Abstract
This method was developed using ultrasonic-assisted hydrophobic deep eutectic solvent based dispersive liquid liquid microextraction (UA HDES DLLME) to determine trace levels of indigo carmine in food samples prior to analysis by UV Vis spectrophotometer at 610 nm. Some important analytical parameters such as pH, and sample volume and influence of foreign components were considered for optimization studies. A suitable extraction medium for indigo carmine was obtained at pH 3.0 and the mentioned above optimum conditions were determined. For instance, 15 mL sample volume, 1 mL final volume and a short centrifucation and ultrasonication time have been found. The limit of detection and limit of quantification were determined to be 2.79 ng mL−1, 9.31 ng mL−1 respectively. Moreover, the performed UA HDES DLLME process implemented to beverage, colored chocolate, chewing gum and nail polish samples. Analyte addition studies were carried out on an energy drink sample to demonstrate the accuracy of the method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Many food additives are used by food manufacturers to add flavour and appearance to foods, the most important of which are colours. These are produced naturally or synthetically and are widely used in almost all food industries (Minioti et al. 2007; Gholami et al. 2021). Synthetic colours are preferred to natural colours because they are more stable, last longer, produce brighter colours and are relatively cheap for the food industry. Synthetic food colours are synthesized by various chemical processes and are classified into five main colour groups according to their structure such as the triarylmethane group, azo compounds, indigo sulfonate, chinophthalon derivative and xanthine (Gholami et al. 2021; Bogdanova et al. 2022).
As well as being one of the most popular synthetic dyes, indigo carmine is also extracted naturally from the leaves of Indigofera tinctoria and is widely used in the food and pharmaceutical industries, textiles, food, leather, plastics, paper and cosmetics (Quintero and Cardona 2010; Karapanagiotis et al. 2006;Pan et al. 2019; Chowdhury et al. 2020; El-Kammah et al. 2022; Tabti et al. 2022; Tabti et al. 2022; Zhan et al. 2022). It's also utilized as a redox indicator and photometric detector in chemical experiments and as a microscopic stain in biological applications (Lakshmi et al. 2009; Genazio Pereira et al. 2017; Edwin et al. 2021; Mo et al 1992; Adam et al. 2022; Gokul Eswaran et al. 2022). Indigo carmine is derived from indigo dye by a chemical reaction with sulphuric acid (H2SO4). Its IUPAC systematic name is disodium (2E)-3-oxo-2-(3-oxo-5-sulphonato-2,3-dihydro-1H-indol-2-ylidene)-2,3-dihydro-1H-indole-5-sulphonate (C16H8Na2N2O8S2) and its CAS Registry Number, EINECS Number and Colour Index Number are 860-22-0, 212-728-8 and 73015 respectively (EFSA 2014).
Researchers have been studying the toxic effects of food colouring since 1975, in particular indigo carmine due to the fact that it can cause several diseases such as allergic problems, asthma, reproductive problems, hypersensitivity, migraine, some types of central tumours, permanent damage to the cornea, eye and skin irritations. Because of its harmful effects on humans, the accepted daily intake in the light of these studies is 0–5 mg kg−1 (Otterstatter 1999; FAO and WHO 2002; Crini 2006; Forgacs et al. 2004; Mittal et al. 2006; Gupta and Suhas 2009; EFSA 2014; Güray 2019; Altunay 2021).
Therefore, it is important to monitor the additives in foods containing synthetic food dyes and to determine their concentrations because of their adverse health effects (Otterstatter 1999; Güray 2019). Various studies have been carried out for the analysis of indigo carmine such as chromatography, electrophoresis, electroanalytical methods, thin layer chromatography (TLC), 3D paper chromatography, high performance liquid chromatography photodiode array detection (HPLC–PDA) and spectrophotometry. UV–vis spectrophotometry is the fastest, cheapest, simplest and easiest of these analytical methods for the determination of indigo carmine (Güray 2019; Tsai et al. 2015; Iammarino et al. 2019; Deroco et al. 2018; Gharaghani et al. 2020).
