Abstract
High-internal-phase-emulsion polymers (polyHIPEs) show great promise as solid-phase-extraction (SPE) materials because of the tremendous porosity and highly interconnected framework afforded by the high-internal-phase-emulsion (HIPE) technique. In this work, polyHIPE monolithic columns as novel SPE materials were prepared and applied to trace enrichment of cytokinins (CKs) from complex plant samples. The polyHIPE monoliths were synthesized via the in-situ polymerization of the continuous phase of a HIPE containing styrene (STY) and divinylbenzene (DVB) in a stainless column, and revealed highly efficient and selective enrichment ability for aromatic compounds. Under the optimized experimental conditions, a method using a monolithic polyHIPE column combined with liquid chromatography–electrospray tandem mass spectrometry (LC–MS–MS) was developed for the simultaneous extraction and sensitive determination of trans-zeatin (tZ), meta-topolin (mT), kinetin (K), and kinetin riboside (KR). The proposed method had good linearity, with correlation coefficients (R 2) from 0.9957 to 0.9984, and low detection limits (LODs, S/N = 3) in the range 2.4–47 pg mL−1 for the four CKs. The method was successfully applied to the determination of CKs in real plant samples, and obtained good recoveries ranging from 68.8 % to 103.0 % and relative standard deviations (RSDs) lower than 16 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-internal-phase emulsions (HIPEs) are highly viscous, paste-like emulsions in which the main, “internal” phase, usually defined as constituting more than 74 % of the volume (i.e. an internal-phase volume ratio (Φ) of 0.74 or greater) and possibly constituting up to 99 %, is dispersed within the continuous, minor “external” phase [1, 2]. The external phase is converted into a solid polymer and the emulsion droplets are removed, yielding (in most cases) a highly interconnected network of micron-sized pores of quite well defined diameter [3]. Because of their unique structure, HIPEs are particularly useful as templates for the construction of highly porous and permeable polymeric materials with a well-defined porosity [4–6]. The resulting materials are often termed a polymerized HIPE or polyHIPE and are suitable for a wide variety of applications, including tissue engineering and cell culture scaffolds [3, 7, 8], pure protein scaffolds [9, 10], enzyme immobilization [11, 12], water purification [3, 13], fluid separation [14], CO2 capture [15], hydrogen storage [3], and sensor materials [3, 16], because of their readily controllable pore and pore-throat size, ease of functionalization, and flexible synthesis.
Polymeric monoliths as sample-preparation materials have been successfully used in extraction, separation, and enrichment of analytes in environmental, food, and biological matrices, and have many potential advantages over more conventional particulate materials, especially in miniaturized and automated sample preparation [17–19]. HIPEs as templates have been used to prepare porous polymer monoliths, and the resulting monolithic column had high porosity, good mechanical stability, and good separation of proteins [20] and alkylbenzenes [21] in a very short time, similar to the separation obtained by use of commercial monoliths. As far as we are aware, however, there are no reports on polyHIPE monolith for the extraction and enrichment of trace analytes from complex plant samples.
Cytokinins (CKs) are a highly complex family of N6-substituted adenine derivatives and chemically unrelated phenylurea-type regulatory molecules. They are a class of plant-specific hormones that control almost every aspect of plant growth and development, including promoting cell division, nutrient mobilization, and leaf longevity, increasing grain yield, and responses to environmental stresses [22–25]. More than 40 CKs have been found and identified, and they typically present at very low concentration (usually below 30 pmol per gram fresh weight) in plants [26], which makes accurate qualitative and quantitative analysis difficult. Therefore an appropriate sample-preparation procedure is crucial for determination of CKs, and a variety of sample-preparation methods have been developed for enrichment of trace CKs from crude extract that contains thousands of other substances at far higher levels [26–28]; these methods include immunoaffinity purification (IAP) [29], liquid–liquid microextraction (LLME) [30], magnetic solid-phase extraction (MSPE) [31, 32], molecularly imprinted monolith solid-phase extraction (MIM-SPE) [33], polymer monolith solid-phase extraction (PM-SPE) [34–36], among others. These sample-preparation methods have their own advantages and disadvantages for the extraction and enrichment of CKs from plant samples [26]. Compared with traditional SPE, monolith-based SPE possess numerous outstanding advantages including simple preparation, excellent reusability, versatile surface chemistry, fast mass transfer, and easily automated for combination with analytical instruments, and have been successfully used for the extraction and enrichment of trace CKs from plant samples [34–36].
