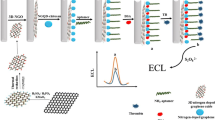
Abstract
In this study, reduced graphene oxide (rGO) hybridized high internal phase emulsions were developed and polymerized as porous carriers for aptamer (5’/5AmMC6/-AGT CCG TGG TAG GGC AGG TTG GGG TGA CT-3’) modification to enrich human α-thrombin from serum. The structure and properties of the materials were confirmed by scanning electron microscope (SEM), Fourier transform infrared spectroscope (FT-IR), and X-ray photoelectron spectra (XPS). The adsorption ability and selectivity were studied and the thrombin was detected with liquid chromatography-mass spectrometry (LC–MS). The adsorption of thrombin onto the sorbent was achieved within 30 min and the desorption was realized using 5.0 mL of acetonitrile/water (80/20, v/v). The thrombin was quantified by LC–MS according to its characteristic peptide sequence of ELLESYIDGR.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid phase extraction (SPE) has been broadly used for sample pretreatment in biological and pharmaceutical analysis [1] and a wide variety of materials have been studied and applied for SPE column preparation. High internal phase emulsion (HIPE) is a kind of paste liquid containing continuous phase and dispersed phase with extremely high viscosity, and the droplets approached to a polyhedral shape [2, 3]. The porous, interoperable, and highly permeable materials will be formed after HIPEs are successfully polymerized (polyHIPEs) [4]. In recent years, polyHIPEs had been approved as an ideal carrier in SPE column for separation and purification procedure [5, 6]. Unfortunately, the organic monomers and crosslinking reagents in emulsions made the polyHIPEs fragile and easy to be collapsed in most cases. In order to overcome the shortage, some inorganic and organic particles like graphene oxide (GO) have been used and doped into emulsions to form a more robust structure and endowed the materials with a large number of functional groups [7].
In another aspect, polyHIPE-based SPE columns generally suffer from poor selectivity towards the target analytes. To date, antibodies [8], molecularly-imprinted polymers [9], nickel oxide nanoparticle-deposited silica [10], and aptamer (Apt) [11] modifications have been developed to improve the selectivity of SPE columns. Benefitting from the abundant activate functional groups, Apt has attracted great attention of scientists because of its specific capture capability for the targets [12, 13]. The modification of Apt on substrates including electrospinning material [14], organic–inorganic hybrid silica material [15], nanospheres [16], and graphene oxide [17], and thus could be tentatively used in biological sample pretreatment and proteomic analysis.
Thrombin is the main effector protease in blood coagulation cascade reaction and it is a special serine protease with the function of promoting coagulation and anticoagulant [18]. At present, most of the methods for thrombin analysis were achieved by anchoring aptamers on multifunctional substrates such as gold nanoparticles [19, 20], 2D titanium carbides (MXenes) [21], silica nanoparticles [22], and cellulose paper [23]. In comparison with other materials for thrombin enrichment, the unique morphology and hierarchical porosity of polyHIPEs endow the material with good permeability and reusability when used as an SPE separation for separation. Here, GO hybridized polyHIPEs (polyGO/HIPEs) were firstly fabricated by polymerizing the continuous and dispersed phases, and then polyGO/HIPEs was aminated and reduced with NaClO, NaOH and ethylenediamine (polyrGO-NH2/HIPEs). Finally, the Apt was modified on the Apt-polyrGO-NH2/HIPEs by glutaraldehyde activation. The Apt-modified polyHIPEs SPE column was applied for the specific adsorption of thrombin from serum sample. In this work, we described and evaluated the improved performance of Apt-polyrGO-NH2/HIPEs for selective extraction of thrombin from serum sample and followed by the determination by liquid chromatography coupled to mass spectrometry.