However, in instrumental analytical methods it is generally necessary to use a preconcentration technique such as solid phase extraction and liquid phase extraction to eliminate the interfering effect of matrix components and to reach the low concentration range of the species to be analyzed (Bogdanova et al. 2022). That's why several environmentally friendly methods have been widely used, such as dispersive liquid liquid microextraction (DLLME), cloud point extraction (CPE), liquid phase microextraction (LPME), supercritical fluid extraction (SFE), homogeneous liquid liquid microextraction (HLLME). (Güray 2019; Gholami et al. 2021). In these techniques, some environmentally friendly liquids such as ionic liquids, deep eutectic solvents, natural deep eutectic solvents, supercritical liquids, supramolecular solvents are used for the extraction process. The selection of a green solvent in the DLLME method is very important both for the extraction efficiency and to contribute to the development of analytical methods within the framework of Green Chemistry (Sajid and Alhooshani 2018; Li and Row 2019). In this context, the Deep Eutectic Solvent (DES) system, which has undergone rapid development in recent years and and is a DLLME method, is used in this research.
DESs are one of the most important green solvents due to their tunable physical properties . They are also a eutectic mixture system, formed by combining two or three solvents in a particular concentration ratio, and are generally linked together by hydrogen bonds (Omar and Sadeghi 2022; Shishov et al. 2019; Zhang et al. 2012; El Achkar et al. 2021). DESs can be easily obtained by heating at low temperatures (60–90 °C) for about an hour or less in the presence of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) (Abbott et al. 2004; Rub and Konig 2012; Chen et al. 2019). HBAs such as tetrabutylammonium bromide (TBAB), tetrabutylammonium chloride, betaine, choline chloride, betaine hydrochloride and HBDs such as ethylene glycol, urea, glycerol, butylene glycol and propanol are used in the synthesis of DES (Hashemi et al. 2018; Martins et al. 2019; Rodríguez-Ramos et al. 2021; Santana-Mayor et al. 2021). Designed green solvents, DESs, have some properties such as low electrical conductivity, low vapour pressure, high viscosity and high thermal stability (Maugeri and Domínguez de María 2012; Tang and Row 2013). Moreover, DESs have been motivated as either hydrophilic or hydrophobic deep eutectic solvents (HDESs). The HDESs are generally utilized to extraction of food dyes from the aqueous phase. Although photocatalytic degradation studies of indigo carmine have been widely carried out by the researhers, a HDES system has been employed in this work because there has been limited research on the preconcentration and extraction of indigo carmine in food, beverage and cosmetic samples (Nakamura et al. 2003; Yazdi et al. 2018; Chowdhury et al. 2020).
In this study, the ultrasonic assisted dispersive liquid–liquid microextraction (UA HDES DLLME) method has been improved for determination and preconcentration of indigo carmine in real samples as due to above mentioned deficiency. That’ s why, DES is a new environmentally friendly solvent used for indigo carmine analysis due to some advantages such as having higher efficiency and better specificity, being used as both extraction and separation agent (Santana-Mayor et al. 2021). The developed method has been used both extractions, preconcentration and separation. The effects of critical parameters on the determination of indigo carmine were examined and also optimized. Consequently, the wavelength of all samples was measured at 610 nm using UV Vis spectrophotometer after the liquid phase microextraction method.
2 Experimental
2.1 Instrumentation
A vortex mixer (Thermomac, Turkey) was used to get homogeneous solutions for all chemicals throughout the study. Indigo carmine absorbance measurements were carried out using UV Vis spectrometer (Perkin-Elmer Lambada 25; Norwalk, CT, USA). pH adjustmnts were carried out with a Hanna HI 2211, USA pH meter. In addition, a J.P. Selectra (ade in Spain) brand ultrasonic bath was used to obtain cloudy solutions. The distilled water, has been utilized in UA HDES DLLME method, was obtained from the Nuve Water Distiller ND-4.