The objective of this study was to develop a new sample-preparation method based on a polyHIPE monolithic column for determination of CKs in plant samples. For this purpose, a polyHIPE monolithic column as SPE sorbent was prepared in a stainless column by in-situ polymerization of the continuous phase of a HIPE, and coupled with liquid chromatography–electrospray tandem mass spectrometry (LC–MS–MS) for analysis of four CKs. The properties of the polyHIPE monolith and the extraction conditions were investigated. The experimental results indicated that the proposed method using polyHIPE-monolith-based SPE coupled with LC–MS–MS could simultaneously determine four trace CKs in plant samples.
Experimental
Chemicals and reagents
trans-Zeatin (tZ), meta-topolin (mT), kinetin (K), and kinetin riboside (KR) were all purchased from J&K Scientific Ltd (Beijing, China). Surfactant sorbitan monooleate (Span 80), styrene (STY), divinylbenzene (DVB), potassium persulfate (K2S2O8, ≥99.0 %), toluene (T), 4-picolinic acid (PA), gibberellic acid (GA3), tetracycline (TC), and doxycycline (DC) were purchased from Aladdin Chemistry Co. Ltd (Shanghai, China). Acenaphthylene (AN) and fluorene (F) were kindly provided by Shenzhen Academy of Metrology and Quality Inspection (Shenzhen, China). SYS and DVB were purified by 10 % NaOH, deionized water, and anhydrous sodium chloride to remove the inhibitors before use. HPLC-grade acetonitrile, methanol, and concentrated formic acid (≥96 %) were purchased from Dikma (Dikma Corporation, Beijing, China). The chemical structures of 11 investigated compounds are shown in Fig. S1 in the Electronic Supplementary Material (ESM).
Individual stock solutions (1.0 mg mL−1) of K, KR, tZ, and mT were prepared in acidified methanol solution (pure methanol–water–concentrated formic acid, 80:19.9:0.1, v/v). Working standard solutions (1.0 μg mL−1) were prepared by mixing the individual stock solutions and then diluting the mixture solution with the acidified methanol solution for the following LC–MS–MS analysis. All stock solutions were stored under refrigerated conditions (4 °C). Deionized water from Arium® 611UV ultrapure water system (Sartorius Stedim Biotch GmbH, Germany) was used throughout the work. Other chemical reagents were analytical grade and were used without further purification.
Plant materials and sample extraction
Tobacco was grown in a greenhouse at 25–30 °C under sunlight, and 90-day-old tobacco leaves were manually wounded and then collected, weighed, and ground into powder. Seven-day-old mung-bean leaves were obtained by cultivating mung bean at room temperature (approximately 26 °C), and they also were manually wounded before collection. Fresh leaf samples (100 mg, tobacco or mung-bean leaves) were immediately immersed in 2.0 mL cold modified Bieleski’s solvent (methanol–formic acid–water, 15:1:4, v/v, −20 °C) in a centrifuge tube. The samples were extracted overnight at −20 °C and then centrifuged at 11,000 g for 15 min. The obtained supernatant was dried under vacuum for 24 h at ambient temperature with the aid of P2O5. The obtained residue was redissolved with 2 mL formic acid–ammonia buffer solution (pH 6.0), which was prepared by adding concentrated ammonia into 0.1 % (v/v) aqueous formic acid solution, and then filtered through a 0.22 μm nylon membrane filter (Agela Technologies Inc., Tianjin, China) for further purification and enrichment.
For the recovery experiments, tobacco leaves were spiked with standard tZ, mT, K, and KR before extraction.
Preparation of STY-DVB polyHIPE monolith
The monolithic STY-DVB polyHIPE column was prepared by in-situ polymerization of the continuous phase of a HIPE consisting of organic (continuous) and water (discontinuous) phase in a stainless column (4.6 × 50 mm I.D.), following the method described by Dizge et al. [37] with minor modifications. Briefly, span 80 (0.177 mL), STY (0.445 mL), and DVB (0.150 mL) were placed in a 10 mL centrifuge tube. The mixture was stirred with an overhead stirrer at 300 rpm for 10 min. The aqueous phase was prepared separately by dissolving 1.00 g K2S2O8 in deionized and degased water (45 mL). Aqueous phase (6.392 mL) was added dropwise to the organic solution under constant stirring. Once all aqueous phase was added, stirring was continued for a further 1.0 min at 3000 rpm to produce a uniform W/O emulsion. Afterward, the emulsion was transferred into a stainless column and heated at 60 °C for 24 h. The resulting polyHIPE monoliths were washed with 5.0 mL water, 5.0 mL methanol, and 5.0 mL acetonitrile by turns, respectively, three times by the HPLC system.