Materials and methods
Chemicals and reagents
Ethylhexyl acrylate (EHA, 99%), divinylbenzene (DVB, 98%), sorbitan monooleate (Span 80), acrylamide (AAm, 99%), ethylenediamine (EDA, 99%), TPCK-trypsin (BAEE (N-benzoyl-L-arginine ethyl ester) > 10,000 Unit/mg), cytochrome C (≥ 95%), graphite powders (99.95%), and azobisisobutyronitrile (AIBN) were obtained from Aladdin Chemistry Co. Ltd (Shanghai, China). Polyvinylpyrrolidone (PVP, K-30) was purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Myoglobin, human serum albumin, hemoglobin, and myoglobin were supplied by Solarbio Science & Technology Co. Ltd (Beijing, China). Human α-thrombin was purchased from Haematologic Technologies Inc (Vermont, America). The aptamer targeting human α-thrombin with an amine terminal group (5’/5AmMC6/-AGT CCG TGG TAG GGC AGG TTG GGG TGA CT-3’) was purchased from Sangon Biotech Co. Ltd (Shanghai, China). Anonymized serum samples were donated from Kingmed Diagnostics Group Co. Ltd (Guangzhou, China). Signal polypeptide of thrombin ELLESYIDGR was purchased from China Polypeptides Co. Ltd (Shanghai, China). Acetonitrile and methanol used in this work were HPLC grade and purchased from Dikma Corporation (Guangzhou, China). All reagents and chemicals were of analytic or HPLC grade without further purification.
Human α-thrombin was dissolved with NH4HCO3 buffer solution (25 mM, pH 7.4) and stocked at –20 °C. The stock solution was thawed at 0 °C before use. The water used in this work was purified by ultrapure water system (Yuechun Technology Co. Ltd, Chengdu, China).
Characterization
The morphology and microstructure of the materials were observed by SU5000 field emission SEM (Hitachi Ltd, Japan). The Fourier transform infrared (FT-IR) spectra were obtained with IS10 FTIR spectrometer (Thermo Fisher Scientific Co., USA). X-ray photoelectron spectra (XPS) were studied with an Axis Ultra DLD (Kratos Ltd, U.K.) paired with a monochromatic Al Kα X-ray source (1486.6 eV).
Preparation of Apt-polyrGO-NH2/HIPEs SPE column
Preparation of GO referred to Hummer’s method [24], detailed steps on the preparation and modification of GO and synthesis of polyGO/HIPEs skeleton materials were mentioned in Electronic Supplementary Material (ESM). After polyGO/HIPEs were prepared, amide groups were transformed with the assistance of Hoffman reaction [25]. Briefly, 1.0 g of polyGO/HIPEs was initially dispersed into 10 mL of ice water, 2.0 mL of NaClO (5.5% effective chlorine), and 2.0 mL of NaOH (1.0 mol/L) were added to the solution with reacting for 6.5 h in 0 °C, and then the mixed solution was gently shaken for another 1.5 h at 70 °C. After being cooled to room temperature, the excess NaClO and NaOH were removed with ultrapure water, then the polymer was lyophilized at − 50 °C and < 10 Pa for 24 h with a freeze dryer (Beijing Boyikang Laboratory Instruments Co., China). To ensure the polymer was more stable and further aminated, the GO that embedded in polyGO/HIPEs was reduced by EDA (GO/EDA = 1/5, w/w) at 85 °C for 12 h to obtain rGO. Finally, the monolithic polyrGO-NH2/HIPEs was achieved.
After the monolithic polyrGO-NH2/HIPEs were gently crushed, 15 mg of them filled a blank SPE column and covered with porosity frits. The SPE column was activated by 10% glutaraldehyde (100 mM PBS solution, pH 8.0, v/v) for 1 h, 5.0 mL of NaBH4 (5.0 mg/mL) was circulated through the SPE column for 1 h, and followed by cleaning with 5.0 mL of NH4HCO3 buffer solution (25 mM, pH 7.4) [15]. For Apt modification, 10 nmol of Apt solution (NH4HCO3, 25 mM, pH 7.4) was circulated through the SPE column for 1 h at room temperature, and the unreacted Apt was flushed with 25 mMof NH4HCO3 buffer (pH 7.4). Schematic illustration of the preparation process of Apt-polyrGO-NH2/HIPEs and enrichment of the thrombin are shown in ESM Fig. S1.
