Abstract
Supercritical fluid extraction (SFE) of tea seed oil was performed to study the effects of various parameters such as pressure, temperature, extraction time (dynamic) and modifier (ethanol) on the yield and composition of the oil. The results were also compared with those obtained by Soxhlet extraction, ultrasonic extraction, and DGF (Deutsche Gesellschaft für Fettwissenschaft) standard method B-I5 (87) in lab conditions. The yield of tea seed oil obtained using SFE was similar to or higher than the other methods. The results from SFE showed that the modifier and pressure have significant effects on the extraction efficiency. The oil extracted by SFE in the absence of modifier was clearer than the oils obtained in other conditions. The fatty acid composition of each extract was determined by gas chromatography. Palmitic (C16:0), stearic (C18:0), oleic (C18:1), linoleic (C18:2) and gadoleic (C20:1) fatty acids were observed in the oil samples. Since it contains high-unsaturated fatty acids (UFA) and low saturated fatty acids (SFA), edible tea seed oil is also relatively healthy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipids have important uses in all cells as structural components, and nutritional interest in the lipid compositions of vegetable oils has been steadily increasing in recent years [1].
Camellia tea seeds have been utilized in China for more than a thousand years. Tea oil is the main cooking oil in China’s southern provinces, especially Hunan, where more than 50% of the vegetable cooking oil comes from camellia. Tea oil is a high quality cooking oil, like olive oil, and it stores well at room temperature. Tea oil is a good raw material for industrial use and is used to manufacture soap, margarine, hair oil, lubricants, paint, in the syntheses of other high-molecular weight compounds, and in rustproof oil [2].
Several studies have been devoted to tea seed oil composition. Palmitic (C16:0), stearic (C18:0), oleic (C18:1), and linoleic (C18:2) fatty acids have been found in tea seed oil obtained from different areas [3, 4, 5].
The extraction of natural products using carbon dioxide in the near- or supercritical state (supercritical fluid extraction, SFE) has received much recent attention. Supercritical separation technology that uses carbon dioxide as the solvent allows not only the design of environment-friendly processes, but also the processing of biological materials (carbon dioxide has a near-ambient critical temperature), and the possibility of obtaining products free of solvent residuals [6]. Because SFE has several distinctly advantageous properties, such as relatively low viscosity and high diffusivity, it is regarded as a promising alternative to conventional solvent extraction methods. SFE can penetrate into the pores of solid materials more effectively than techniques based upon liquid solvents, so it enables much faster mass transfer, resulting in faster extractions. For instance, the extraction time can be reduced from hours or days for a liquid-solid extraction (LSE) to a few tens of minutes for SFE, with comparable or better recoveries. Also, in SFE, fresh fluid is continuously pumped through the samples, so it can provide quantitative or complete extraction, and the solvation power of the fluid can be manipulated by changing pressure and/or temperature, facilitating a remarkably high selectivity. Solutes dissolved in supercritical CO2 can be easily separated by depressurization, and use of SFE eliminates or significantly reduces the need for environmentally hostile organic solvents [7, 8, 9, 10].
Over the last few years, numerous applications of the extraction of oily substances from different raw materials such as hazelnut [1], celery seed [11], cherry seed [12], microalgae spirulina [13], ground beef [14], grape seed [15], meat and cheese products and oilseeds [16], as well as coriander seed oil [17] have been reported in the literature.
The object of this work was to compare the results from SFE with those from Soxhlet extraction, ultrasonic extraction, and DGF standard method B-I 5(87). The influences of parameters such as temperature, pressure, dynamic extraction time, and modifier on the SFE of tea seed oil were also studied. The aim was to achieve the following goals: (i) to find suitable extraction parameters for the SFE of tea seed oil; (ii) to determine the fatty acid composition of tea seed oil.
Experimental
Materials
Tea seeds (Lahijan variety) were obtained from the Lahijan Tea Research Center of Iran. The seeds were dried in the shade until they reached a moisture content of 7%, and then these dried seeds were maintained at −10 °C until test time. Standards for palmitic, stearic, oleic, linoleic, gadoleic, and margaric acids were obtained from Aldrich or Sigma (USA). Hexane and petroleum benzene were of analytical grade and were obtained from Merck Chemical Co. (Germany). An Elma Transsonic model 690/H ultrasonic bath (Germany) was used to extraction the tea seed oil by ultrasonication.