2.2 Reagents
The UA HDES DLLME method was carried out using analytical grade chemicals. The analyte, indigo carmine dye stock solution as 1.0 × 10−4 M was prepared in ethanol. Model and calibration solutions were obtained by dilution of this solution. Tetrabutylammonium bromide (TBAB) and choline chloride (ChCl), have been utilized as HBA and also decanoic acid (Da) (HBD) were purchased from Sigma-Aldrich (USA). Urea (Isolab Chemicals (Wertheim, Germany), betaine (Sigma-Aldrich, China), sucrose (Karlsruhe- Germany) were used to investigate the effect of DESs formation on the extraction efficiency of Indigo carmine. pH adjustment in 2–4 was done via buffer solutions prepared as acetate, ammonium and phosphate formations.
2.3 Synthesis of HDES
Two or more HBAs and HBDs combine to form a new solvent system with different properties called DES. Hence, a DES formation consisting of TBAB and Da was carried out for the preconcentration/seperation of indigo carmine. First, the solvents (TBAB:Da) were mixed at different ratios of 2:1 at the temperature commonly used in the literature for DES preparation (60–80 °C) until they were completely dissolved (Chen et al. 2019). Once a fully homogeneous solution was obtained, the HDESs were cooled to room temperature for use in the optimization of the UA HDES DLLME method.
2.4 UA HDES DLLME procedure
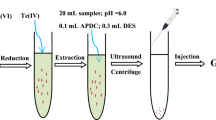
Model solutions including indigo carmine were prepared in 5 mL sample volume. Firstly, their pH values have been arranged as 3 with buffer solutions. Then, 500 µL of DES was added to the pH adjusting sample solutions and shortly after they were mixed by means of vortex procedure. 300 µL of tetrahydrofuran (THF), an aprotic solvent, was utilized in order to extract the dye from the aqueous phase. The resulting solutions were placed in an ultrasonic bath for 3 min to cloud. The turbidity of these solutions increased regularly owing to the dispersion of the TBAB: Da into the aqueous phase. The mixtures were centrifuged at 4000 rpm for 10 min and then the analyte collected at the top of the tube. As soon as the indigo carmine passed into the DES phase by centrifugation, the aqueous phase was removed by means of an injector. The extraction phase was analysed by adding 1 mL of ethanol and finally using a UV Vis spectrophotometer at 610 nm (Fig. 1). Blank solutions were also prepared using the same UA HDES DLLME method.
2.5 Sample preparation
Samples of beverages, colored chocolate, chewing gum and nail polish were prepared for the analysis of indigo carmine with the help of both addition recovery studies and the developed method. These samples were purchased from markets in Gaziantep, Turkey.
Initially, 1.41 g and 1.21 g of draje chocolate, 2.5 g of chewing gum and nail polish samples were weighed into the tubes prior to the extraction studies. The samples were then blended with of 5 mL and 10 mL of ethanol by sonication procedure for half an hour. When the transfer of indigo carmine to the aqueous phase was complete, the tubes were centrifuged for 10 min. The solid phase settling to the bottom was separated from the ethanol phase. After sample preparation, 0.1 mL of the liquid phase containing indigo carmine was collected in centrifuge tubes for the implementation of the UA HDES DLLME method described in Sect. 2.3. In addition, beverages were analyzed directly by applying the UA HDES DLLME method without sample preparation procedure and stored at 4 °C. The presence of indigo carmine in these samples was then determined using a UV Vis spectrophotometer at 610 nm.
3 Results and discussion
3.1 Effect of pH
pH is one of the most crucial parameters in order to optimize solution ambient conditions in enrichment and extraction studies (Soylak and Koksal 2019; Faraj and Fakhre 2023; Jagirani and Soylak 2022). Therefore, the procedure described in Sect. 2.3 was applied to the model solutions, which were adjusted between pH 2 and 4. pH regulation was achieved by use of buffer solutions. The recoveries calculated from the absorbances measured with the UV Vis spectrophotometer are shown in Fig. 2. It is clear that quantitative recoveries were obtained when microextraction studies were carried out at pH 3.0. Due to the high recovery values, pH 3.0 was chosen for subsequent studies.
