Abstract
Previous works on organic phase enzyme electrodes (OPEE) applied methods that worked mostly under stirred conditions. The aim of our present work was to develop a flow-through measuring set-up for determination of cholesterol content in organic media. For determination of free cholesterol content cholesterol oxidase (COD) was used, while for measuring the total cholesterol content a bi-enzyme cell containing immobilised cholesterol esterase (CE) and cholesterol oxidase was developed. Enzyme immobilisation took place on a natural protein membrane, by a glutaraldehyde cross-linking method, in a thin-layer enzyme cell made from Teflon. The enzyme cell was connected into a stopped-flow injection system (SFIA), with a flow-through amperometric detector. The parameters of biochemical and electrochemical reactions were measured. The effect on amperometric detection of different organic salts as electron mediator or conducting salts was studied. The optimal concentration of (TBATS) was 2.4 mg L−1 while for (FMCA) an optimal concentration was found at 0.4 mg L−1. The minimum amount of water, necessary for enzymatic activity in the organic phase, was also determined. Changing the concentration of toluene in acetonitrile carrier solution, the peaks increased definitely in the range 10–40% toluene. Since CE and COD were immobilised together in the enzyme cell, the conversion rate was found to be about 0.7–0.8 when the toluene content was higher then 30%. The linear measuring range for cholesterol oleate and cholesterol was 0.1–0.5 mM. Total cholesterol content of lard, butter and pasta samples were determined. It is concluded that an organic phase bi-enzyme cell may be suitable for cholesterol determination in food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cholesterol is essential to the human body in small amounts for the manufacture of hormones. However, a high level of blood cholesterol increases the risk of heart diseases. Saturated fats raise cholesterol levels in the blood while unsaturated fats help lower it. Cholesterol is also a constituent of animal foods such as eggs, meat and diary products. Determination of the cholesterol content in food is of primary importance to select a diet for low intake of cholesterol. In the analytical practice well-known GC and HPLC methods are used for cholesterol measurement, but they are time- and labour-intensive.
In the last few years a lot of research has gone into establishing rapid, routine methods for fast determination of the free and bound forms of cholesterol. The measurement of cholesterol is usually performed by employing the enzymes cholesterol oxidase (COD) together with cholesterol esterase (CE) to monitor the native and esterified cholesterol level by biosensors. COD catalyses the oxidation of cholesterol, whereas CE catalyses the hydrolysis of esterase esterified cholesterol, which is an important factor for the determination of total cholesterol, since about 70% of the cholesterol is found to be esterified [1].
Ram [2] described a biosensor where COD and CE enzymes were bound to a collagen membrane or immobilized on a conducting polymer matrix. The electrochemical redox processes of the enzyme-layered films deposited either on platinum or ITO coated glass plate were investigated. Amperometric determination of cholesterol was carried out in the water phase by platinised electrodes vs. Ag/AgCl using artificial (FMCA) and natural (flavin nucleotides) mediators [3]. Gobi and Mizutani [4] constructed a direct amperometric biosensor by a layer-by-layer nanothin film formation using COD and poly(styrenesulfonate) on a monolayer of microperoxidase covalently immobilised on Au-alkanethiolate electrodes.
Situmorang and co-workers [5] studied the conversion of esterified cholesterol by flow injection potentiometry using a tungsten electrode, which followed the ferricyanide–ferrocyanide conversion and gave well-defined peaks for cholesterol samples. FIA measurements were carried out by immobilisation of the enzymes (COD, HRP) within a polymeric film on the surface of pyrolytic graphite electrode [6].
Different research groups investigated a FIA method for the determination of total and free cholesterol by immobilising the enzymes by covalently binding to the silica in packed-bed reactors (IMMER) and using an amperometric peroxidase electrode or photometric determination of hydrogen peroxide [7, 8, 9].
Buckland and co-workers [10] reported first that the conversion of cholesterol to cholestenone could be done in the presence of a very high concentration of water-immiscible solvents of low polarity. The use of CCl4 resulted in a much faster reaction rate than was observed without organic solvent present. Liu [11] found that long-chain hydrocarbons with high logP values, such as hexane, dodecane and hexadecane gave much higher conversions when using COD than those of solvents with low logP values, such as ethyl acetate and diethyl ether. Narayan and Klibanov [12] found that neither solvent apolarity nor water-immiscibility in itself is essential for optimal enzymatic activity. When it was first observed that enzymatic activity was higher in hydrophobic solvents than in hydrophilic ones, the proposed explanation for this was that hydrophilic solvents have a much higher propensity than hydrophobic solvents to strip the essential water from enzyme molecules, thereby lowering their activity. Kazandjian and co-workers [13] studied enzymatic determination of cholesterol in organic solvents. Water indeed had a strong activating influence; upon addition of 0.1 mM aqueous phosphate buffer (pH 7.0), the rate of cholesterol oxidation accelerated to reach a maximum of about 27 nM/min at 0.4% water. This is due to the fact that the water essential for the enzymatic activity is rather tightly bound to the enzyme mono-molecules and may remain bound even when the bulk water is replaced with an organic solvent [14].