Brunauer–Emmett–Teller (BET) experiment
The specific surface area, pore volume, and pore-size distribution of porous polyHIPE monoliths were evaluated using nitrogen (N2) as adsorbate with a Micromeritics ASAP (accelerated surface area and porosimetry)-2020 (Micromeritics, USA). The N2 adsorption–desorption isotherm was measured using Micromeritics ASAP-2020 at liquid nitrogen temperature (77 K). Before the measurement, the sample was degassed at 60 °C for 4 h. The specific surface area was measured using the BET method.
PolyHIPE-monolith-based SPE procedure for enrichment of CKs
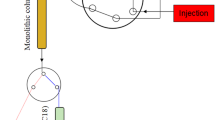
The extraction process is shown in Fig. S2 in the ESM. LC-20A (Shimadzu, Japan) liquid-chromatography equipment was used for the delivery of sample solution, washing solution, and desorption solvent.
Before extraction, the prepared monolithic polyHIPE column was first washed with 2.0 mL 80 % methanol (methanol–water, 80:20, v/v) and 2.0 mL formic acid–acetonitrile (20:80, v/v) solution in turn to remove leftover components to avoid matrix effects and interference, and then washed with formic acid–ammonia buffer solution (pH 6.0) for 15 min at a flow of 0.30 mL min−1. Sample solution (2.0 mL) was injected into a six-port valve equipped with a 2.0 mL sample loop and then loaded onto the washed polyHIPE monolith at a flow of 0.10 mL min−1. Target analytes retained on the polyHIPE monolith were then eluted with 4 mL elution solution (formic acid–acetonitrile, 20:80, v/v). The elution solution was collected and then dried under vacuum at ambient temperature. Finally, the monolithic polyHIPE column was stored in methanol after washing with 2 mL water and 2 mL methanol.
To evaluate the adsorption ability and extraction efficiency of the polyHIPE monolith for CKs, 2.0 mL of the CK-standard mixture solution, containing 50 ng mL−1 of each CK in formic acid–ammonia buffer solution (pH 6.0), was passed through the polyHIPE monolith at a flow of 0.05 mL min−1, and then continually eluted with 4.0 mL formic acid–acetonitrile (20:80, v/v) at a flow of 0.10 mL min−1. The elution solution was collected in 1.0 mL tubes and analyzed by LC–MS–MS.
Extraction conditions including sample solution, eluting solvent, and flow rate of sample loading were optimized to obtain high extraction efficiency, by evaluating mean recoveries of tZ, mT, K, and KR under different experimental conditions. The pH of the sample solution was increased from 4.0 to 7.0, acetonitrile solution containing different formic acid concentrations (10 %, 20 %, 40 %, and 60 %, v/v) was used as eluting solvent, and the flow rate of sample loading was set at 0.05, 0.1, and 0.15 mL min−1. All fractions in the eluting steps were collected and dried under vacuum at 40 °C. The residues were redissolved in 200 μL 80 % (v/v) acetonitrile containing 0.1 % formic acid and then filtrated by a 0.22 μm membrane filter for the LC–MS–MS analysis. Under the optimized conditions, the column-to-column reproducibility and the reusability of the proposed polyHIPE monolith were evaluated by investigating recoveries of four CKs. All data presented in this work were obtained by averaging at least three replicates unless otherwise noted.
LC–MS–MS analysis
The UHPLC–MS–MS system consisted of an Accela UHPLC system (Accela 1250 pump and Accela autosampler) coupled with a TSQ Vantage triple-quadrupole mass spectrometer, using LC Device (2.4.0), TSQ Series (2.2.0), and XCalibur (2.1.0) software (Thermo Fisher Scientific, San Jose, USA).
