Abstract
The usefulness of different bread improvers (α-amylase, sourdough, κ-carrageenan, hydroxypropylmethylcellulose) in an interrupted baking process was evaluated by using a differential scanning calorimeter as an oven. The thermal transitions of the wheat starch produced during the part-baking process, frozen storage at –18 °C, finish baking and aging of the baked dough at 4 °C were registered. The thermal properties of wheat starch during gelatinisation measured by differential scanning calorimetry were slightly affected by the dough formulation: only the peak temperature and the onset temperature underwent an increase, whereas the gelatinisation enthalpy decreased. The presence of the bread improvers minimised the negative effect of the frozen storage observed in the control sample, which showed an increase in the retrogradation temperature range. Concerning the aging of the baked dough after freezing and re-baking, all the improvers decreased the retrogradation enthalpy of the amylopectin, retarding the staling. Bread improvers can act effectively in the interrupted baking processes with frozen storage of the part-baked breads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade a number of additives and technological aids have been developed for improving the breadmaking process and the quality of the fresh bread. Some bread improvers are focussed on improving dough machinability, like pentosanases [1], others on bread volume and crumb texture, such as different emulsifiers like sodium/calcium stearoyl lactylate, and mono/diglycerides [2, 3]; moreover, other improvers, such as hydrocolloids [4, 5, 6] and enzymes (namely different α-amylases, hemicellulases and lipases) [7, 8, 9] are added for extending the freshness of the product during storage .
Simultaneously, breadmaking has undergone numerous changes trying to meet the new consumer requirement of bread freshness at any time of the day. The interrupted baking method (part-baking) has been useful for improving the bread quality [10, 11, 12, 13], but the resulting partly baked bread has a very limited shelf-life. However, the application of freezing temperatures to the partly baked breads has solved that problem [14, 15, 16]. The interrupted breadmaking process differs from the full process mainly in the baking time; it is recommended to bake bread dough up to two-thirds of the time required for full baking [15]. In addition, lower baking temperatures are sometimes used in the interrupted breadmaking process [15].
Due to the rapid growth of the part-baked bread market, the bread improvers used in the full baking processes have been directly applied to the interrupted baking process without any supporting information about their usefulness, despite the different baking time and sometimes low baking temperatures. In addition, there is a lack of information about the effectiveness of these bread improvers when freezing is applied to the breadmaking process, such as in the case of an interrupted breadmaking process with following frozen storage. It is already established that all the compounds that interact with water can affect the quality of the resulting product; in fact, the effects of compounds like dextrins, sugars, proteins, hydrocolloids, as well as exogenous enzymes like carbohydrases, that modify the water balance and in turn the polysaccharide structure have been assessed [6, 9, 17, 18, 19, 20, 21, 22].
The objective of the present work was to analyse the effect of several bread improvers such as α-amylase, sourdough and hydrocolloids [κ-carrageenan and hydroxypropylmethylcellulose (HPMC)] on the behaviour of thermal transitions of wheat starch during the pre-baking process of dough, frozen storage of pre-baked bread and amylopectin retrogradation of bread during aging or storage.
Differential scanning calorimetry (DSC) has been used to perform this research, because bread quality changes during storage and the modifications associated with loss of consumer acceptance are usually related to the physical changes accompanying the retrogradation of starch [9, 23], and DSC allows the study of the enthalpy associated with starch gelatinisation and amylopectin recrystallisation [23]. The thermal behaviour of the starch is highly dependent on the water content and its relative mobility, especially in systems with a limited amount of water, like bread dough [24, 25]. In consequence, DSC is an appropriate technique to simulate the baking process, with the calorimeter acting as an oven, and removal of the interference of water loss by using hermetic capsules [16, 26, 27].
Materials and methods
Materials
Commercial Spanish flour was obtained from the local market. Compressed yeast was used as a starter. Fungal α-amylase (Fungamyl 1500 BG) was generously donated by Novo Nordisk (Madrid, Spain). Hydrocolloids as HPMC (Methocel K4 M) from Dow (Germany), κ-carrageenan (Genugel type UPC from Copenhagen Pectin) and sourdough (Sarl PHIL XN 290) from PHILXN (France) were used in the bread formulations.