Non-cofactor-requiring steroid oxidation in mono-phasic solvents has been performed in toluene using COD adsorbed to glass beads. Efficient conversion of cholesterol to cholestenone was carried out in organic solvents using p-anizidine as colour reactant for determination of the hydrogen peroxide produced [15]. Pineiro-Avila and co-workers [16] presented a method by using a non-covalently co-immobilised bi-enzyme reactor of HRP and COD, operating in a continuous flow system of toluene saturated with p-anizidine-containing buffer (pH 7.0) for cholesterol determination in animal fats.
Kumar et al. [17] described a new immobilisation support, Formvar, for the preparation of enzyme membrane in the presence of organic solvents. The immobilised enzyme membrane has a long life due to its hydrophobic nature, as compared with other ones. Recently, Hall and Turner [18] described an amperometric OPEE using cholesterol oxidase enzyme for determining cholesterol concentration in a chloroform/hexane mixture. Campanella and co-workers [19] suggested the use of a supporting electrolyte, TBATS, because of the high resistance to the passage of current through the water-saturated chloroform solution. Pena and co-workers [20] reported a bi-enzyme amperometric composite biosensor for determination of free and total cholesterol in food samples. COD and HRP, together with potassium ferrocyanide as a mediator, were incorporated into a graphite–70% Teflon matrix. The compatibility of this biosensor design with predominantly non-aqueous media allows the use of reversed micelles as working medium formed with ethyl acetate. Determination of free and total cholesterol content in food samples such as butter, lard, egg yolk was carried out.
Klibanov [21] investigated a biosensor in which COD was deposited onto glass beads together with HRP enzyme. Poorly water-soluble analytes present in minute concentrations could be assayed by this approach if they are extracted from a large volume of water with a small volume of a water-immiscible solvent. For example, cholesterol is enriched during extraction, and the analyte that originally was present below the sensitivity level can thereby become readily measurable in organic solvents. Selective extraction may also prove useful for separating interfering compounds. Rong-Zhen-Zhang and co-workers [22] presented an improved method for sample preparation for cholesterol determination in egg yolk. Egg yolk was diluted and then cholesterol was extracted with ether and petroleum ether and quantified by reversed phase HPLC using a mobile phase of acetonitrile and 2-propanol (4:1). No differences in cholesterol concentration were observed between egg yolk samples with and without saponification. Rapid and reproducible quantification of cholesterol in egg yolk can be completed with this simplified method. Johnson et al. [23] studied the sample preparation for the determination of cholesterol in a wide range of matrices. A solution of n-hexane/2-propanol was substituted for the traditional methanol-chloroform extractions.
In previous works on cholesterol determination, such methods have been applied that worked mostly in the water phase, or in the organic phase but under stirred conditions using electrochemical detection or an FIA system using optical detection. The present study demonstrates the feasibility of amperometric cholesterol testing in a stopped-FIA system in a non-aqueous medium. We developed an enzyme-based biosensor used in organic solution for measuring free and bound forms of cholesterol. We investigated food samples to show the simplicity of sample preparation and measurement by the biosensor.
Instrumentation and materials
Materials and reagents
Cholesterol esterase (EC. 3.1.1.13.) 683 U g−1, lyophilised from bovine pancreas, cholesteryl oleate and ferrocene monocarboxylic acid (FMCA) were obtained from Sigma (St. Louis, MA, USA). Cholesterol oxidase (EC 1.1.3.6.) 150 U mg−1, lyophilised from Pseudomonas, was obtained from ICN Biomedicals (Aurora, Ohio, USA). Tetrabutylammonium-p-toluenesulfonate (TBATS) was obtained from Aldrich (Steinheim, Germany). All other reagents were commercially available and of analytical grade.
Acetate buffer (0.2 M) was used at pH 7 for the experiments. The standards were prepared at various concentrations as described below, and were always diluted with adequate carrier solution.
