Abstract
Capillary electrophoresis (CE) and mass spectrometry have been used to verify the formation of lactosylated casein variants. CE was performed in a hydrophilically coated capillary at low pH and in the presence of urea. Lactosylated α-casein, β-casein, and κ-casein migrate shortly after the unmodified proteins. Evidence for casein lactosylation was obtained by electrospray ionization-mass spectrometry (ESI-MS). Lactosylated β-casein can be monitored not only in milk and milk powders but also in the complex protein mixture of processed cheese. The formation of lactosylated β-casein A1 and A2 during heating of processed cheese was found to correlate with the furosine content. Consequently, CE of casein may be a possible method for directly assessing the extent of the early Maillard reaction in dairy products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteins in dairy products are often subjected to alterations by the Maillard reaction during processing and storage. These changes may or may not be desired dependent on the particular product. During early stages of this complex reaction mainly lysine residues react with the reducing sugar to the corresponding aminoketoses. Such lactosylation of β-lactoglobulin is well known during processing of milk and whey, where multiple lactosylation of β-lactoglobulin has been demonstrated to occur under mild conditions [1–3]. Recently, capillary electrophoresis (CE) proved to be a suitable tool for the separation of various lactosylated forms of β-lactoglobulin [4]. Additional CE peaks migrating shortly after whey proteins were observed and shown to be due to lactosylation. CE and mass spectrometry (MS) were used to identify lactosylation of β-lactoglobulin during the manufacturing process of skimmed milk powder [5]. It has been demonstrated that CE of the whey protein fraction can be used for monitoring storage conditions [6]. Very recently, multiple lactosylation of purified ovine caseinomacropeptide was obtained in a model reaction and characterized using CE, RP-HPLC and MS [7]. For major casein variants separated by CE at low pH using a hydrophilically coated capillary and an urea containing buffer, additional CE peaks migrating shortly after the caseins were found in half skimmed UHT milk [8]. Although not discussed by the authors, these peaks were also found in skimmed milk powder [5] and in processed cheese [9].
Processed cheese contains a rather complex mixture of modified milk proteins, including low levels of β-lactoglobulin, which does not separate well from para-κ-casein in CE [10]. Unmodified α-casein variants are often difficult to quantify since they migrate in the time window of various derivatives such as hydrolysis products. κ-Casein is usually present in very small amounts itself and its modifications are therefore difficult to measure. However, β-casein variants, A1 and A2 in particular, are usually present in considerable quantities and the peaks separate well from other proteins and peptides that occur in significant amounts, indicating that CE might also be used to monitor casein modification in processed cheese.
In this context, the purpose of this study was to prove by mass spectrometry that the additional CE peaks migrating shortly after the major casein variants are also due to lactosylation. Furthermore, our intention was to assess whether these additional peaks derived from casein could be utilized for monitoring the extent of the early Maillard reaction during the manufacture of complex dairy products such as processed cheese.
Materials and methods
Materials
Calibration and tuning chemicals for ESI-MS (Ultramark 1621, caffeine, l-methionyl arginyl phenylalanyl alanine acetate hydrate, reserpine), urea (Electrophoresis Grade), tris(hydroxymethyl)aminomethane (Electrophoresis Grade), ethylenediamine-tetraacetic acid (99.7%), α-casein (85%), β-casein (90%), κ-casein (80%), and dithiothreitol (Clelands's Reagent, 99%) were obtained from Sigma (Sigma-Aldrich Chemie GmbH, Steinheim, D). Methyl 2-hydroxyethyl cellulose was from Aldrich (Aldrich Chemical Company, Inc., Milwaukee, WI, USA). Citric acid and tri-sodium citrate dihydrate were obtaind from Fisher (Fisher Scientific U.K. Ltd., Loughborough, UK) and 3-morpholino propanesulfonic acid from Fluka (Fluka Chemie AG, Buchs, CH). Syringe filters 0.2 and 0.45 μm (GHP Acrodisc ®) were from Gelman (Pall Gelman Laboratory, Ann Arbor, MI, USA). Sodium hydroxide (50% solution, p.a.) was obtained from Baker (Mallinckrodt Baker B.V., Deventer, NL).