LC separations were performed with a Zorbax 300SB-C18 column (2.1 × 150 mm I.D., 3.5 μm; Agilent Technologies) and a binary mobile phase (solvent A: 0.1 % (v/v) formic acid in water; solvent B: acetonitrile). The gradient elution program, at a flow of 200 μL min−1, was: 0.0 min, 10 % B; 10 min, 50 % B; 14 min, 10 % B; 17 min, 10 % B. The column temperature used for LC was 30 °C. All samples were filtered by a 0.22 μm membrane filter before LC–MS–MS analysis.
Electrospray ionization (ESI) in positive-ion mode and selected reaction monitoring (SRM) mode were used to detect transition pairs. The first quadrupole mass analyzer (Q1) was operated in high-resolution mode with a full width half maximum (FWHM) of 0.1 Da, whereas the second quadrupole mass analyzer (Q3) was operated at 0.70 FWHM resolution. For selective identification of the four CKs, four SRM transitions were monitored (collision energies (CE) in eV are given in the brackets): 220.00 → 136.40 (CE = 21) for tZ, 242.00 → 107.10 (CE = 21) for mT, 216.00 → 148.00 (CE = 20) for K, and 348.00 → 216.10 (CE = 36) for KR. The ion-source settings were optimized as: spray voltage 3,000 V, vaporizer temperature 400 °C, capillary temperature 300 °C, sheath gas pressure 30 psi, ion sweep gas pressure 0 psi, auxiliary gas pressure 20 psi, de-clustering voltage 0 V, and argon collision gas pressure 1.5 mTorr. The data acquisition software used was Xcalibur 2.1.0 software.
Results and discussion
Characteristics of polyHIPE monolith
The morphology of the prepared polyHIPE monolith was observed by scanning electron microscope (SEM). The SEM images shown in Fig. 1 revealed that the polyHIPE was highly interconnected, having pore throats less than 4.0 μm in diameter. According to the SEM results, the average values of pore size and interconnected throat size were approximately 12 μm and 2.0 μm, respectively; however, the highly interconnected pore structure made it difficult to clearly differentiate pores from pore throats. Larger numbers of pore throats resulted in highly permeable polyHIPE monoliths. In addition, the obtained results revealed that the back-pressure of the monoliths was not higher than 3.0 MPa when the flow was 2.0 mL min−1, which suggested that the polyHIPE monolith had high permeability.
The mechanical stability of the polyHIPE monolith was investigated by comparing the microstructure before and after use for more than 100 extraction cycles when the flow was maintained at 2.0 mL min−1. The obtained SEM results revealed that the interconnecting pores and pore throats were the same as those of the original, which suggested that the mechanical performance of polyHIPE was good and the crush strength was more than 3.0 MPa. The obtained results indicated that the prepared polyHIPEs were stable as SPE materials.
The surface properties of the polyHIPE monolith which are not accessible by electron-microscopy techniques were investigated by surface-area (BET) analysis and are summarized in Table S2 in the ESM. The results indicated that the polyHIPE monolith possessed high surface area (57.1747 m2 g−1) because of the presence of mesopores in the materials, which led to transference of target analytes from bulk solution to the polyHIPE adsorbent surface. In addition, the N2 adsorption–desorption isotherm of the polyHIPE monolith (Fig. S3 in the ESM) presented a type iv isotherm according to the IUPAC classification, revealing the porous characteristics of the prepared monoliths.
The adsorption ability of the polyHIPE monolith for analytes was estimated from the elution order of the four CKs. The results revealed that tZ was first found in the first 1.0 mL tube of elution solution, KR was first present in the second tube, and mT and K were detected at the same time in the third tube. This suggested that tZ was the first eluted from the polyHIPE monolith, KR was the second, and mT and K were the last, revealing the last two CKs (mT and K) to have the strongest adsorption affinity with polyHIPE. The non-covalent interaction between CKs and polyHIPE, including Van der Waals type interactions, π–π interaction, and the hydrophobic effect, was affected by the protonation of CKs, and the degree of protonation of tZ at pH 6.0 was higher than that of the other three CKs, leading to poor interactions between tZ and polyHIPE and easy elution from the polyHIPE monolith. Moreover, Fig. 2 shows that the recovery of tZ in the first tube was 29.2 % and reached a maximum of 42.2 % after washing with 3.0 mL elution solvent. The recoveries of mT, K, and KR were 91.4 %, 93.1 %, and 99.6 %, respectively, after washing with 4.0 mL elution solvent, which indicated that the four CKs retained on the monolithic polyHIPE column can be almost completely released by eluting with 4.0 mL formic acid–acetonitrile (20:80, v/v) solution.