The adsorption and desorption of thrombin
Apt-polyrGO-NH2/HIPEs SPE packed column was rinsed with 5.0 mL of NH4HCO3 buffer (25 mM, pH 7.4), and then a suitable amount of standard thrombin was cyclically transported through SPE columns. After incubation for 30 min at room temperature, the column was washed 3 times with 5.0 mL of ultrapure water to remove unbounded thrombin. The bonded thrombin was eluted with 5.0 mL of acetonitrile/water (80/20, v/v), and the eluent was collected and dried with nitrogen gas at room temperature. Thereafter, 900 µL of NH4HCO3 buffer (25 mM, pH 7.4) and 80 µL of trypsin solution (2.5 mg/mL, NH4HCO3, 25 mM, pH 7.4) were added to dissolve the residual, and hatched in water bath at 37℃ for 20 min for protein digestion. Then, 20 µL of acetonitrile/formic acid solution (9/1, v/v) was used to terminate the enzymatic reaction. For real sample analysis, healthy human serum was diluted 40-fold with NH4HCO3 (25 mM, pH 7.4), and 2.0 mL of serum diluent was loaded and enriched with SPE procedure. After digestion with trypsin, the resulting solution was filtered by a 0.22-µm membrane and analyzed by LC–MS.
LC–MS analysis
The thrombin analyses were conducted by LCMS-8050 mass spectrometer (Shimadzu, Japan) with a Shim-pack XR-ODS III (2.2 μm, 2.0 mmi.d × 200 mm, Shimadzu) at a flow rate of 0.4 mL/min. A 25℃ column temperature and a positive mode were adopted for this work. The mobile phase was composed of 0.1% formic acid aqueous solution (A) and acetonitrile (B). The elution gradient was as follows: 0–2 min: 5–10% B; 2–5 min: 10–40% B; 5–6 min: 40–40% B; 6–12 min: 40–5% B. Additionally, nebulizing gas flow: 3 L/min, heating gas: 10 L/min, DL temperature: 250˚C, heat block temperature: 400˚C, driving gas flow: 10 L/min. The concentration of thrombin was obtained according to the intensity of signal polypeptide which was proportional to the concentration of thrombin in the calibration equation.
Qualitative and quantitative analyses of thrombin were set as follows: precursor mass/charge ratios (m/z) was 597.8, and product m/z was 215.15 and 232.25. Precursor ion splitting process was monitored as ELLESYIDGR to GR which came from m/z of 215.15. In this study, structures of α-thrombin (No. NP_001298186.1) are listed in ESM Fig. S2; we employed characteristic peptide m/z of 597.80 with sequence ELLESYIDGR to quantify thrombin.
Results and discussion
Characterization of composites
To understand the porous characteristic and surface morphology of Apt-polyrGO-NH2/HIPEs, SEM image of the polymer was investigated. As shown in Fig. 1a, Apt-polyrGO-NH2/HIPEs show an interconnected open-cell structure with high porosity, which is an obvious character of polyHIPEs materials [26]. The smooth wall of the Apt-polyrGO-NH2/HIPEs material is observed with almost uniform pores with diameter of 2.0 µm, which guarantees a rapid mass transfer speed.