Samples
Different sorts of commercial butter, lard and margarine samples, egg yolks and pasta with different egg content were bought. Different extracting methods were used for different samples. Acetonitrile solution used for the sample dilution contained 0.067 g FMCA and 4 mL acetate buffer in 100 mL.
Several types of butter, lard and margarine samples were from commercial sources. Butter I, II, III were the same sort from different producers. Margarine I. was a hard sample containing only vegetable fat, while margarine II and III were light products containing both animal and vegetable fat. Half g of the homogenised samples were weighed into a glass test-tube, then shaken with 5 mL of toluene for 10 min. The solution was centrifuged (800×g, 10 min) and 2 mL of it was diluted with 3 mL of the acetonitrile solution.
Typical Hungarian dry pasta products prepared with 0, 4, 6 and 8 eggs/1000 g were bought. Powdered pasta (2g) was weighed and mixed with 20 mL of HCl (1:1) and shaken for 10 min. After that 10 mL of toluene was added and shaken for 10 min. The toluene phase was washed twice with distilled water, and 2 mL of the organic phase was diluted with 3 mL of the acetonitrile solution.
Two hen eggs from different farms were investigated. The egg yolk was separated, homogenised and 0.2 g of it was diluted and mixed with 20 mL of distilled water for 10 min. The recovery of cholesterol oleate standard was studied by adding standard solution to the egg yolk samples before diluting them. After mixing, 10 mL of toluene was added and shaken for 10 min. The toluene phase was washed twice with distilled water, and 2 mL of the organic phase was diluted with 3 mL of the acetonitrile solution.
Instrumentation
A stopped-flow injection analyser (SFIA) consisting of a buffer reservoir, a HPLC pump (ESA, USA), an injection valve (Rheodyne, Cotati, CA, USA), an enzyme cell and a thin-layer amperometric cell (Mo. 5040, ESA, USA) connected to an electrochemical detector (Coulochem II. ESA, USA) with recorder were the basic instrumentation of our research. The polarisation potential was ensured and fixed (+590 mV). The complete tubing of the flow system was solvent resistant. The temperature of the enzyme cell was set at 28 °C.
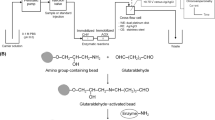
The thin-layer enzyme cell developed in our laboratory [24], consisting of a natural protein membrane for immobilising the enzymes, was fixed together with a Teflon plate supplied with a channel to ensure the flow-through between two Teflon blocks. The protein membrane (pig small intestine) was washed in buffer solution, tightened on the Teflon block and dried at room temperature. The enzyme solution (for measuring free cholesterol, 12 mg of COD and 5 mg of BSA suspended in 0.1 mL of acetate buffer, pH 7, while for conversion of esterified cholesterol 12 mg of CE, 12 mg of COD and 5 mg of BSA) was layered onto the membrane and immobilised with glutaraldehyde solution (0.05 mL 2.5% glutaraldehyde). After preparation and drying, the sample loop of the injector was replaced by the enzyme cell. The sample was filled directly into the enzyme cell directly and injected into the flow system only after the enzymatic reaction was completed. The system worked as a stopped-flow analyser but there was no need for complicated timing. In our approach, we always measured the hydrogen peroxide produced in the enzymatic reaction after a well-defined delay time in the enzyme cell. In our earlier research [25], different solvents were investigated and maximal signals were obtained for acetonitrile as solvent, with a flow rate of 0.8 mL min-1.
Results and discussion
Effect of incubation time in the enzyme cell
We determined the kinetics of cholesterol oxidation as a function of elapsed time as the sample remained in the enzyme cell when only COD was immobilised (Fig. 1). The increase of the signals showed that after 4 min the reaction rate was high enough to measure the hydrogen peroxide produced when using 40% toluene in acetonitrile solution. Comparing the results measured in organic phase we found that both in aqueous buffer solution and in buffer solution with Triton X-100 solution (used for dissolving cholesterol in buffer) the signal was very low.
Effect of conducting salts on cholesterol measurement
As conducting salts in non-aqueous solutions have an effect on the electrochemical measurements, optimization of the salt concentration was unavoidable. FMCA and TBATS in different concentrations were added to the organic solvents containing 2.4% acetate buffer (pH 7) and 40% toluene. As expected, added salts in the eluent increased the peaks in each case. When using TBATS in the solution the peaks in the concentration range 7.5–15.0 mg L−1 increased slowly. The peaks became about twice as big when the TBATS concentration was 15.0–25.0 mg L−1; at a higher amount only a small decrease could be observed. The optimal concentration of TBATS in the carrier solution was 24.0 mg L−1 (Fig. 2).