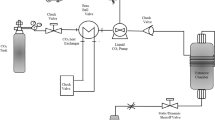
A Suprex MPS/225 system (Pittsburg, KS, USA) operated in the SFE mode was used for all of the extractions. In this study, extractions were accomplished with a 10 ml extraction vessel. Nine extractions were carried out at constant static time of 20 min, temperatures of 60, 70, and 80 °C, pressures of 300, 350, and 400 atm, and dynamic times of 20, 30, and 40 min. Ethanol at two different concentrations (7.5 and 15%) were used as modifier. Table 1 shows the SFE experimental conditions for tea seed oil extractions. A Duraflow manual variable restrictor (Suprex, USA) was used in the SFE system to collect the extracted oils. The supercritical carbon dioxide flow rate through the Duraflow restrictor was approximately 1.0±0.1 ml/min (compressed).
The fatty acid methyl esters (FAMEs) were prepared by following the procedure described by Metcalf et al [18]. 50 mg of extracted oil was saponified with 5 ml methanolic NaOH (2%) solution by refluxing for 10 min at 90 °C. After the addition of 2.2 ml BF3-methanolic, the sample was boiled for 5 min. The FAMEs were extracted from a salt-saturated mixture with hexane. The FAMEs were then analyzed using a gas chromatograph (UNICAM model 4600, England) coupled with a FID detector. The column used for oil separation was a fused silica BPX70 column, 30 m×0.22 mm i.d.×0.25 μm film thickness (from SGE). The oven temperature was held at 180 °C during separation; the injector and detector temperatures were 250 and 260 °C, respectively. The carrier gas (helium) flow rate was 1 ml/min. Two microliters of methyl esters of free fatty acids were injected into the split injector. The split ratio was adjusted to 1:50. The compounds were identified by comparison of retention time with authentic compounds. The internal standard C17:0 was used in the quantitative analysis of the extracted oils.
DGF standard method B-I (87)
5 g of the seeds were milled using a laboratory mill. 20 ml of light petroleum benzene (50–70 °C) was used during milling, and a further 50 ml was used to transfer the ground sample into a filter cup. The extraction was then carried out for 4 h in Twisselmann extraction apparatus. After extraction, the solvent was evaporated and the extract was dried at 103 °C to remove residual solvent, cooled for 30 min in a desiccator, and then weighed. This procedure was repeated until a constant extract weight was obtained [19].
Soxhlet extraction
For Soxhlet extraction, 2 g of milled tea-seeds were weighed out and then dried in an oven at a temperature of 103 °C. The dried sample was placed in an extraction thimble and then Soxhlet extracted for 7.5 h using 150 ml petroleum benzene (50–70 °C). After extraction, the solvent was evaporated and the extract was dried at 103 °C to remove residual solvent, cooled for 30 min in a desiccator, and weighed. This procedure was repeated until a constant extract weight was obtained.
Sonication procedure
5 g of the milled seeds were mixed with 70 ml petroleum benzene (50–70 °C). The mixture was then sonicated in an ultrasonic bath for 30 min. After extraction the solvent was evaporated and the extract was dried according to the method above.
Supercritical fluid extraction (SFE)
Exactly 2.0000 g (±0.1 mg) of powdered plant material (mesh 40) was weighed out, and after mixing with an appropriate amount of glass beads, was placed in the extraction vessel (10 ml). The extraction was then performed with supercritical carbon dioxide under the nine conditions mentioned in Table 1. The extracted analytes were collected in 3 ml of hexane in 5 ml volumetric flasks. The final volumes of the extracted analytes were adjusted to 5 ml with hexane at the end of the extraction. In order to increase collection efficiency, the volumetric flask was placed in an ice bath during the dynamic time. For all of the modifier studies, ethanol was spiked directly into the extraction vessel with charged samples prior to extraction.
Results and discussion
Soxhlet extraction has traditionally been used to extract oils. In this study, we compare the efficiency of this method with other extraction methods, especially SFE.