To investigate the extraction selectivity of the polyHIPE monolith, three aromatic compounds (T, AN, and F) with non-polar groups and three aromatic compounds (PA, TC, and DC) and one non-aromatic compound (GA3) with polar groups were studied by comparing the recoveries of the four CKs and those of the selected seven analytes (T, PA, AN, F, TC, DC, and GA3, shown in Fig. S1 in the ESM). Figure 3 shows that the recoveries of mT, K, KR, T, AN, and F were all in the range 91.4–101.2 %, those of tZ, TC, and DC were approximately 40 %, and PA and GA3 were not detected. The high recoveries of mT, K, KR, T, AN, and F might be caused by the strong hydrophobic interaction and π–π interaction between analytes and the polyHIPE, and the low extraction recoveries of tZ, TC, and DC might be caused by the protonation of analytes, because protonation or deprotonation could weaken the interactions between the three analytes and the polyHIPE. GA3 was not detected in the elution solution, which indicated that the adsorption of the polyHIPE monolith for GA3 was very poor; this was ascribed to a lack of π–π interaction between GA3 and the polyHIPE. The above results suggested that the aromatic compounds without strong protonation or deprotonation were selectively extracted by the polyHIPE monolith.
Mean recoveries of four CKs (K, KR, tZ, and mT) and the seven analytes (T, PA, AN, F, TC, DC, and GA3, shown in Fig. S2 in the ESM) using monolithic polyHIPE column
Optimization of extraction conditions with polyHIPE monolith
The pH of the sample solution is a critical factor for effective extraction of CKs because the pH affects the form of CKs via protonation and deprotonation in solution [33, 38]. As seen in Fig. 4a, different recoveries of the four CKs were found in the pH range 4.0–7.0, and the recovery at pH 6.0 was higher than the recovery at other pH values, resulting from the stronger hydrophobic interaction and, especially, π–π interaction between polyHIPE and CKs when the neutral form of CKs predominates at pH ~6.0. Therefore, the extracted sample was redissolved in formic acid–ammonia buffer solution at pH 6.0 for the following purification with polyHIPE monolith.
To select an appropriate elution solution for desorption of CKs retained on the polyHIPE monolith, concentrated formic acid (≥96 %)–methanol–water (10:80:10, v/v), concentrated formic acid–methanol (10:90, v/v), and concentrated formic acid–acetonitrile (10:90, v/v) were investigated. The obtained CK recoveries were lower than 30 % when using formic acid–methanol–water or formic acid–methanol as elution solvent, and the CK recoveries were higher than 40 % with formic acid–acetonitrile as elution solvent. With increased formic acid in the formic acid–acetonitrile solution, the CK recoveries increased as shown in Fig. 4b, and high recoveries were obtained using 20 % acidified acetonitrile and 40 % acidified acetonitrile as elution solvent. On the basis of the recoveries of the four CKs, 20 % acidified acetonitrile (formic acid–acetonitrile, 20:80, v/v) was the optimum eluent solution for desorption of these four CKs from polyHIPE monolith.
The optimum sample flow rate must be enough to enable the interactions between the analytes and polyHIPE monolith, and at the same time should be compatible with the kinetics of adsorption–desorption between the analytes and binding sites. To obtain higher recovery for the four CKs, the effect of sample flow rate on the polyHIPE monolith extraction was evaluated in the range 0.05–0.15 mL min−1. The obtained recoveries, shown in Fig. 4c, indicated that the higher flow rate was not optimum for extracting CKs from samples, because the binding interaction time between CKs and polyHIPE monolith was not enough to enable equilibration, and the CKs bound on polyHIPE were partially eluted by the sample solution at high flow rate. On the basis of the operating time and extraction efficiency, a sample flow rate of 0.10 mL min−1 was selected for further work.
Under the optimized conditions, the mean recoveries of tZ, mT, K, and KR in the standard solution on the polyHIPE monolith were 74.6 %, 86.7 %, 105.6 %, and 91.8 %, respectively, and the enrichment factors (EFs) were in the range 17–75 (Table 1), which indicated that the selected extraction conditions were appropriate for the extraction and enrichment of the four CKs from sample solutions. The maximum column capacity of the polyHIPE monolith was approximately 131.6 ng for tZ, 255.0 ng for mT, 300.1 ng for K, and 357.9 ng for KR, determined by investigating the amount extracted from 2.0 mL standard sample solution at a concentration of 500 ng mL−1 under the optimum extraction conditions.
