Abstract
Common sea bass ( Dicentrarchus labrax) of a Greek cage-culture origin, sampled in December and July, were stored in ice, and their sensory, microbiological and chemical spoilage patterns were studied as well as their seasonal differentiation. The sensory storage life was determined at 15 days of ice storage. The microbial population in the muscle reached levels of 105 at the acceptability limit. The ATP breakdown pattern showed a quick depletion of inosine-monophosphate (IMP) in the first 8–9 days of ice storage and slower depletion thereafter. Inosine (INO) and hypoxanthine (Hx) were formed at significant quantities. TVBN and TBA, as indicators of protein breakdown and lipid oxidation, respectively, showed low and delayed increase. A seasonal effect was observed with summer fish showing higher rates of K-value increase during early spoilage, while winter fish showed higher K-values, microbial populations and TVBN at late spoilage stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The common sea bass ( Dicentrarchus labrax) consists together with the gilthead sea bream ( Sparus aurata), the two most important economically cultured fish species in the Mediterranean area. The total Mediterranean production of sea bass in 2000 exceeded 50,000 tones [1].
Freshness is the attribute of major importance when the quality of fish is assessed. The spoilage patterns of fresh fish and shellfish have been extensively reviewed [2,3]. The freshness and spoilage pattern of ice-stored cultured gilthead sea bream have recently been intensively investigated [4, 5, 6, 7, 8, 9, 10]. A recent study also related gilthead bream spoilage pattern to the season [11]. Attention has also been given to the common sea bass in relation with its ice-storage qualities [12, 13, 14, 15, 16]. However, no study exists relating the spoilage pattern of sea bass with the season of sampling. This study is an attempt to determine whether there is a seasonal impact in the spoilage pattern of ice-stored sea bass.
Material and methods
Commercial-size fish were collected in December and July from a cage-culture unit in western Greece (Sea Farm Ionian, Ionian sea). The average water temperature at the two sampling periods was 14 and 25 °C, respectively. All fish were fed with a commercial extruded diet containing 45% protein, 15% fat, at a daily intake defined by the feed manufacturer (Europa Marine, TROUVIT, Italy). The standard ice-killing procedure was followed. Fish were stored in ice (0 °C) and periodically analysed.
Whole fish weights, total lengths, and gonads weights were measured. The gonadosomatic index was calculated as the gonad weight percentage of the total body weight. The fat deposition was also measured as total perivisceral and peritoneal fat (the fat quantities concentrated in and around the peritoneal cavity, respectively). Both of these deposit fat forms were manually removed and weighed and expressed as their percentages to the total weight. Somatometric indexes were measured in 20 fish. Ten of them were subsequently manually filleted and fillets proximate composition analyses took place according to AOAC (1984) [17].
Taste panel assessments took place in the fish sampled in December, for both raw whole fish and cooked fish fillets. Five trained assessors were used for all sensory analyses. For freshness evaluation of whole raw fish, a modified TFRU sensory assessment [16] was used. Fish fillets were prepared after manually filleting and microwave cooking for 3 min (in a covered plate). For the sensory evaluation of cooked fillets, the Torry scoring scheme [18] was used, modified for sea bass [16].
Microbiological analyses were conducted in triplicate by a previously described methodology [11] and included total plate count (TPC) and aerobial plate count (APC) in 1 g of white flesh from the dorsal area.
K-values and ATP breakdown products were determined by an HPLC methodology [19]. The instruments consisted of a Waters 717plus autosampler set at 10 °C injection temperature, a Waters column C18 Hypersil (5 μm, 100 RP, 4×250 mm), a 484 Waters UV detector set at a wavelength of 254 nm and Millennium32 Waters Chromatography computing integrator (Waters, Milford MA 01757). The mobile phase was 0.04 M potassium dihydrogen orthophosphate (KH2PO4), 0.06 M dipotassium hydrogen orthophosphate (K2HPO4), and the flow rate 2 ml/min with an injection volume 5 μl. The K-values were calculated according to Saito et al. (1959) [20].
Chemical indexes also included total volatile basic nitrogen (TVBN) as an index for protein degradation, and TBA reactive substances (TBArs) as lipid oxidation index. TVBN determination took place in a Kjeltec unit by direct steam distillation over boric acid, following the extraction procedure of the Israeli Standard method (1976, IS 281) [21]. Titration took place with 0.05 N HCl. The total volatile basic nitrogen content was expressed in mg/100 g tissue. The TBARS were determined by a modification [22] of the method of Vyncke [23]. The TVBN and TBA analyses in all fish took place for dorsal white muscle.
All analyses for spoilage indexes determination took place in at least three individuals each time. Multiple comparisons of the means were conducted by the use of one-way analysis of variance (ANOVA), and the Tukey test [24]. In all cases, confidence levels were set at 95%.