Chemical characters of the material were testified though FTIR analysis. The FT-IR spectrum (Fig. 1b) of polyrGO-NH2/HIPEs (II) revealed the C-H stretching (2950–2865 cm−1) and bending (1457–1370 cm−1) in alkyl chains. The absorption at 1731 cm−1 corresponded to C = O stretching vibration. The adsorption at 2721 cm−1 revealed the presence of aldehyde groups, demonstrating the successful modification of glutaraldehyde. The FT-IR spectrum of polyrGO-NH2/HIPEs also showed strong absorption bands from 1003 to 1152 cm−1, which belonged to the C-N stretching in the amine groups and C-O stretching in the hydroxyl groups. After the Apt modification, the aldehyde groups induced absorption at 2721 cm−1 disappeared and the absorption ranging from 1003 to 1152 cm−1 became much weaker in the FT-IR spectrum of Apt-polyrGO-NH2/HIPEs because of the formation of amide groups and the change of nitrogen content in the material. We tried to use the P-O, P = O, and O = P-OH induced absorption bands to give more direct evidence for illustrating the Apt modification; however, the overlap of these absorption bands with other functional groups in the FT-IR spectra made it difficult to provide convincing results.
High-resolution P2p XPS analysis was performed to provide more insight on the chemical composition of Apt-polyrGO-NH2/HIPEs. Peaks of P2p assigned to the phosphate groups from Apt were found in the Apt-polyrGO-NH2/HIPEs (Fig. 2a), whereas P2p peak could not be found in polyrGO-NH2/HIPEs (Fig. 2b). This observation illustrated that the Apt had been successfully immobilized to the polymer material. The survey scan in Fig. S3 shows that the presence of elemental peaks for C, N, O, and P. High-resolution C1s, N1s, and O1s spectra for polyrGO-NH2/HIPEs and Apt-polyrGO-NH2/HIPEs are illustrated as ESM Figs. S4, S5, and S6. The nitrogen in polyrGO-NH2/HIPE was mainly assigned to amine nitrogen and amide nitrogen (399.7 eV). The peaks at 398.7 eV and 400.6 eV were reasonably assigned to pyridinic-N and pyrrole-N, respectively, which presumably derived from rGO (PVP modification and reduction by ethylenediamine). The nitrogen in Apt-polyrGO-NH2/HIPE was still dominated by amine nitrogen and amide nitrogen, and the increase in the component at lower binding energy might be caused by the increase of pyridine- and lactam-N contents in Apt [27, 28].
Improvement and specific adsorption of thrombin on Apt-polyrGO-NH2/HIPEs
The adsorption of thrombin on Apt-polyrGO-NH2/HIPEs was mainly attributed to the specific adsorption between Apt and thrombin because of specific 3D folded structure of Apt, as previously described [29]. In this work, polyrGO-NH2/HIPEs, Apt-polyHIPEs, and Apt-polyrGO-NH2/HIPEs were arranged to perform adsorption experiments under the same conditions and the adsorption efficiencies were 10.7%, 65.2%, and 85.6%, respectively (Fig. 3a). The adsorption efficiency increased by more than 70% after modification of Apt, which demonstrated that one strand of doped Apt has high binding affinity to a thrombin fragment because of homologous sequence [30]. On the other hand, the rGO doped material could improve the adsorption efficiency as compared with non-rGO in the presence of Apt. One explanation for this difference was that the carboxyl groups on the pristine GO provided additional active sites for Apt modification and thrombin adsorption (ESM Fig. S1). In a word, the rGO doping and Apt modification greatly improve the extraction efficiency of thrombin.
The effect of different materials on adsorption rate of polyrGO-NH2/HIPEs, Apt-polyHIPEs, and Apt-polyrGO-NH2/HIPEs, respectively (a). Selectivity of Apt-polyrGO-NH2/HIPEs for thrombin (b), concentration of thrombin, myoglobin, human serum albumin, hemoglobin, and cytochrome C in 2.0 mL enrichment solution was 100 ng/ mL and the mass of the material was 15.0 mg
The abundant matrixes in biological sample generally greatly interfered the specific adsorption of thrombin; thus, we monitored the effect of anti-interference ability on the adsorption of thrombin by Apt-polyrGO-NH2/HIPEs. Several proteins including myoglobin (pI 7.07), human serum albumin (pI 4.7 ~ 4.9), hemoglobin (pI 7.23), and cytochrome C (pI 4.47 ~ 4.57) were selected and studied. Herein, the interference of myoglobin, human serum albumin, hemoglobin, and cytochrome C was evaluated by directly testing the adsorption efficiency of thrombin in the coexistence of these substrates. As shown in Fig. 3b, the adsorption efficiency to thrombin decreased by 17.01% in the presence of these interferents compared with the control experiment. The result indicates the slight influence of myoglobin, human serum albumin, hemoglobin, and cytochrome C on selective capture of thrombin to Apt-polyrGO-NH2/HIPEs. The relatively lower adsorption efficiency of the Apt-polyrGO-NH2/HIPEs in the interference experiment was ascribed to the interaction of the charges between thrombin and the extraneous proteins [31].
