 Maggio 1995, II:381–386
Sacchi R, Patumi M, Fontanazza G, Barone P, Fiordiponti P, Mannina L, Rossi E, Segre AL (1996) J Am Oil Chem Soc 73:747–758
Mariani C, Venturini S, Gasparoli A, Tagliabue S, Bondioli P (2000) Riv Ital Sostanze Grasse LXXVII:49–59
Spyros A, Dais P (2000) J Agric Food Chem 48:802–805
Pérez-Camino MC, Moreda W, Cert A (2001) J Agric Food Chem 49:699–704
Serani A, Piacenti D, Staiano G (2000) Riv Ital Sostanze Grasse LXXVIII:525–528
Fronimaki P, Spyros A, Christophoridou S, Dais P (2002) J Agric Food Chem 50:2207–2213
Leone AM, Santoro M, Liuzzi VA, La Notte E, Gambacorta G (1998) Riv Ital Sostanze Grasse LXV:613–622
Kennedy EP (1961) Fed Proc Am Soc Exp Biol 20:934–940
Barton HR, O'Connor CJ (1997) Aust J Chem 50:355–361
Santinelli F, Damiani P, Christie WW (1992) J Am Oil Chem Soc 69:552–556
Damiani P, Cossignani L, Santinelli F, Simonetti MS (1997) Riv Ital Sostanze Grasse LXXIV:439–442
Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis.
Damiani P, Santinelli F, Simonetti MS, Castellini M, Rosi M (1994) J Am Oil Chem Soc 71:1157–1162
Damiani P, Rosi M, Castellini M, Santinelli F, Cossignani L, Simonetti MS (1994) Ital J Food Sci 6:113–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cossignani, L., Luneia, R., Damiani, P. et al. Analysis of isomeric diacylglycerolic classes to evaluate the quality of olive oil in relation to storage conditions. Eur Food Res Technol 224, 379–383 (2007). https://doi.org/10.1007/s00217-006-0327-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0327-8