Results and discussion
The proximate composition and somatometric indexes of cultured sea bass are presented in Table 1. The fat deposition and the proximate analysis results are within the values’ range detected in other studies for the same species [13, 25, 14]. The gonadosomatic index for winter fish was found to be about 1.4, indicating that fish of commercial size do not reach maturity. Maturity has been reported to be reached long after attaining marketable size [26], and it has been related to gonadosomatic index of >2% for male bass that consist the 70–90% of the total aquacultured population in the Mediterranean region [27].
Sensory assessment took place up to the 15th day of ice storage for both raw and cooked fish fillets. The Torry sensory score decreased with storage. A Torry score of 4, at day 15, indicates fish acceptability limit (Fig. 1). The best fit for this reduction was given by the second-order polynomial regression equation y=−0.0198x2–0.1033x+10.261 (r2=0.98). The TFRU sensory score for whole raw fish increased with storage time linearly (r2=0.98) and a TFRU score of ~30 corresponded to the limit of acceptability as previously detected (Fig. 1). The Torry score, being almost stable for fish stored up to the sixth day, indicates that cooked fillet evaluation does not have sensitivity in detecting early storage alterations, while sensory changes in raw fish indicated them. Comparing the present sensory results to other studies on the same fish species, the shelf life has been found to be somewhat shorter than in previous studies that ranged it from 16 [16] to 18 days [14].
Sensory quality changes of cultured sea bass sampled in winter and stored in ice. Changes of whole raw fish by TFRU (grading 0–38) and cooked fillet by Torry system (grading 10–0). The r2 value of linear regression is 0.98 for the TFRU and the r2 value of second-order polynomial regression is 0.99 for the Torry scheme. Each point represents the average sensory score of five trained assessors
The fillet was found to be sterile for at least up to the sixth day for both winter and summer fish. The microbial population development coincides with the cooked flesh sensory reduction and therefore explains the second-order polynomial best fit for Torry scheme organoleptic evaluation. The presence of off-odours and off-flavours in fish muscle has been related to microbial development [3]. The limit beyond acceptability corresponds to a microbial population of the order of 105 cfu/g of muscle found for fish stored for 15 days in ice (Fig. 2). Aerobial spoilage was found dominant (Fig. 2), and this comes to agreement with other microbiological findings in Mediterranean fish species where Pseudomonas sp. dominated [28, 29].
The K-value increased over the storage period (Fig. 3), and a value of 60%, reached within 15 days for summer fish and 13 days for winter fish, seems to be indicative for the acceptability limit.
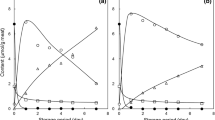
The ATP, ADP and AMP concentrations, were found in very small and relatively fluctuating quantities during ice storage, indicating the rapid breakdown of ATP after death, and with ADP always present at highest concentrations of the three. Inosine mono-phosphate (IMP) was present at high concentrations even from the beginning of storage life (Figs. 4, 5). IMP levels decreased rapidly in the first 9 days of ice storage, corresponding to a depletion from initial 9.21 μmol/g muscle to 3.06 μmol/g in winter fish and from initial 7.47 to 1.4 μmol/g muscle in summer fish, respectively. The depletion of IMP continued thereafter, but at a slower rate (Figs. 4, 5). This initial IMP depletion in the 8–9 days of storage coincides with the initial organoleptic quality reduction. Sea bass formed hypoxanthine (Hx) at a relatively high rate, reaching a concentration of 2.24 μmol/g and 0.98 μmol/g, for 13 and 15 days of ice storage in winter and summer fish, respectively. Inosine (INO) was formed and reached the concentration of 3.6 μmol/g for 13 days of ice storage in winter fish and 2.4 μmol/g for 15 days of ice storage in summer fish.
Comparing our results to those on the same species and storage conditions [16], we found a slightly different IMP depletion pattern, with the former study showing a steady IMP decrease over time for the 22 days of ice storage. Also, present results do not confirm the low Hx formation at the end of the 22-day storage period mentioned in the former reference. This ATP breakdown pattern also differs from the pattern of the other broad-cultured Mediterranean species—the gilthead bream that showed a higher preservation of IMP and lower INO and Hx formation levels [11].
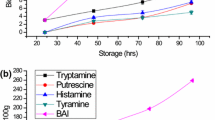
Both TVBN and TBArs levels showed a very small increase during ice storage (Table 2). The TVBN levels at the acceptability limit have been found approximately 19–20 mg/100 g tissue. TBArs levels remained low throughout the storage period, a fact that is correlated with the relatively low muscle fat content (3.9% in summer fish and 4.5% in winter fish). The TVBN increase in advanced spoilage fish as well as the low rancidity indicated by low TBA-values agrees with previously found results on the same species [13,14].