Optimization of adsorption and desorption conditions
Extraction time was one of fundamental parameters that governed the efficiency of the process, and a rapid adsorption process was highly favored. As seen in Fig. 4a, the adsorption efficiency to thrombin increased with adsorption time from 10 to 30 min, and tended to plateau after 30 min. Therefore, 30 min was chosen as the optimum adsorption time for the following operations.
Effect of adsorption time (a) and enrichment volume (b) on adsorption efficiency. Effect of acetonitrile concentration (c) and desorption volume (d) on desorption of thrombin. Peak areas of enzyme hydrolysis solution under different concentrations of trypsin and time of enzyme hydrolysis (e). The mass of the material was 15.0 mg
A reasonably designed enrichment volume could achieve satisfactory adsorption efficiency of material against target during the enrichment process. The various volumes of enrichment solutions that ranged from 1.0 to 10.0 mL containing 200 ng of thrombin were tested. Figure 4b revealed that the adsorption of thrombin increased with the enrichment volume from 1.0 to 5.0 mL then decreased from 5.0 to 10.0 mL. The high concentration of thrombin at a small volume enhanced the electrostatic interaction and steric blocking between Apt-polyrGO-NH2/HIPEs and thrombin, which weakened the thrombin adsorption [32]. The adsorption efficiency gradually decreased after reaching the maximum, which might be because the capture of thrombin was more difficult for Apt-polyrGO-NH2/HIPEs in low concentrations of thrombin.
Eluent was a critical factor in the SPE process and polarity of the solution would affect the interaction between aptamers and proteins. The elution efficiency of the thrombin was investigated using different volume ratios of acetonitrile/water eluents (Fig. 4c). As the concentration of acetonitrile increased, the desorption efficiency of thrombin gradually increased and then approached to plateau beyond 80% acetonitrile (v/v). The effect of desorption volume was studied by comparing the desorption efficiency for the same content of analytes in 1.0, 2.0, 4.0, 5.0, and 6.0 mL acetonitrile/deionized water (80/20; v/v). Figure 4d shows that the desorption efficiency increased with the increase of the eluent volume that ranged from 1.0 to 5.0 mL, and the desorption efficiency showed little changes when the volume was greater than 5.0 mL. To achieve a better desorption of thrombin from Apt-polyrGO-NH2/HIPEs column, the eluent used for the subsequent experiments was 5.0 mL of acetonitrile/deionized water (80/20; v/v).
In this study, the concentration and time for thrombin hydrolysis were studied because they directly affected the determination of thrombin. As plotted in Fig. 4e, the peak area of characteristic polypeptides decreased with the increase of trypsin concentration (0.3–1.0 mg/mL). The reason for this trend was that polypeptides after hydrolysis were further digested under the high concentration of trypsin; hence, the target could not be detected by LC–MS. In addition, the peak area was enhanced by extending the time for thrombin hydrolysis (from 5 to 20 min) when the concentration of trypsin was 0.2 mg/mL, and there were no apparent changes after 20 min. According to the experimental results, we concluded that thrombin shall be hydrolyzed under 0.2 mg/mL of trypsin for 20 min prior the detection by LC–MS.