Method validation
A method using a monolithic polyHIPE column coupled with LC–MS–MS was developed for the determination of four CKs. For method validation, the calibration curve, linear range, limit of detection (LOD), and limit of quantification (LOQ) were investigated; the obtained results are summarized in Table 1. The linear range, tested by varying the concentration of the standard solution, was 0.20–20 ng mL−1 for tZ and 0.050–20 ng mL−1 for mT, K, and KR. The calibration equations were calculated from the peak area of the corresponding SRM of the strongest specific fragment ions of each CK over the corresponding concentration range. Good linearity, with squared regression coefficients (R 2) in the range 0.9957–0.9984, was obtained. The LOD and LOQ were evaluated on the basis of the signal-to-noise ratio (S/N) and the corresponding calibration curve. The LODs (S/N = 3) obtained for the four CKs were in the range 2.4–47 pg mL−1, and the LOQs (S/N = 10) were in the range 7–157 pg mL−1.
The recoveries of the proposed method were evaluated by standard-addition of the CKs, and the results are shown in Table 2. The recoveries for the four CKs ranged from 68.8 % to 103.0 %, with relative standard deviations (RSDs) lower than 16 %. Compared with the recoveries of the four CKs in standard stock solution without matrix interference, the average recoveries for tobacco leaves were not obviously different, which indicated that the sample matrix did not obviously affect the extraction efficiency.
The reproducibility of the developed method was estimated by five repeated recovery determinations of samples spiked with 500 pg per 200 mg fresh sample. The variation coefficients of the recoveries were less than 15.0 % for all target analytes, indicating that the repeatability of the method was satisfactory. In addition, the column-to-column reproducibility and reusability were evaluated by determination of recoveries of CKs on three monolithic polyHIPE columns, and the obtained results revealed satisfactory repeatability both intra-batch (RSDs from 1.2 to 4.6 %) and inter-batch (RSDs 2.4 to 7.1 %). Moreover, the reusability was investigated by repeatedly using the same monolithic polyHIPE column for purification of specific samples. After purification of 100 standard CKs solutions (10.0 ng loading amount), the recoveries with the same monolithic polyHIPE column were not decreased and the column back-pressure was not increased. For purification of CKs from tobacco-leaf samples, the polyHIPE monoliths used in these experiments could be used 50 times without detectable loss of extraction effectiveness compared with the corresponding initial recovery values with the same monolithic column, which suggested that the prepared monolithic polyHIPE column was suitable for at least 50 extraction cycles without any change in its extraction performance.
All the validation experiments indicate that the proposed method is accurate and reliable for the simultaneous analysis of four CKs in real plant samples.
Analysis of real samples
To investigate the applicability to real complex plant samples, the developed monolithic polyHIPE column was used to extract CKs from mung-bean-leaf and tobacco-leaf samples. After one-step purification with the polyHIPE monolith, the obtained sample solution was successfully screened based on the ion transitions and retention times of tZ, mT, K, and KR. Under optimized LC–MS–MS conditions, the typical SRM chromatograms and the corresponding MS–MS spectra of four CKs were as shown in Figs. S4 and S5, respectively, in the ESM. The results revealed that the concentrations of tZ, mT, K, and KR were, respectively, 2663, 131, 144, and 161 pg g−1 fresh weight in mung-bean leaves, and 5920, 75, 100, and 120 pg g−1 fresh weight in tobacco leaves. The content of the four CKs in tobacco leaves was different from that reported elsewhere [33], because the wounding induced changes in CK levels in the leaves [39, 40].
Conclusions
In this study, we proposed an effective approach to simultaneously determinate four CKs in plant samples by SPE based on a polyHIPE monolithic column coupled with LC–MS–MS. The results revealed that the prepared polyHIPE monolith had good selectivity and high enrichment capability for aromatic compounds, and it was used for the first time to enrich CKs from crude plant extract by one-step purification, which greatly simplified the sample-pretreatment procedure and improved the analytical sensitivity. The developed method had good linearity and satisfactory recovery and repeatability, and was suitable for the analysis of CKs in complex plant samples. It is important to stress that, although the work used poly(STY-DVB) monolith as the SPE sorbent material for enrichment of CKs from plant extracts, polyHIPE monolithic columns could be used to extract other aromatic compounds without strong protonation or deprotonation. It is expected that more and more novel polyHIPEs as SPE sorbent materials could emerge, and these materials are promising for the extraction and enrichment of trace analytes in complicated samples.