Model reaction of casein variants and lactose
The protein (5 mg/ml of α-, β-, and κ-casein, respectively) and lactose (50 mg/ml) were dissolved in 100 mM pyridine-acetic acid. The pH was adjusted to 5.7. The solution was filtered through a 0.45 μm membrane filter and heated at 80 °C under gentle stirring. The reaction was stopped after 30, 60, 120, and 180 min, respectively. A control experiment was conducted by heating the protein solution without lactose for 180 min but otherwise as described above. The reaction mixtures were diluted with water (10 ml), freeze dried twice in order to completely remove the pyridine-acetic acid buffer, and directly analyzed by ESI-MS and by CE.
ESI-MS of unmodified and modified casein variants
A LCQ mass spectrometer (Finnigan MAT, San Jose, CA, USA) with ion trap detection and electrospray interface (ESI) was used. Typical MS are: Capillary temperature 250°C, source voltage +2.9–3.1 kV; capillary voltage −6 V, tube lens offset −15.0 V, octapole 1 offset 1.75 V, octapole 2 offset 6.50 V, interpole lens offset 28.0 V. The maximum ionization time was 200 ms. Full-scan spectra were acquired from m/z 800 to 2000. The instrument scale for the mass to charge ratio (m/z) was calibrated with the known ions of caffeine, MRFA (l-methionyl-arginyl-phenylalanyl-alanine acetate-hydrate) and Ultramark 1621 in methanol/water (1:1, v/v) containing 1% acetic acid was used. A solution of 50 ng/ml reserpine in methanol/water (1:1, v/v) containing 1% acetic acid was used for tuning the ESI system. The samples (1 mg/ml) dissolved in methanol/water (1:1, v/v) were delivered into the mass spectrometer by a syringe infusion pump through a fused silica capillary (10 μm i.d.) at a flow rate of 1 μl/min. In the probe, solutions were sprayed through a stainless steel capillary held at 2.9–3.1 kV with positive ionization. All protein signals were obtained from the averaging of multiple scans.
Preparation of processed cheese
Typical processed cheese ingredients (cheese, butter, milk protein powders, melting salts, water, sodium chloride) were mixed until a homogenous product was obtained comprising the following key composition: 22.0% fat, 12.2% protein, 5.9% lactose, and 46.0% total solids. Heating conditions for industrial processed cheeses vary over a wide rage (e.g., temperatures of between 78°C and 145°C) depending on the desired product type and quality. Pasteurization or ultra-high temperature treatment is applied to make the product stable against microbial deterioration. The heating time depends on the temperature regimen used and on whether a post-creaming process to obtain the final product texture is required or not [11]. In this study, the following conditions were selected: The pH was adjusted to 5.7±0.05 and the resulting mixture was kept at 80 °C under gentle stirring for 2 h. Samples taken after 1, 30, 60, 90, and 120 min of heating were used for the determination of furosine and for protein analysis by CE.
Determination of furosine
Furosine was determined according to the method of Resmini et al. [12]. Briefly, samples were hydrolyzed in the presence of 6 N hydrochloric acid for 23 h at 110 °C. Aliquots of the hydrolyzates were purified using solid-phase extraction on RP-18 cartridges, followed by ion-pair reversed-phase chromatography on a RP-8 column with UV detection at 280 nm. Composition of buffers and conditions of chromatography were as described in [12]. External calibration was performed using a commercially available furosine standard (Neosystems, Strasbourg, F). Samples were analyzed in triplicates. Inter assay variation was typically below 5%.
CE of casein and processed cheese
CE was performed using a hydrophilically coated capillary at low pH and in the presence of urea. CE buffers were prepared according to Recio and Olieman [8] except that a higher concentration of dithiothreitol was used for the sample buffer. The electrophoresis buffer [6 M urea, 320 mM citric acid, 20 mM tri-sodium citrate dihydrate, 0.5 g methyl 2-hydroxyethyl cellulose/l; pH 3.0] was filtered through a 0.2 μm filter. Processed cheese (320 mg) was filled into glass vials (5 ml, with screw cap) and homogenized in 4 ml of the sample buffer [6 M urea, 167 mM tris(hydroxymethyl)aminomethane, 42 mM 3-morpholino propanesulfonic acid, 67 mM ethylenediamine-tetraacetic acid, 50 mM dithiothreitol, 0.5 g methyl 2-hydroxyethyl cellulose/l; pH 8.6 adjusted with sodium hydroxide] for 2 h at room temperature using a head-over-head tumbler after adding three glass beads (∅ 5 mm) to each vial. The suspensions were then centrifuged for 10 min at 4000 rpm. An aliquot of the supernatant was filtered through a 0.2 μm filter prior to the analysis. The freeze-dried products of the model reactions between casein variants and lactose were dissolved in the sample buffer (1 ml), centrifuged and filtered as described for processed cheese. CE separations were performed essentially according to Recio et al. [10] but with minor modifications. An Agilent CE (Agilent Technologies, Inc., Waldbronn, D) was used. The capillary (CElect™- P150 CE column 1 m, 363 μm o.d., 50 μm i.d.; Supelco, Bellefonte, PA, USA) was cut to a length of 0.47 m (0.55 m total length). The cassette temperature was 30°C. The samples were injected for 20 s at 50 mbar. The voltage was 23 kV (positive polarity). Detection was at 214 nm. The capillary was flushed with water and electrophoresis buffer for 10 min each prior to any electrophoretic separation.