Bread dough
The bread dough recipe consisted of flour (50 g), water (up to optimum consistency, 500 Brabender units), yeast (2%) and salt (2%) on a flour basis (f.b.). Ingredients were mixed in the 50 g bowl of the farinograph (Brabender, Duisburg, Germany) for 4 min; the resulting dough was proofed (up to three times the initial dough volume) at 28 °C and 85% relative humidity. When bread improvers were studied they were added in the concentrations usually employed in breadmaking: fungal α-amylase 0.0014% (f.b.), sourdough 1.25% (f.b.), κ-carrageenan 0.5% (f.b.) and HPMC 0.5% (f.b.).
The moisture content of the dough samples was measured in two steps, following the standard method [28].
Differential scanning calorimetry
DSC was used to simulate the oven in a baking process as previously reported by Leon et al. [26], although with slight modifications in order to imitate the interrupted breadmaking process [16]. Starch behaviour was investigated during pre-baking (part-baking), after frozen storage, during the baking process (complete-baking) and after refrigeration storage.
Part-baking process
To simulate the partial baking of dough a DSC-7 (Perkin Elmer) was used. Dough samples of 18–20 mg were weighed in stainless steel pans (PE 0319–0218). After sealing, the capsules were heated from 25 to 90 °C and then cooled to 40 °C at 10 °C/min. An empty capsule was used as a reference. For each sample, 27 replicates were run. Thermal transitions of starch samples were defined as T o (onset), T p (peak of gelatinisation) and T c (conclusion). The enthalpy associated with starch gelatinisation (ΔH g) was calculated as the area enclosed by the straight line and endotherm curve, and it was expressed in joules per gram of dry sample. The ratio ΔHg/(T p−T o), designated as “peak height index” (PHI), previously defined by Biliaderis et al. [29] was used for describing the relative shape of the endotherm.
Frozen storage
Capsules were quickly placed in a freezer at –35 °C for 15 min and then stored at –18 °C for 7, 15 and 30 days.
Re-baking, or finish baking
At different frozen storage times capsules were defrosted at 25 °C for 15 min and then heated again in the calorimeter from 25 to 110 °C at 10 °C/min to complete the baking process. Nine replicates for each sample were run.
Aging or refrigeration storage
After the complete baking, the capsules were stored at 4 °C for 2, 4 and 7 days. Aging during storage was evaluated by analysing the starch retrogradation in the differential scanning calorimeter, heating the capsules from 25 to 110 °C at 10 °C/min. The retrogradation enthalpy (ΔH r) was measured to assess the amylopectin retrogradation. The retrogradation index (RI) was defined as (ΔH r/ΔH g) [30]. Three replicates for each sample were run.
Results and discussion
Effect of bread improvers on thermal parameters of wheat starch during the part-baking process
The differential scanning calorimeter was used as an oven to bake the bread dough inside the capsules. This procedure allows determination of the thermal behaviour of the wheat starch during the part-baking process using hermetic capsules. When the temperature of the fermented dough increased from 25 to 90 °C simulating the part-baking process, two endotherms appeared in the thermograms of all the samples (Fig. 1) due to the starch heating in the presence of a limited amount of water [31, 32, 33, 34]. The first peak of the thermogram corresponds to the gelatinisation process of the amorphous phase of the starch [26, 35, 36], and the second endotherm is related to the melting of the more stable crystalline structure of starch [26, 35, 36].
The effect of the bread improvers on the thermal properties of the wheat starch during part-baking are summarised in Table 1 and Table 2. The bread improvers promoted a rise of the onset temperature (T o) compared to the control dough. The largest increase was observed with the addition of α-amylase, which could be attributed to the various sugars and polyhydroxy compounds that increase the melting temperature of starch [30, 37]. The same tendency was observed with the gelatinisation peak temperature, showing an increase with the addition of the bread improvers tested. Conversely, the final temperature (T c) was not significantly (P<0.05) affected by the presence of the different bread improvers, excepting the sample with κ−carrageenan added.