3.2 Optimization of the type and volume of DES
In this part, the effect of various type of DESs, have been scrutinized on extraction effiency for indigo carmine. The DESs were prepared from a mixture of 1:1 ratio of ChCl (HBA): urea (HBD) (DES1), ChCl (HBA): sucrose (HBD) (DES2), betaine (HBA): sucrose (HBD) (DES3), TBAB (HBA): Da (HBD) (DES4). The UA HDES DLLME method has been sucritinized by trying the above-mentioned type of DES. Figure 2 shows that the highest extraction efficiency was obtained by using TBAB/Da DES solvent mixture, which was choosen as the extraction solvent for this method.
Furthermore, the solvents, TBAB and Da, were carried out in the ratio of (DES5) 2:1, (DES6) 3:1 in order to obtain the optimum DES ratio. From Fig. 3, it can be seen that the most successful results in the recovery of indigo carmine correspond to DES5. Therefore, ongoing studies have been carried out with the use of DES5.
On the other hand, the volume of quantitative DES5 was investigated in order to obtain high recovery values. For this, the devised UA HDES DLLME method was applied to sample solutions containing different volumes of DES5. 100, 200, 300, 400, 500, and 700 μL volumes of DES were used to extract indigo carmine from working and real samples. The optimal volume of DES was found to be 500 μL (Fig. 4).
3.3 Effect of THF volume
Generally, various aprotic solvents such as dichloromethane, acetonitrile, dimethylsulfoxide or acetone are utilized in order to ensure phase separation from aqueous to organic phase in deep eutectic solvent based liquid phase microextraction procedures. In this study, THF was used as an aprotic solvent for the extraction of indigo carmine to the DES phase. THF was added to the sample solution in volumes between 100 and 500 μL in the wake of fixed volume of DES as 500 μL. At this stage the DES phase, including the analyte, was allowed to self-aggregate to the top of the tube. All recovery results up to % 95 for indigo carmine extraction efficiency were shown in Fig. 5. This was followed by optimisation studies using 300 μL THF .
3.4 Optimization of sonication and centrifugation time
In the UA HDES DLLME method, an ultrasonic bath was used to accelerate the extraction of indigo carmine into the DES phase. For this purpose, model solutions prepared according to the UA HDES DLLME method were kept in an ultrasonic bath between 3–10 min. at certain intervals. According to the results illustrated in Fig. 5, the best extraction efficiency was obtained at 3 min.
Furthermore, blurred solution formation was observed after the addition of the aprotic organic solvent THF to the sample solutions in the UA HDES DLLME method. Therefore, centrifugation should be performed to separate the DES phase from these solutions. In order to achieve a good separation of the DES phase, the study of the centrifugation time is crucial. That’s why, centrifugation process was carried out at 3–10 min. In light of the results shown in Fig. 6 it seems that the centrifugation time of 10 min. was sufficient. As a result of the time experiments, the optimum sonication and centrifugation times for the preconcentration/ separation of indigo carmine dye were chosen as 3 and 10 min. respectively.
3.5 Sample and final volume
Before the spectrophotometric measurement of the dye, we enriched according to the developed UA HDES DLLME method, the final volume was investigated to reduce the organic solvent volume and achieve a high preconcentration factor (Ghaedi et al. 2016; Mortada et al. 2023; Hoseinzadeh et al. 2023). In this context, the sample volume on the extraction efficiency of the dye has been made the most effective use of the process. The effect of sample volume on the extraction efficiency of the dye was investigated in order to make the most effective use of the process. Sample volumes in the range of 5–20 mL were studied to enrich indigo carmine from real products. It could be explicable thanks to Fig. 7 that the highest recovery value was at 15 mL. Once and for all, the preconcentration factor was obtained as 15 according to the final and sample volumes of 1, 15 mL respectively.