When FMCA was added into the carrier solution, the changes were more definite, as in the case of TBATS. The peaks increased in the concentration range 1.0–4.0 mg L−1 but if the eluent contained more FMCA (4.5 mg L−1) the peaks of the standards decreased dramatically, the cholesterol oxidase enzyme showed reduced activity (Fig. 3). We assumed that not only the stripping of water from the active site of the enzyme but also the presence of FMCA contributed to the loss of activity of the COD enzyme. The optimal concentration of FMCA in the carrier solution was 3.8 mg L−1.
Effect of toluene concentration on cholesterol measurement
It could be concluded from the literature and from the practical applications using COD and CE in organic solvents that the activity of these enzymes is highest in apolaric solvents. But in non-polar organic solvents only a small amount of added buffer is dissolved, the amount of which is not enough to stabilise the hydrated form of the enzymes, to dissolve the hydrogen peroxide produced and for transfer to the electrode. Toluene exerted a significant effect on the peaks representing cholesterol. When toluene content was less than 10%, the sensitivity was low. The peaks increased significantly when the toluene concentration was changed between 10 and 40%, but the signals decreased using a higher amount of toluene (Fig. 4). However, when using a higher concentration of toluene in the carrier solution the conditions were better for the enzymatic reaction [16], but the amperometric signal for the hydrogen peroxide produced was much smaller.
Effect of buffer content on cholesterol measurement
The buffer quality and content is known to affect not only the enzymatic activity in organic media but also the sensitivity of measuring the hydrogen peroxide produced during the reaction. In the SFIA measurements, stability of the carrier solution during the whole process is a very important requirement. Using acetonitrile with FMCA and 40% toluene, we observed the stability of the solution when adding different quantities of buffer. The signal for hydrogen peroxide produced increased by adding an increasing volume of buffer into the solution. At over 3% buffer content in the carrier solution, a quick separation of the solution into two phases was found. The stability of carrier solution was ensured by using less buffer and a magnetic stirrer during the whole measurement. In our measurements 2.4% buffer was used.
Conversion of cholesterol oleate to cholesterol
Since CE and COD were immobilised together in the enzyme cell, the conversion of cholesterol oleate to cholesterol was studied. During the measurements, the conversion rate increased significantly when the toluene concentration was changed between 10 and 50% (Fig. 5). The highest conversion rate was found to be about 0.7–0.8; when the toluene concentration was higher than 30%, the conversion rate was about the same up to 50% toluene, but the amperometric signal of hydrogen peroxide produced became smaller. After the optimisation steps detailed above, we used 40% toluene in the carrier solution in all the experiments.
Statistical parameters of cholesterol measurement
After the biochemical and electrochemical parameters were optimised for the cholesterol measurement, the linear concentration range of free and total cholesterol determination and the statistical parameters of the calibration curves were determined. The linear measuring range was between 0.05 and 0.5 mM for both standards but the signals for cholesterol oleate were smaller than for cholesterol because of the 0.7–0.8 conversion rate of cholesterol oleate to cholesterol. The correlation coefficients for the regression curve for free and total cholesterol were 0.967 and 0.972, respectively.
Determination of cholesterol content in food samples
Egg yolk, pasta samples and milk products were investigated, as detailed above, to determine their total cholesterol content.
The results for total cholesterol content in egg yolk are summarised in Table 1. The recovery of 0.1 mM cholesteryl oleate standard added to the samples was 86–93%, which shows that the sample preparation was adequate for a quick determination. The total cholesterol content was 1135 and 1310 mg 100g−1, respectively, these results are in agreement with the data (1120 mg 100g−1) from the food composition table [26].
As illustrated in Fig. 6, there is a linear relationship between the total cholesterol content of pasta and the amount of egg content. Our results showed that this measurement could be used for quality control of pasta.
Results for margarine, lard and butter are summarised in Fig. 7. Margarine I sample, containing only vegetable fat, showed a small signal, while for margarines II and III higher cholesterol contents were measured (45–50 mg 100g−1). Margarine sample I contained sterols typical for sunflower oil, e.g. β-sitosterol, brassicasterol, camposterol, stigmasterol [27].