Gelatinisation enthalpy was also modified with the addition of bread improvers in the bread formulation. This reduction was from 7.7% for HPMC to 35.7% for α-amylase. Rojas et al. [38] found similar results in the gelatinisation enthalpy of wheat flour suspension with different hydrocolloids added. However, Biliaderis et al. [37] found that the addition of 1% (w/w, starch basis) guar gum and xanthan gum produced a slight increase in the gelatinisation enthalpy of the wheat starch suspension.
The PHI, related to the shape of the endotherm, was only significantly affected by the addition of α-amylase. A reduction in the gelatinisation temperature range (ΔT g )(T c-T o) was also observed (results not shown), more evident in the sample with α-amylase added; this narrowness in the gelatinisation temperature range together with the increase of the peak temperature suggest a modification of the starch structure, indicating that the enzyme hydrolysed the starch during the part-baking process. This result supports the previous findings of Martin and Hoseney [20] who showed the effect of amylase on wheat starch during the breadmaking process. The amylolytic enzyme reached maximum activity during the part-baking, where the starch was partially gelatinised.
The peak of the second endotherm, related to the melting of the more stable crystalline starch, appeared at 88.1–88.6 °C with enthalpies ranging between 0.39 and 0.55 J/g (dry basis) (Table 2). These second endotherms were also observed in the second scan that simulated the re-baking process (Fig. 1).
The addition of the bread improvers tested produced a slight displacement in the peak temperature; however, no change was observed in the sample with HPMC added (Table 2). Regarding the melting enthalpies, the incorporation of α-amylase, sourdough and κ-carrageenan produced an increase in this transition. Similar results were found by Andreu et al. [36] when adding starter (Lactobacillus) in the formulation of wheat dough. This increase in the melting enthalpy has been related to a greater heterogeneity in starch granule populations [39], which would be caused by hydrolysis of starch during the part-baking process in the sample with added α-amylase. The increase in the melting enthalpy has also been related to a more ordered starch structure [26], which may be the case when hydrocolloids are present, since the hydrocolloids interact with gelatinised starch and amylose, increasing the forces exerted on the starch granules [38, 40].
Effect of different bread improvers on thermal properties of wheat starch from simulated part-baked bread during frozen storage
Capsule samples of part-baked bread were stored at frozen temperatures for a period of time, then samples were scanned again in the differential scanning calorimeter in order to simulate the finish baking. The thermogram registered during this process presented only one peak that corresponded to the melting or fusion endotherm (Fig. 1). In all the samples analysed, the frozen storage did not produce a detectable retrogradation peak, which agrees with previous findings of Ferrero et al. [35] and Ferrero and Zaritzky [41]. They stated that rapid freezing prevented crystallisation of both amylose and amylopectin, leading to a homogeneous starch structure upon thawing, without any amylopectin retrogradation peaks [35].
In order to detect the possible effects of frozen storage on the starch, samples were aged by storing them at 4 °C and then scanned again. In the aging thermogram, two peaks were detected; one corresponded to the retrogradation endotherm and the second one to the melting endotherm (Fig. 1). The thermal parameters of the retrogradation endotherm were affected by the frozen storage period (Table 3). In the control, the onset temperature decreased with the time of frozen storage, but the peak and the conclusion temperatures remained constant; in consequence the retrogradation temperature range increased with the frozen storage time. The same trend was observed when bread dough was stored at freezing temperatures for long periods [27]. This result could be attributed to a redistribution of the water through the system [42], since the amylopectin endotherm is highly dependent on the water available in the system [26, 43].
When the bread improvers tested were added, the effect on the thermal parameters varied depending on the improver. Excepting κ-carrageenan, all the improvers tested decreased the onset temperature at 7 days of frozen storage, and the peak and conclusion temperatures were only modified in the presence of α-amylase. As a consequence, the retrogradation temperature range (ΔT r) increased in the presence of α-amylase and sourdough. Those changes observed in the thermal properties of starch may have been owing to modification of the amount of water available, which controls the amylopectin melting [24, 26, 27, 43]. This effect could be intensified in the presence of dextrins released from α-amylase, and also in the presence of the different metabolites from sourdough, which can affect the spatial populations of water with different mobilities [25]. Concerning the storage time at frozen temperatures, the increase in the retrogradation temperature range observed in the control was absent in the presence of the various improvers.