3.6 Matrix effects
In the presence of foreign ions or food additives, it is very important for separation/preconcentration methods to investigate this parameter in order to extract the dye to be analyzed without being affected in the aforementioned matrix environment. Due to the fact that matrix components determine the selectivity of the devised method, effects of some dyes (chlorophyll, β-carotene, carmoisine, brillant blue FCF) and some ions (Mg2+, Ca2+, Li+, Na+, Zn2+) on the method were investigated. The UA HDES DLLME method was applied by adding different concentrations of organic and inorganic species to the samples containing a certain amount of indigo carmine dye. The extraction phases obtained were measured by UV Vis spectrophotometer and the recovery values of the analytes were calculated. All quantitative results in tolerance concentration are presented in Table 1. Thus, the UA HDES DLLME method was performed with high selectivity for the real samples.
3.7 Real samples analysis
For the analysis of indigo carmine, the optimized method was studied on real samples such as energy drink, colour chocolate, chewing gum, candy, nail polish, fruit soda. In addition, the accuracy of the method was demonstrated by adding the standard solution of indigo carmine to the energy drink at known concentrations. Increasing concentrations of indigo carmine were added to model solutions prepared in 3 parallels under optimal conditions. The fact that the recoveries are over 95% shows both the accuracy and the success of the method. The results in Tables 2 and 3 show that the method is applicable, accurate and successful.
3.8 Analytical performance
Some analytical performance criteria such as relative standard deviation (RSD), preconcentration factor (PF), limit of quantification (LOQ), correlation coefficient (R2), limit of detection (LOD), and also linear range (LR) have indicated that the method has been successfully applied. Limit of detection (LOD formula: 3Sb/m), limit of quantification (LOQ formula: 10Sb/m), calculated as 2.79 ng·mL−1, 9.31 ng·mL−1 respectively according to the measurement results of the blank solution. After repeated measurements at the same analyte concentration, the RSD which is the formula Sb/x was determined to be 0.8–2.7 (Sb: standart deviation, x: concentration, m: slope). And also (R2), preconcentration factor (PF) were obtained as 0.9975, 15. Finally, linear equation obtaining from calibration curve was found A = 1.6634C + 0.0039 (A: absorbance and C: dye concentration).
Furthermore, analytical performance criteria of the UA HDES DLLME method were compared with other studies in literature which were summarized in the Table 4. When the values were examined, it was explicitly seen that the method has high PF factor, low LOD and LOQ values, and a short extraction time.
4 Conclusions
Dark blue indigo carmine, known as E 132, is mainly used in food products such as confectionery, gelatine, ice cream, biscuits and medicines. It is very important to determine the amount of indigo carmine in these products, as using more than the recommended dose can have a toxic effect. For this purpose, the liquid phase method was developed in this study. Optimisation studies such as sample volume, extraction time and pH were carried out. The method was successfully applied to real samples under optimal conditions such as pH 3.0, 0.5 mL Des, 0.3 mL THF, 3 min extraction time, 15 mL maximum sample volume where recoveries of more than 95% were obtained (Figs. 2, 4, 5, 6, 7). In addition, the concentration of indigo carmin was determined in samples such as energy drink, colour chocolate, chewing gum, candy, nail polish, fruit soda (Table 3). On the other hand, the addition/recovery studies, one of the most important experiments, were carried out by adding indigo carmine at a known concentration to the energy drink. According to the results shown in the Table 2, 100% recovery of indigo carmine proved that the method was quite successful and accurate. In the light of these recovery values, the devised UA HDES DLLME method was flawlessly applied to real samples without any interfering effect. The main advantages of this study are to cut down on the consumption harmful chemicals, to present low LOD, LOQ values, acquire high preconcentration factor (PF), and ensure brief extraction time.
Data availability
The data will be available upon request.