Method validation and confirmation
Serum samples were pretreated with Apt-polyrGO-NH2/HIPEs every day for 1 month to research the stability of the material, the recoveries of thrombin remained between 65.1 and 70.5%, and the RSD was 8.7%. The recovery of thrombin maintained at around 68% even after 30 times recycling. These results demonstrated that the Apt-polyrGO-NH2/HIPEs have good stability and intrinsic selectivity of aptamers to thrombin. In addition, the calibration curves for different concentrations of thrombin are depicted in ESM Table S1. The peak area was directly proportional to the thrombin concentration within the range from 25 to 400 ng/mL with LOD of 1 ng/mL (R2 = 0.9997), which exhibited the high sensitivity of this method for quantitative detection of thrombin in serum sample.
Compared with previous reports on the aptamer functionalized adsorbent for thrombin enrichment and determination (summarized in Table 1) [14, 22, 33,34,35, 36], our method presented an excellent performance in shortening the extraction time in virtue of the hierarchical structure and good permeability of Apt-polyrGO-NH2/HIPEs. The combination of high selectivity of Apt-polyrGO-NH2/HIPEs to thrombin and the low detection limit of LC–MS served as an efficient method for thrombin assay.
Application of complex sample and LC–MS analysis
In order to locate the target quickly and conveniently, extracted ion chromatograms were obtained for quantitative and qualitative identification of the target. The extracted ion chromatograms of signal peptides at different concentrations are illustrated in Fig. 5. The MS/MS spectra of [M + 2H]2+ of ELLESYIDGR (m/z 597.80) from database searching and the chromatogram of hydrolyzed products by enzyme (50.0 ng/mL) are shown in ESM Fig. S7 and Fig. S8, respectively.
The inhibitors in serum could lead to a low recovery of spiked thrombin. Appropriate dilution of serum solution could reduce the complex background and enhance the recovery rate [37]. In order to examine if the developed method was suitable for analysis of a complicated sample, several amounts of thrombin were added into 40-fold diluted human serum for standard addition experiments. The spiked recoveries of thrombin were within the range of 53.8–66.0% (as presented in Table 2), which declared that this assay might have great potential for clinical use. Anyway, the recoveries were not the best; we would continue to optimize experimental factors such as the doping ratio of rGO and the type of eluent solvent in future experiments to achieve more efficient desorption of thrombin from the sorbent.
Conclusions
In this work, we have successfully developed Apt-modified polyrGO-NH2/HIPEs SPE adsorbent coupled with LC–MS for adsorption and determination of thrombin in human serum. The rGO doping and Apt modification enhanced the adsorption efficiency as well as the selectivity of Apt-polyrGO-NH2/HIPEs packed column to thrombin. The 3D macroporous structure of Apt-polyrGO-NH2/HIPEs enabled the fast mass transfer and facilitated the rapid adsorption of thrombin. In short, we have proposed a method coupling the selective enrichment by Apt-polyrGO-NH2/HIPEs and the highly sensitive determination by LC–MS for effective analysis of thrombin in human serum. We envision that this assay will meet the requirements of clinical medicine and contribute to the disease diagnosis.
References
Hashemi B, Zohrabi P, Shamsipur M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta. 2018;187:337–47.
Lissant KJ, Mayhan KG. A study of medium and high internal phase ratio water/polymer emulsions. J Colloid Interface Sci. 1973;42:201–8.
Zhang W, Ruan G, Li X, Jiang X, Huang Y, Du F, Li J. Novel porous carbon composites derived from a graphene-modified high-internal-phase emulsion for highly efficient separation and enrichment of triazine herbicides. Anal Chim Acta. 2019;1017:17–24.
Silverstein MS. PolyHIPEs: recent advances in emulsion-templated porous polymers. Prog Polym Sci. 2014;39:199–234.
Du F, Sun L, Zhen X, Nie H, Zheng Y, Ruan G, Li J. High-internal-phase-emulsion polymeric monolith coupled with liquid chromatography-electrospray tandem mass spectrometry for enrichment and sensitive detection of trace cytokinins in plant samples. Anal Bioanal Chem. 2015;407:6071–9.