Effect of bread improvers and frozen storage on the aging of baked bread during storage at 4 °C
The amylopectin retrogradation of baked samples was evaluated by measuring the transition temperatures and the enthalpy of the retrogradation endotherm (ΔH r) after 2, 4, and 7 days of storage at 4 °C (Table 4). Retrogradation involves fast crystallisation of amylose and slow recrystallisation of amylopectin [23, 44].
The presence of the bread improvers produced an increase in the onset temperature and a decrease in the conclusion temperature, although the latter was only significant (P<0.05) in the case of HPMC. Therefore the overall effect was a narrowing of the endotherm, decreasing the retrogradation endotherm range. It is probable that the aging increases the differences produced by the presence of the improvers. The hydrocolloids lowered the retrogradation index, indicating a lower tendency to retrograde. This finding agrees with data reported by Rojas et al. [21, 38], who described the anti-staling effects of κ-carrageenan and HPMC due to their water retention capacity and a possible inhibition of the amylopectin retrogradation.
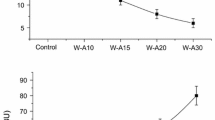
When the retrogradation enthalpy during storage was analysed, an increase with aging time was found, this being accentuated in the control (Fig. 2). However, after 4 days of storage, retrogradation enthalpy achieved an asymptotic behaviour. The control had higher retrogradation enthalpy than the samples with bread improvers. The magnitudes of retrogradation enthalpy values obtained for HPMC, α-amylase and κ−carrageenan were very similar, and lower than the one obtained for the sourdough sample. Starch-based formulation are usually stabilised with low proportions of hydrocolloids, which help to minimise the negative effects of freezing and frozen storage [41].
Effect of different bread improvers on the amylopectin retrogradation during baked dough aging at 4 °C. Baked dough was obtained after part-baking, frozen storage at –18 °C for 7, 15 and 30 days and re-baking. Circles Control, triangles α-amylase, squares sourdough, diamonds κ-carrageenan, stars hydroxypropylmethylcellulose
It was also observed that the control was the most affected by the frozen storage time, obtaining higher retrogradation enthalpy during aging. The presence of improvers, mainly α-amylase and the two hydrocolloids minimised the negative effect of the frozen storage period, leading to only a slight increase, likely because they contribute to the redistribution of water and also avoid interactions between starch and gluten [25].
In conclusion, during the part-baking process starch is partly gelatinised; there remain some starch granules that melt during the finish baking; this behaviour is due to the low water content in the dough. The frozen storage of part-baked dough produces an increase in the retrogradation temperature range, which is counteracted by the presence of the bread improvers. Concerning the aging or staling during storage, the bread improvers tested reduce the amylopectin retrogradation, and they would therefore also be useful in interrupted baking processes with frozen storage.