Since various parameters can potentially affect the extraction process, optimization of the experimental conditions is a critical step in the development of the SFE method. In fact, the fluid pressures and temperatures, the percentage of modifier and the extraction times are generally considered to be the most important factors. The optimization of the method can be carried out step-by-step or by using an experimental design. Table 1 shows different conditions for SFE experiments carried out to extract tea seed oil according to the Taguchi experimental design [20]. All of the selected factors were examined using a three-level orthogonal array design with a L9 (34) matrix. In this study, interactions among variables were not incorporated into the matrix, and we focused on the main effects of the four most important factors [20, 21].
The extraction yields from the different methods—SFE, Soxhlet, Sonication and DGF method B-I 5(87)—are shown in Table 1. The results shown in Table 1 were transformed into those in Table 2 after analysis of variance (ANOVA) was carried out on the results. The ANOVA results from this experiment indicate that the modifier and the pressure play an important role in SFE.
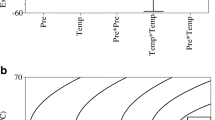
The mean extraction yields for the corresponding factors at each level were calculated according to the assignment of the experiment (Fig. 1). For example, the extraction yields of the three trials at 300 atm were evaluated as mean values of the corresponding three runs. The mean values of the three levels of each factor (such as pressure) reveal how the extraction yield changes when the level of that factor is changed. Figure 1 shows the variation in extraction yield as a function of changes in the factors studied. Pressure and modifier were found to be the most important factors, where higher levels of modifier and pressure significantly increase the yield of oil, as illustrated in Fig. 1b and Fig. 1c. At lower pressures, the solubility of oil was affected by the vapor pressure of the oil; apparently the CO2 acts like an ideal gas that does not have any special solvent characteristics in this case. However, at higher pressures, the solubility of the oil increased due to the increased density of CO2. As the density increased, the distance between molecules decreased and the interaction between the oil and CO2 increased, leading greater oil solubility in CO2 [22].
Depending on the properties of the samples and the desired compounds, the best modifier can usually be determined via preliminary experiments. At least 17 modifiers have been studied in SFEs of natural products. Among these modifiers, methanol is the most commonly used because it is an effective polar modifier and is up to 20% miscible with CO2 [7]. However, as our preliminary experiments showed, methanol wasn’t a good modifier in this case. We decided upon ethanol instead, because it is safe, commonly available, and inexpensive [15]. When the modifier was used in the SFE, the extraction yield was higher and the oil was darker. This influence of the modifier on the oil can be explained by the fact that the modifier (ethanol) increases the extraction of some polar compounds (such as polyphenols). The temperature did not influence the yield of oil (Fig. 1d, Table 2) significantly, although higher temperatures appear to be unfavorable to the extraction of UFAs.
Soxhlet extraction has been traditionally used to determine total oil. Therefore the yield of oil in the Soxhlet extraction may be more than the other methods. The yield of oil from Soxhlet extraction was found to be 30.3±0.2% (see Table 1). This indicates that the SFE efficiency is about 54% (16.4% with pure CO2) of that of Soxhlet extraction, while the color is almost the same. Also, the yields gained by sonication and the DGF standard method were found to be 21.0±0.2 and 23.3±0.3 %, respectively (see Table 1). This indicates that the SFE efficiency is about 78% of that of sonication, and about 70% of that of the DGF standard method, with the same color and fatty acid compositions. These results are acceptable, since pure CO2 was used as supercritical fluid in this case. Addition of 15% modifier (ethanol) increased the extraction yield from SFE to the same level as Soxhlet—higher than the sonication and DGF standard methods, although the oil darkened. Our future work on the tea seeds may include improving the purification of the oil.
In Table 3, the fatty acid contents of the oils extracted by the different methods—SFE, Soxhlet, sonication and DGF standard method B-I 5(87)—are shown. Palmitic (C16:0), stearic (C18:0), oleic (C18:1), linoleic (C18:2) and gadoleic (C20:1) fatty acids were identified in the tea seed oil by gas chromatography, as previously reported [3]. The major fatty acid (at 50% of total oil) in the oil was oleic acid. The proportions of unsaturated fatty acids (UFA) and saturated fatty acids (SFA) in the extracted oils were 58.1–71.7% and 17.4–23.7%, respectively. As shown in Table 3, the differences in the compositions of the SFAs and UFAs from oils extracted with solvent and pure SF CO2 (or modified CO2) were minor.