Results and discussion
Model reactions between casein variants and lactose and their analysis by CE
The native proteins α-, β-, and κ-casein were modified by heating their aqueous solutions containing 5% lactose. Additional peaks migrating shortly after the corresponding unmodified variants were observed in the electropherograms of the resulting reaction products. This is illustrated for β-casein in Fig. 1 (data for α- and κ-casein not shown). It should be noted that slight shoulders (Fig. 1B) were formed in β-casein without the addition of lactose after extended heating (3 h). This may be due to small amounts of lactose present in the protein preparation which was no more than 90% pure. Such small extent of modification could not be detected by ESI-MS, presumably due to insufficient sensitivity of the equipment used (Fig. 2, left). Nevertheless, a considerable increase in the newly formed derivatives with increasing heating time was observed for α-, β-, and κ-casein in the presence of lactose. Therefore, lactosylation of casein seems to occur in analogy to β-lactoglobulin which had been reported earlier [1, 4]. However, there has been no report of MS data providing an unambiguous evidence for such modification of casein.
Capillary electrophoresis: Effect of heating time on the lactosylation of β-casein in solution (100 mM pyridine/acetic acid, pH 5.7) incubated at 80 °C: A 0 min without lactose (native control); B 180 min without lactose (heated control); C 60 min with 5% lactose; D 180 min with 5% lactose (C and D contain slightly less protein than B due to the presence of lactose); A1: unmodified β-casein A1, A2: unmodified β-casein A2, A1m: modified β-casein A1, A2m: modified β-casein A2
ESI-MS of model reaction between of β-casein and lactose
ESI-MS generated multiply charged ions for native β-casein (Fig. 2, left). The degree of ionization was derived for each peak from the known molecular weight of casein. A modification of the relative mass of native β-casein was observed for the heated mixture with lactose that differed from that of the native form by m/z = 10 to 20 depending on the ionization. This is shown for a heating time of 60 min in Fig. 2 (right). A modification of β-casein by m=324 was calculated from all different ionization steps. The difference in mass for β-casein corresponds to the covalent binding of one lactose molecule to the protein molecule. Lactosylation could be deduced from all ionized states of the protein molecule that were obtained. The lactosylated complex could be quantified by integration of the respective peak areas. It was increasingly formed as heating progressed. A corresponding result was obtained for α-casein confirming its lactosylation. Unfortunately, a clear spectrum for κ-casein was not obtained.
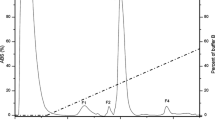
Furosine content in processed cheese heated at 80 °C as a function of heating time (▵) and relative content of unmodified (A1: (□); A2: (○)) and modified (A1m: (▪), A2m: (•)) β-casein A1 and A2 in processed cheese heated at 80°C as a function of heating time, analyzed by CE (abbreviations as in Fig. 1)
Furosine analysis of processed cheese
Furosine [Nɛ-(furoylmethyl)-l-lysine] is formed during the hydrolysis of early Maillard products of proteins and widely used as a sensitive indicator for the assessment of heat treatment in food, dairy products in particular (see for example [12–16]). Therefore, furosine was chosen as a control for the extent of the Maillard reaction during the heating of processed cheese. Furosine was generated and quantified in the processed cheese samples that were taken after heating at 80 °C for 1, 30, 60, 90, and 120 min, respectively. The furosine content as a function of heating time is shown in Fig. 3. Clearly, the formation of furosine in the heated processed cheese is directly proportional to the time of heating (R 2=0.997).