References
Martinez-Anaya MA, Jiménez T (1997) Z Lebensm Unters Forsch 205:569–583
Twillman TJ, White PJ (1988) Cereal Chem 65:253–257
Stampfli L, Nersten B (1995) Food Chem 52:353–360
Armero E, Collar C (1996) Food Sci Technol Int 2:323–333
Davidou S, Le Meste M, Debever E, Bekaer D (1996) Food Hydrocolloids 10:375–383
Rosell CM, Rojas JA, Benedito de Barber C (2001) Eur Food Res Technol 212:473–476
Martinez-Anaya MA, Devesa A, Andreu P, Escriva C, Collar C (1999) Food Sci Technol Int 5:263–273
Rosell CM, Haros M, Escriva C, Benedito de Barber C (2001) J Agric Food Chem 49:2973–2977
Haros M, Rosell CM, Benedito C (2002) Eur Food Res Technol 215:425–430
Stephan H (1977) Getreide Mehl Brot 31:100–102
Smolitskii VE, Rakhmankulova RG, Sidorenko SI (1979) Khlebopek Konditer Prom 1:12–14
Labutina NV, Puchkova LI, Gubiev-Yuk, Ilyasov SG, Kats AM (1981) Khlebopek Konditer Prom 8:27–28
Morgenstern G (1985) Getreide Mehl Brot 39:46–49
Ferreira PBM, Watanabe E, Benassi VT (1999) Braz J Food Technol 2:91–95
Fik M, Surowka K (2002) J Sci Food Agric 82:1268–1275
Barcenas ME, Haros M, Benedito C, Rosell CM (in press) Food Res Int
Guarda A, Rosell CM, Benedito C, Galotto MJ (2003) Food hydrocolloids10.1016/S0268–005X(03)00080–8
Schiraldi A, Piazza L, Brenna O, Vittadini E (1996) J. Therm Anal 47:1339–1360
He H, Hoseney RC (1990) Cereal Chem 67:603–607
Martin ML, Hoseney RC (1991) Cereal Chem 68:503–507
Rojas JA, Rosell CM, Benedito de Barber C (2001) Eur Food Res Technol 212:364–368
Rosell CM, Rojas JA, Benedito de Barber C (2001) Food Hydrocolloids 15:75–81
Biliaderis CG (1992) Food Technol 6:98–100, 102, 104, 106, 108–109, 145
Zeleznak KJ, Hoseney RC (1987) Cereal Chem 63:407–411
Czuchajowska Z, Pomeranz Y (1989) Cereal Chem 66:305–309
Leon A, Duran E, Benedito de Barber C (1997) Z Lebensm Unters Forsch 204:116–120
Ribotta PD, Leon AE, Añon MC (2003) Food Res Int 36:357–363
AACC (1995) Approved methods of AACC, 9th edn, method 44–15A. The American Association of Cereal Chemists, St Paul, Minn., USA
Biliaderis CG, Page CM, Maurice TJ, Juliano, BO (1986) J Agric Food Chem 34:6–14
Duran E, Leon A, Barber B, Benedito de Barber C (2001) Eur Food Res Technol 212:203–207
Donovan JW (1979) Biopolymers 18:263–275
Biliaderis CG, Maurice TJ, Vose JR (1980) J Food Sci 45:1669–1680
Califano A, Añon MC (1990) J Food Sci 55:771–773
Jovanovich G, Zamponi RA, Lupano CE, Añon MC (1992) J Agric Food Chem 40:1789–1793
Ferrero C, Martino MN, Zaritzky NE (1993) Int J Food Sci Technol 28:481–498
Andreu P, Collar C, Martinez-Anaya MA (1999) Eur Food Res Technol 209:286–293
Biliaderis CG, Arvanitoyannis I, Izydorczyk MS, Prokopowich DJ (1997) Starch 49:278–283
Rojas JA, Rosell CM, Benedito de Barber C (1999) Food Hydrocolloids 13:27–33
Biliaderis CG, Zawistowski J (1990) Cereal Chem 67:240–246
Christianson DD (1982) Hydrocolloid interactions with starchesIn: DR Lineback, GE Inglett (eds) Food carbohydrates. IFT basic symposium series. AVI, Westport, Conn., pp 399–419
Ferrero C, Zaritzky NE (2000) J Sci Food Agric 80:2149–2158
Berglund PT, Shelton DR, Freeman TP (1991) Cereal Chem 68:105–107
Lu W, Grant LA (1999) Cereal Chem 76:663–667
Qian J, Rayas-Duarte P, Grant L (1998) Cereal Chem 75:365–373
Acknowledgements
This work was financially supported by Ministerio de Ciencia y Tecnologia Project (MCYT, AGL2002–4093) and Consejo Superior de Investigaciones Científicas (CSIC), Spain. M.E. Bárcenas would like to thank the University of the Americas, Puebla, Mexico for her grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bárcenas, M.E., Haros, M. & Rosell, C.M. An approach to studying the effect of different bread improvers on the staling of pre-baked frozen bread. Eur Food Res Technol 218, 56–61 (2003). https://doi.org/10.1007/s00217-003-0816-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-003-0816-y