Jiang X, Gui Ruan, Deng H, Gan Z, Zhang W, Du F, Chen Z. Synthesis of amphiphilic and porous copolymers through polymerization of high internal phase carboxylic carbon nanotubes emulsions and application as adsorbents for triazine herbicides analysis. Chem Eur J. 2021;415:129005.
López-Diaz D, Merchán MD, Velázquez MM. The behavior of graphene oxide trapped at the air water interface. Adv Colloid Interface Sci. 2020;286:102312.
Verdian A, Fooladi E, Rouhbakhsh Z. Recent progress in the development of recognition bioelements for polychlorinated biphenyls detection: antibodies and aptamers. Talanta. 2019;202:123–35.
Du F, Ruan G, Liang S, Xie F, Liu H. Monolithic molecularly imprinted solid-phase extraction for the selective determination of trace cytokinins in plant samples with liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem. 2012;404:489–501.
Yu Q, Liu S, Zheng F, Hua Xiao, Guan H, Feng Y. Identification and quantification of benzimidazole metabolites of thiophonate-methyl sprayed on celery cabbage using SiO2@NiO solid-phase extraction in combination with HPLC-MS/MS. Chin Chem Lett. 2020;31:482–6.
Jiang D, Hu T, Zheng H, Xu G, Jia Q. Aptamer-functionalized magnetic conjugated organic frameworks for selective extraction of trace hydroxylated polychlorinated biphenyls in human serum. Chem Eur J. 2018;24:10390–6.
Climent E, Rurack K. Combining electrochemiluminescence detection with aptamer-gated indicator releasing mesoporous nanoparticles enables ppt sensitivity for strip-based rapid tests. Angew Chem Int Ed. 2021;60:2–13.
Liu L, Yang K, Gao H, Li X, Chen Y, Zhang L, Peng X, Zhang Y. Artificial antibody with site-enhanced multivalent aptamers for specific capture of circulating tumor cells. Anal Chem. 2019;91:2591–4.
Du F, Alam MN, Pawliszyn J. Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal Chim Acta. 2014;845:45–52.
Deng N, Liang Z, Liang Y, Sui Z, Zhang L, Wu Q, Yang K, Zhang L, Zhang Y. Aptamer modified organic-inorganic hybrid silica monolithic capillary columns for highly selective recognition of thrombin. Anal Chem. 2012;84:10186–90.
Xue J, Zhao Q, Yang L, Ma H, Wu D, Liu L, Ren X, Ju H, Wei Q. Dual-mode sensing platform guided by intramolecular electrochemiluminescence of a ruthenium complex and cationic N, N-Bis(2-(trimethylammonium iodide)propylene) perylene-3,4,9,10-tetracarboxydiimide for estradiol assay. Anal Chem. 2021;93:6088–93.
Chang M, Wang Q, Qin W, Shi X, Xu G. Rational synthesis of aptamer-functionalized polyethylenimine modified magnetic graphene oxide composites for highly efficient enrichment and comprehensive metabolomics analysis of exosomes. Anal Chem. 2020;92:15497–505.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64.
Jiang N, Zhu T, Hu Y. Competitive aptasensor with gold nanoparticle dimers and magnetite nanoparticles for SERS-based determination of thrombin. Microchim Acta. 2019;186:747.
Xing Y, Han J, Wu X, Pierce DT, Zhao JX. Graphene/gold nanoparticle composites for ultrasensitive and versatile biomarker assay using single-particle inductively-coupled plasma/mass spectrometry. Analyst. 2020;145:7932–40.
Cui H, Fu X, Yang L, Xing S, Wang XF. 2D titanium carbide nanosheets based fluorescent aptasensor for sensitive detection of thrombin. Talanta. 2021;228:122219.
Chen Z, Sun M, Luo F, Xu K, Lin Z, Zhang L. Stimulus-response click chemistry based aptamer-functionalized mesoporous silica nanoparticles for fluorescence detection of thrombin. Talanta. 2018;178:563–8.