CE of lactosylated casein in processed cheese
The effect of heating time on the modification of the protein variants as measured by CE is illustrated in Fig. 4. Although the resolution in the α-casein region (migration time range of 32 to 37 min) is rather poor, especially for the longer heating times, β-casein A1 and A2 as well as their modified, lactosylated forms (A1m, A2m) are sufficiently resolved from other proteins. The resulting contents of the unmodified and modified β-casein forms are shown in Fig. 4. The contents were quantified using a peak-split method (software 3D-CE ChemStation Rev. A.0803, base line was manually set, a perpendicular was manually set with the peak split option at shoulder onset or lowest point of peak separation) and are relatively expressed with regard to the total area of the four peaks (A1, A2, A1m, and A2m) that were analyzed. Relative standard deviations of three replicates were between 0 and 6%. The contents of both unmodified β-casein variants, A1 and A2, decreased as heating progressed, i.e. the decrease is indirectly proportional to the heating time at constant temperature (\( R_{A1}^2 = 0.993\), \(R_{A2}^2 = 0.988\)). The formation of the lactosylated variants A1m and A2m by contrast was found to be directly proportional to the heating time with good correlation (\(R_{{\rm A}1{\rm m}}^2 = 0.993\), \(R_{{\rm A}2{\rm m}}^2 = 0.990\)). Thus, the decrease of the relative amount of unmodified β-casein correlates indirectly and the increase of the relative amount of lactosylated β-casein correlates directly with an increase of overall furosine content (F) in the processed cheese (\(R_{\rm A1 - F}^2 = - 0.988\), \(R_{\rm A2 - F}^2 = - 0.995\), \(R_{\rm A1m - F}^2 = 0.991\), \(R_{\rm A2m - F}^2 = 0.991\)).
Capillary electrophoresis of processed cheese: Effect of heating time on the lactosylation of β-casein in processed cheese heated at 80 °C: A 1 min; B 60 min; C 120 min (abbreviations as in Fig. 1)
Consequently, the measurement of lactosylated β-casein by CE may be used to quickly assess the extent of heat treatment applied to complex, lactose-containing dairy products such as processed cheese. Further investigation will show whether the degree of casein lactosylation and the furosine contents, based on absolute quantities that correspond to the additional CE peaks, are directly correlated.
References
Burr R, Moore CH, Hill JP (1996) Milchwiss 51:488–492
Léonil J, Mollé D, Fauquant J, Maubois JL, Pearce RJ, Bouhallab S (1997) J Dairy Sci 80:2270–2281
Morgan F, Bouhallab S, Mollé D, Henry G, Maubois JL, Léonil J (1998) Int Dairy J 8:95–98
Otte J, Larsen KS, Bouhallab S (1998) Int Dairy J 8:857–862
Jones AD, Tier CM, Wilkins JPG (1998) J Chromatogr A 822:147–154
De Block J, Merchiers M, Van Renterghem R (1998) Int Dairy J 8:787–792
Moreno FJ, López-Fandiño R, Olano A (2002) J Agr Food Chem 50:5179–5184
Recio I, Olieman C (1996) Electrophoresis 17:1228–1233
Miralles B, Ramos M, Amigo L (2000) J Dairy Res 67:91–100
Recio I, Amigo L, Ramos M, López-Fandiño R (1997) J Dairy Sci 64:221–230
Berger W, Klostermeyer H, Merkenich K, Uhlmann G (1989) Processed Cheese Manufacture — A JOHA ® Guide, BK Ladenburg GmbH, D-68520 Ladenburg
Resmini P, Pellegrino L, Battelli G (1990) Ital J Food Sci 173–183
Erbersdobler HF, Holstein B, Lainer E (1979) Z Lebensmi Unters Forsch 168:6–8
Erbersdobler HF (1986) Twenty years of furosine—better knowledge about the biological significance of Maillard reaction in food and nutrition. In: Fujimaki M, Namiki M, Kato H (eds) Amino-carbonyl reactions in food and biological systems. Elsevier, Amsterdam, pp 481–491
Erbersdobler HF, Dehn B, Nangpal A, Reuter H (1987) J Dairy Res 54:147–151
Villamiel M, Muñoz MM, Hernández A, Corzo N (1998) Milchwiss 53:434–436
Acknowledgements
We thank Mrs. M. Schulze for her skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steffan, W., Balzer, H.H., Lippert, F. et al. Characterization of casein lactosylation by capillary electrophoresis and mass spectrometry. Eur Food Res Technol 222, 467–471 (2006). https://doi.org/10.1007/s00217-005-0177-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0177-9