References
Cameron NR (2005) High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 46:1439–1449
Silverstein MS (2014) PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog Polym Sci 39:199–234
Kimmins SD, Cameron NR (2011) Functional Porous Polymers by Emulsion Templating: Recent Advances. Adv Funct Mater 21:211–225
Ikem VO, Menner A, Horozov TS, Bismarck A (2010) Highly Permeable Macroporous Polymers Synthesized from Pickering Medium and High Internal Phase Emulsion Templates. Adv Mater 22:3588–3592
Wu RT, Menner A, Bismarck A (2013) Macroporous polymers made from medium internal phase emulsion templates: Effect of emulsion formulation on the pore structure of polyMIPEs. Polymer 54:5511–5517
Silverstein MS (2014) Emulsion-templated porous polymers: A retrospective perspective. Polymer 55:304–320
Hayward AS, Sano N, Przyborski SA, Cameron NR (2013) Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol Rapid Commun 34:1844–1849
Hayward AS, Eissa AM, Maltman DJ, Sano N, Przyborski SA, Cameron NR (2013) Galactose-Functionalized PolyHIPE Scaffolds for Use in Routine Three Dimensional Culture of Mammalian Hepatocytes. Biomacromolecules 14:4271–4277
Pulko I, Smrekar V, Podgornik A, Krajnc P (2011) Emulsion templated open porous membranes for protein purification. J Chromatogr A 1218:2396–2401
Li ZF, Xiao MD, Wang JF, Ngai T (2013) Pure Protein Scaffolds from Pickering High Internal Phase Emulsion Template. Macromol Rapid Commun 34:169–174
Pierre SJ, Thies JC, Dureault A, Cameron NR, van Hest JCM, Carette N, Michon T, Weberskirch R (2006) Covalent Enzyme Immobilization onto Photopolymerized Highly Porous Monoliths. Adv Mater 18:1822–1826
Pulko I, Kolar M, Krajnc P (2007) Atrazine removal by covalent bonding to piperazine functionalized PolyHIPEs. Sci Total Environ 386:114–123
Kimmins SD, Wyman P, Cameron NR (2014) Amine-functionalization of glycidyl methacrylate-containing emulsion-templated porous polymers and immobilization of proteinase K for biocatalysis. Polymer 55:416–425
Tebboth M, Menner A, Kogelbauer A, Bismarck A (2014) Polymerised high internal phase emulsions for fluid separation applications. Curr Opin Chem Eng 4:114–120
He HK, Li WW, Lamson M, Zhong MJ, Konkolewicz D, Hui CM, Yaccato K, Rappold T, Sugar G, David NE, Damodaran K, Natesakhawat S, Nulwala H, Matyjaszewski K (2014) Porous polymers prepared via high internal phase emulsion polymerization for reversible CO2 capture. Polymer 55:385–394
Zhao CT, Danish E, Cameron NR, Kataky R (2007) Emulsion-templated porous materials (PolyHIPEs) for selective ion and molecular recognition and transport: applications in electrochemical sensing. J Mater Chem 17:2446–2453
Namera A, Nakamoto A, Saito T, Miyazaki S (2011) Monolith as a new sample preparation material: Recent devices and applications. J Sep Sci 34:901–924
Namera A, Saito T (2013) Advances in monolithic materials for sample preparation in drug and pharmaceutical analysis. Trends Anal Chem 45:182–196
Nema T, Chan ECY, Ho PC (2014) Applications of monolithic materials for sample preparation. J Pharm Biomed Anal 87:130–141
Krajnc P, Leber N, Štefanec D, Kontrec S, Podgornik A (2005) Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media. J Chromatogr A 1065:69–73
Tunç Y, Gölgelioğlu ç, Hasirci N, Ulubayram K, Tuncel A (2010) Acrylic-based high internal phase emulsion polymeric monolith for capillary electrochromatography. J Chromatogr A 1217:1654–1659
Choi J, Choi D, Lee S, Ryu CM, Hwang I (2011) Cytokinins and plant immunity: old foes or new friends. Trends Plant Sci 17:172–179
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179
Naseem M, Wölfling M, Dandekar T (2014) Cytokinins for immunity beyond growth, galls and green islands. Trends Plant Sci 19:481–484
Murai N (2014) Plant Growth Hormone Cytokinins Control the Crop Seed Yield. Am J Plant Sci 5:2178–2187