Conclusions
In conclusion, SFE is a very useful method for extracting valuable pure tea seed oil without any remaining organic solvents (such as hexane or petroleum benzene). The greener nature of SF CO2 makes it a desirable option when compared with traditional organic solvent extractions. Furthermore, it is safer, and a one-step process. SFE uses lower input energy than the Soxhlet method, so the operating cost is lower for SFE. A complete and detailed economic study is required in this area. The yield of oil depends on the pressure and the type and amount of modifier applied during extraction. The extraction efficiency by SF CO2 is 54% of that obtained using Soxhlet, although this can be increased to the same efficiency as Soxhlet by adding 15% ethanol (although the oil becomes darker, probably due to the presence of some contaminant polar compounds).
References
Bernado-Gil MG, Grenha J, Santos J, Gardos P (2002) Eur J Lipid Sci Tech 104:402–409
Ruter JM (2002) Nursery production of tea oil camellia under different light conditions. In: Janick J, Whipkey A (eds) Trends in new crops and new uses (Proc 5th Nat Symp New Crops and New Uses: Strength in Diversity). ASHS, Alexandria, VA, pp 221–224 (see also http://www.hort.purdue.edu/newcrop/ncnu02/pdf/ruter.pdf)
Ataii D, Sahari MA, Hamedi M (2003) J Sci Tech Agr Nat Res 7:1–10
Ravichandran R, Dhandapani M (1992) J Food Sci Tech India 29: 394–396
Sengupta C, Sengupta A, Ghosh A (1976) J Sci Food Agric 27:1115–1122
Corrêa NCF, Arau ME, Machado NT, Franca LF (1999) Proc ENPROMER’99, Florianopolis, Santa Catarina, Brasil, 31 Aug–1 Sept 1999
Lang Q, Wai CM (2001) Talanta 53:771–782
Ramsey ED (1998) Analytical supercritical fluid extraction techniques. Kluwer, London
Hawthorne SB (1990) Anal Chem 62:633–642
Majors RE (1990) LC-GC 8:734–743
Papamichail I, Louli V, Magoulas K (2000) J Supercrit Fluid 18:213–226
Bernado-Gil MG, Oneto C, Antunes P, Rodrigues MF, Empis ME (2001) Eur Food Res Technol 212:170–174
Canela RF, Rosa TV, Marquses OM, Meireles MA (2002) Ind Eng Chem Res 41:3012–3018
Taylor SL, Eller FJ, King JW (1997) Food Res Int 30:365–375
Cao X, Ito Y (2003) J Chromatogr A 1021:117–124
Valcárcel M, Tena MT (1997) Fresen J Anal Chem 358:561–573
Illes V, Daood HGM, Perneczki S, Szokonya L, Then M (2000) J Supercrit Fluid 17:177–186
Metcalf LC, Schmitz AA, Pelka JR (1966) Anal Chem 38:514–515
Matthaüs B, Brühl L (1999) Fett–Lipid 101:203–206
Roy RK (1990) A primer on the Taguchi Method. Van Nostrand Reinhold, New York
Khajeh M, Yamini Y, Seidkon F, Bahramifar N (2004) Food Chem 86:584–591
De Castro MDL, Variance M, Tena MT (1994) Analytical Supercritical fluid extraction. Springer, Berlin Heidelberg New York
Acknowledgements
The authors thank Tarbiat Modarres University for providing financial support. We are also grateful to Mr. Kazemi and Mr. Bahramifar for technical assistance and to Dr. M. A. Sahari for supplying the tea seed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajaei, A., Barzegar, M. & Yamini, Y. Supercritical fluid extraction of tea seed oil and its comparison with solvent extraction. Eur Food Res Technol 220, 401–405 (2005). https://doi.org/10.1007/s00217-004-1061-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-1061-8