The sampling season was found to have some effects on spoilage pattern of sea bass in the present study. Summer fish were found to keep slightly better than fish sampled in December. This was confirmed from the higher microbial population (Fig. 2), K-values (Fig. 3) and TVBN in winter fish at later spoilage stages. Furthermore, winter fish showed a higher hypoxanthine concentration and a slower IMP decomposition rate than fish sampled in summer (Figs. 4, 5).
A seasonal difference in spoilage pattern has previously been observed for gilthead bream [11]. Better preservation of summer fish in the present study confirms the findings for gilthead bream. However, differences also occur in this seasonal effect for the two species. Thus, muscle microbial population differences were more profound between summer- and winter-sampled gilthead sea bream of the former study than those presently found for sea bass. In the present study, on the other hand, TVBN was found to have significantly higher values in winter-sampled sea bass, contrary to gilthead sea bream where no seasonal effect was observed. Also, seasonal differences of Hx concentration were higher in sea bass than those found for gilthead sea bream.
In summer fish, the higher K-values at early spoilage stages, but lower in the later stages (after the 10th day of ice storage) confirm the assumption made of higher autolytic activity and slower bacterial development in summer fish. [11].
References
Theodorou JA (2002) Rev Fish Sci 10(3–4):529–543
Huss HH (1988) Fresh fish: quality and quality changes. FAO Fisheries Series No. 29, Rome, pp 40–41
Ashie INA, Smith JP, Simpson BK (1996) Crit Rev Food Sci Nutr 36(1–2):87–121
Kyrana VR, Lougovois VP, Valsamis DS (1997) Int J Food Sci Technol 32:339–347
Alasalvar C, Taylor KDA, Oksuz A, Garhtwaite T, Alexis MN, Grigorakis K (2001) Food Chem 72:33–40
Huidobro A, Pastor A, Lopez-Caballero ME, Tejada M (2001) Eur Food Res Technol 212:408–412
Huidobro A, Mendes R, Nunes ML (2001b) Eur Food Res Technol 213:267–272
Tejada M, Huidobro A (2002) Eur Food Res Technol 215:1–7
Lopez-Caballero ME, Huidobro A, Pastor A, Tejada M (2002) Eur Food Res Technol 215:396–400
Alasalvar C, Taylor KDA, Shahidi F (2002a) J Agric Food Chem 50(7):2039–2045
Grigorakis K, Taylor KDA, Alexis MN (2003) Food Chem 81(2):263–268
Pirini M, Gatta PP, Testi S, Trigari G, Monetti PG (2000) Food Chem 68:289–293
Poli BM, Parisi G, Zampacavallo G, Mecatti M, Gualtieri M, Franci O (2001) Aquaculture 202:303–315
Kyrana VR, Lougovois VP (2002) Int J Food Sci Tech 37:319–328
Parisi G, France O, Poli BM (2002) Aquaculture 214:153–167
Alasalvar C, Taylor KDA, Oksuz A, Shahidi F, Alexis M, (2002) J Food Sci 67(9):3220–3226
AOAC (Association of Official Analytical Chemists) (1984) Official methods of analysis, 14th edn. AOAC, Arlington, Virginia
Howgate PF (1992) In: Aitken A, Mackie IM, Merritt JH, Windsor MI (eds) Fish handling and processing. HMSO Press, Edinburgh U.K.
Ryder JM (1985) J Agric Food Chem 33:678–680
Saito T, Arai K, Matsuyoshi M (1959) Bull Jpn Soc Sci Fish 24:749–750
Gelman A, Pasteur R, Rave M (1990) J Sci Food Agric 52:231–247
Grigorakis K (1999) Quality of cultured and wild gilthead sea bream ( Sparus aurata) and sea bass ( Dicentrarchus labrax), PhD Thesis, University of Lincolnshire and Humberside
Vyncke W (1978) Rev Agric 31(6):1119–1121
Zar JH (1984) Biostatistical analysis. Prentice-Hall International Editions, New Jersey, USA
Alasalvar C, Taylor KDA, Zubkov E, Shahidi F, Alexis M (2002) Food Chem 79:145–150
Zanuy S, Carrillo M, Felip A, Rodriguez L, Blazquez M, Ramos J, Piferrer F (2001) Aquaculture 202:187–203
Rodriguez L, Begtashi I, Zanuy S, Show M, Carrillo M (2001) Aquaculture 202:235–248
Koutsoumanis K, Nychas GJE (1999) Appl Environ Microbiol 65:698–706
Koutsoumanis K, Nychas GJE (2000) Int J Food Microbiol 60:171–184
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grigorakis, K., Alexis, M., Gialamas, I. et al. Sensory, microbiological, and chemical spoilage of cultured common sea bass ( Dicentrarchus labrax) stored in ice: a seasonal differentiation. Eur Food Res Technol 219, 584–587 (2004). https://doi.org/10.1007/s00217-004-1005-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-1005-3