Tarkowski P, Ge L, Yong JWH, Tan SN (2009) Analytical methods for cytokinins. Trends Anal Chem 28:323–335
Du FY, Ruan GH, Liu HW (2012) Analytical methods for tracing plant hormones. Anal Bioanal Chem 403:55–74
Tarkowská D, Novák O, Floková K, Tarkowski P, Turečková V, Grúz J, Rolčík J, Strnad M (2014) Quo vadis plant hormone analysis. Planta 240:55–76
Liang Y, Zhu XC, Zhao MP, Liu HW (2012) Sensitive quantification of isoprenoid cytokinins in plants by selective immunoaffinity purification and high performance liquid chromatography–quadrupole-time of flight mass spectrometry. Methods 56:174–179
Campillo N, Viñas P, Férez-Melgarejo G, Hernández-Córdoba M (2013) Dispersive liquid-liquid microextraction for the determination of three cytokinin compounds in fruits and vegetables by liquid chromatography with time-of-flight mass spectrometry. Talanta 116:376–381
Liu Z, Cai BD, Feng YQ (2012) Rapid determination of endogenous cytokinins in plant samples by combination of magnetic solid phase extraction with hydrophilic interaction chromatography–tandem mass spectrometry. J Chromatogr B 891–892:27–35
Cai BD, Zhu JX, Gao Q, Luo D, Yuan BF, Feng YQ (2014) Rapid and high-throughput determination of endogenous cytokinins in Oryza sativa by bare Fe3O4 nanoparticles-based magnetic solid-phase extraction. J Chromatogr A 1340:146–150
Du FY, Ruan GH, Liang SH, Xie FW, Liu HW (2012) Monolithic molecularly imprinted solid-phase extraction for the selective determination of trace cytokinins in plant samples with liquid chromatography–electrospray tandem mass spectrometry. Anal Bioanal Chem 404:489–501
Liu Z, Yuan BF, Feng YQ (2012) Tandem Solid Phase Extraction Followed by Online Trapping-Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry for Sensitive Detection of Endogenous Cytokinins in Plant Tissues. Phytochem Anal 23:559–568
Liu Z, Wei F, Feng YQ (2010) Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal Methods 2:1676–1685
Cai BD, Zhu JX, Shi ZG, Yuan BF, Feng YQ (2013) A simple sample preparation approach based on hydrophilic solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry for determination of endogenous cytokinins. J Chromatogr B 942–943:31–36
Dizge N, Keskinler B, Tanriseven A (2009) Biodiesel production from canola oil by using lipase immobilized onto hydrophobic microporous styrene-divinylbenzene copolymer. Biochem Eng J 44:220–225
Barták P, Bednář P, Stránský Z, Boček P, Vespalec R (2000) Determination of dissociation constants of cytokinins by capillary zone electrophoresis. J Chromatogr A 878:249–259
Sano H, Seo S, Koizumi N, Niki T, Iwamura H, Ohashi Y (1996) Regulation by Cytokinins of Endogenous Levels of Jasmonic and Salicylic Acids in Mechanically Wounded Tobacco Plants. Plant Cell Physiol 37:762–769
Schäfer M, Meza-Canales ID, Navarro-Quezada A, Brütting C, Vanková R, Baldwin IT, Meldau S (2015) Cytokinin levels and signaling respond to wounding and the perception of herbivore elicitors in Nicotiana attenuate. J Integr Plant Biol 57:198–212
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 21265004, 21465008, and 21065003) and the Natural Science Foundation from Guangxi Zhuang Autonomous Region (No. 2014GXNSFAA118063 and 2012GXNSFAA053030) and the project of high level innovation team and outstanding scholar in Guangxi colleges and universities, respectively.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 469 KB)
Rights and permissions
About this article
Cite this article
Du, F., Sun, L., Zhen, X. et al. High-internal-phase-emulsion polymeric monolith coupled with liquid chromatography–electrospray tandem mass spectrometry for enrichment and sensitive detection of trace cytokinins in plant samples. Anal Bioanal Chem 407, 6071–6079 (2015). https://doi.org/10.1007/s00216-015-8782-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8782-3