Kaneko K, Hara M, Nishino T, Maruyama T. One-step biotinylation of cellulose paper by polymer coating to prepare a paper-based analytical device. Anal Chem. 2020;92:1978–87.
Ge H, Bao H, Zhang L, Chen G. Preparation of porous graphene using cuprous oxide microspheres as sacrificial templates for enriching proteins and peptides. Carbon. 2015;82:579–89.
Huang Y, Ruan G, Ruan Y, Zhang W, Li X, Du F, Hu C, Li J. Hypercrosslinked porous polymers hybridized with graphene oxide for water treatment: dye adsorption and degradation. RSC Adv. 2018;8:13417–22.
Kimmins SD, Cameron NR. Functional porous polymers by emulsion templating: recent advances. Adv Funct Mater. 2011;21:211–25.
Yang G, Chen H, Qin H, Feng Y. Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl Surf Sci. 2014;293:299–305.
Inagaki M, Toyoda M, Soneda Y, Morishita T. Nitrogen-doped carbon materials. Carbon. 2018;132:104–40.
Villalonga A, Pérez-Calabuig AM, Villalonga R. Electrochemical biosensors based on nucleic acid aptamers. Anal Bioanal Chem. 2020;412:55–72.
Dolot R, Lam CH, Sierant M, Zhao Q, Liu FW, Nawrot B, Egli M, Yang X. Crystal structures of thrombin in complex with chemically modified thrombin DNA aptamers reveal the origins of enhanced affinity. Nucleic Acids Res. 2018;46:4819–30.
Sandner A, Ngo K, Schiebel J, Pizarroso AIM, Schmidt L, Wenzel B, Steinmetzer T, Ostermann A, Heine A, Klebe G. How a fragment draws attention to selectivity discriminating features between the related proteases trypsin and thrombin. J Med Chem. 2021;64:1611–25.
Lai PX, Mao JY, Unnikrishnan B, Chu HW, Wu CW, Chang HT, Huang CC. Self-assembled, bivalent aptamers on graphene oxide as an efficient anticoagulant. Biomater Sci. 2018;6:1882–91.
Xiong Y, Deng C, Zhang X. Development of aptamer-conjugated magnetic graphene/gold nanoparticle hybrid nanocomposites for specific enrichment and rapid analysis of thrombin by MALDI-TOF MS. Talanta. 2014;129:282–9.
Li D, Song Q, Li T, Shu C, Ji S, Su C, Su Y, Ding L. An LC-MS/MS method for protein detection based on a mass barcode and dual-target recognition strategy. RSC Adv. 2020;10:16094–100.
Guo WJ, Yang XY, Wu Z, Zhang ZL. A colorimetric and electrochemical dual-mode biosensor for thrombin using a magnetic separation technique. J Mater Chem B. 2020;8:3574–81.
Qin B, Yang K. Voltammetric aptasensor for thrombin by using a gold microelectrode modified with graphene oxide decorated with silver nanoparticles. Microchim Acta. 2018;185:407.
Zhao Q, Li XF, Le XC. Aptamer capturing of enzymes on magnetic beads to enhance assay specificity and sensitivity. Anal Chem. 2011;83:9234–6.
Funding
This research was supported by the National Natural Science Foundation of China (No. 21665006); the Natural Science Foundation from Guangxi Zhuang Autonomous Region (No. 2020GXNSFDA297025), the project of improving the basic scientific research ability of young and middle-aged teachers in Guangxi Universities (No. 2020KY12004), and Guangxi science and technology base and talent special project (No. 2019AC20330), respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, W., Hu, H., Ruan, G. et al. Aptamer functionalized and reduced graphene oxide hybridized porous polymers SPE coupled with LC–MS for adsorption and detection of human α-thrombin. Anal Bioanal Chem 414, 1553–1561 (2022). https://doi.org/10.1007/s00216-021-03776-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03776-9