Although in substrate specificity, the 3β-hydroxy configuration of the steroid was essential for the substrates of the COD enzyme, investigations of the substrate specificity of COD in the water phase for cholesterol, dihydroxy cholesterol, ergosterol, stigmasterol, stigmastanol and 7-dehydrocholesterol showed the relative activities to be 100%, 78%, 51%, 40%, 32% and 24%, respectively [28]. However, the most important sterol in vegetable fats and oils was β-sitosterol; under the dynamic conditions in our measuring system we measured only a low response for margarine I. Using OPEE for cholesterol under stirred conditions in a chloroform/hexane mixture, higher response was detected after 30 min [18]. Results for lard and butter agree with the data from food composition tables [26]. Summarising the results, we can state that the new biosensor method offers the possibility of quality control of animal and vegetable fat products.
Conclusions
On the basis of these results, we can conclude that biosensors using enzymes with covalent immobilisation can be used in an organic phase FIA system with the eluent containing acetonitrile, toluene and the optimised quantity of buffer. Utilising the influence of different organic solvents on the immobilised enzyme, the biosensor developed was used successfully to measure cholesterol content in types of food that are only partly soluble in water.
References
Karam WG, Chiang JYL (1994) J Lipid Res 35:1222–2131
Ram MK, Bertoncello P, Ding H, Paddeu S, Nicolini C (2001) Biosens Bioelectr 16:849–856
Vidal JC, Garcia-Ruiz E, Castillo JR (2000) J Pharm Biomed Anal 24:51–63
Gobi K, Mizutani F (2001) Sensors Actuators B 4062:1-6
Situmorang M, Alexander PW, Hibbert DB (1999) Talanta 49/3:639–649
Bongiovanni C, Ferri T, Poscia A, Varalli M, Santucci R, Desideri A (2001) Bioelectrochemistry 54:17–22
Yao T, Sato M, Kobayashi Y, Wasa T (1985) Anal Biochem 149:387–391
Krug A, Suleiman AA, Guilbault GG, Kellner R (1992) Enzyme Microb Technol 14:313–316
Baticz O, Tömösközi S (2002) Nahrung 46:46–50
Buckland BC, Dunnill P, Lilly D (1975) Biotechnol Bioeng 17:815–826
Liu WH, Houng WC, Tsai MS (1996) Enzyme Microb Technol 18:184–189
Narayan VS, Klibanov AM (1993) Biotech Bioeng 41:390–393
Kazandjian RZ, Dordick JS, Klibanov AM (1986) Biotechnol Bioeng 28:417–421
Zaks A, Klibanov AM (1988) Biol Chem 263:3194–3201
Dordick JS (1989) Enzyme Microb Technol 11:194–211
Pineiro-Availa G, Salvador A, de la Guardia M (1998) Analyst 123:999–1003
Kumar H, Kumar A, Kumari P, Jyotirmai S, Tulsani NB (1999) Biotechnol Appl Biochem 30:231–233
Hall GF, Turner APF (1991) Anal Lett 24:1375–1388
Campanella L, Tomassetti M (1996) Food Technol Biotechnol 34:131–141
Pena N, Ruiz G, Reviejo AJ, Pingarron JM (2001) Anal Chem 73(6):1190–1195
Klibanov AM (1986) Chemtech 354–358
Rong-Zhen-Zhang, Long-Li, Shu-Tao-Liu, Ru-Ming-Chen, Ping-Fan-Rao, (1999) J Food Biochem 23:351–361
Johnson JH, McIntyre P, Zdunek J (1995) J Chromatogr A 718:371–81
Váradi M, Adányi N, Nagy G, Rezessy-Szabó J (1993) Biosens Bioelectr 8:339–345
Adányi N, Tóth-Markus M, Szabó EE, Váradi M, Sammartino MP, Tomassetti M, Campanella L (2003) Anal Chim Acta (submitted)
Holland B, Welch AA, Unwin ID, Buss DH, Paul AA, Southgate DAT (eds) (1992) McCance and Widdowson’s The composition of foods. RSC, Cambridge
Choong YM, Lin HJ, Wang ML (1999) J Food Drug Analysis 7(4):279–290
Lee SY, Rhee HI, Tae WC, Shin JC, Park BK (1989) Appl. Microbiol Biotechnol 31:542–546
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adányi, N., Váradi, M. Development of organic phase amperometric biosensor for measuring cholesterol in food samples. Eur Food Res Technol 218, 99–104 (2003). https://doi.org/10.1007/s00217-003-0805-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-003-0805-1