References
Abbott, A.P., Boothby, D., Capper, G., Davies, D.L., Rasheed, R.K.: Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 126, 9142–9147 (2004). https://doi.org/10.1021/ja048266j
Adam, F., Ghoniem, M., Diawara, M., Rahali, S., Abdulkhair, B., Elamin, M., Ben Aissa, M., Seydou, M.: Enhanced adsorptive removal of indigo carmine dye by bismuth oxide doped MgO based adsorbents from aqueous solution: equilibrium, kinetic and computational studies. RSC Adv. 12, 24786-24803 (2022). https://doi.org/10.1039/d2ra02636h
Albalawi, M.A., Gomaa, H., El Hamd, M.A., Abourehab, M.A., Abdel-Lateef, M.A.: Detection of Indigo Carmine dye in juices via application of photoluminescent europium-doped carbon dots from tannic acid. Luminescence 38(2), 92–98 (2023). https://doi.org/10.1002/bio.4417
Altunay, N.: An optimization approach for fast, simple and accurate determination of indigo-carmine in food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 257, 119791 (2021). https://doi.org/10.1016/j.saa.2021.1197911386-1425
Bogdanova, P., Vakh, C., Bulatov, A.: A surfactant-mediated microextraction of synthetic dyes from solid-phase food samples into the primary amine-based supramolecular solvent. Food Chem. 380, 131812 (2022). https://doi.org/10.1016/j.foodchem.2021.131812
Chen, J., Li, Y., Wang, X., Liu, W.: Application of deep eutectic solvents in food analysis: a review. Molecules 24(24), 4594 (2019). https://doi.org/10.3390/molecules24244594
Chowdhury, M., Khandaker, S., Sarker, F., Islam, A., Rahman, M., Awual, M.: Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: a review. J. Mol. Liq. 318, 114061 (2020). https://doi.org/10.1016/j.molliq.2020.114061
Crini, G.: Non-conventional low-cost adsorbents for dye removal: a review. Biores. Technol. 97, 1061–1085 (2006). https://doi.org/10.1016/j.biortech.2005.05.001
Deroco, P.B., Medeiros, R.A., Rocha-Filho, R.C., Fatibello-Filho, O.: Selective and simultaneous determination of indigo carmine and allura red in candy samples at the nano-concentration range by flow injection analysis with multiple pulse amperometric detection. Food Chem. 247, 66–72 (2018). https://doi.org/10.1016/j.foodchem.2017.12.006
Edwin, D.S., Manjunatha, J.G., Raril, C., Girish, T., Ravishankar, D.K., Arpitha, H.J.: Electrochemical analysis of indigo carmine using polyarginine modified carbon paste electrode. J. Electrochem. Sci. Eng. 11(2), 87–96 (2021). https://doi.org/10.5599/jese.953
EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re‐evaluation of Indigo Carmine (E 132) as a food additive. EFSA J. 12(7), 3768 (2014). https://doi.org/10.2903/j.efsa.2014.3768
El-Achkar, T., Greige-Gerges, H., Fourmentin, S.: Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 19(4), 3397–3408 (2021). https://doi.org/10.1007/s10311-021-01225-8
El-Kammah, M., Elkhatib, E., Gouveia, S., Cameselle, C., Aboukila, E.: Enhanced removal of Indigo Carmine dye from textile effluent using green cost-efficient nanomaterial: Adsorption, kinetics, thermodynamics and mechanisms. Sustain. Chem. Pharmacy 29, 100753 (2022). https://doi.org/10.1016/j.scp.2022.100753
FAO and WHO. Pan European Conference on Food Safety and Quality, February (2002) http://www.fao.org
Faraj, H.S., Fakhre, N.A.: Dispersive liquid-liquid microextraction spectrophotometric determination of human salivary nitrite content. Egypt. J. Chem. 66, 157–166 (2023)
Forgacs, E., Cserháti, T., Oros, G.: Removal of synthetic dyes from wastewaters: a review. Environ. Int. 30, 953–971 (2004). https://doi.org/10.1016/j.envint.2004.02.001
Genazio Pereira, P.C., Reimao, R.V., Pavesi, T., Saggioro, E.M., Moreira, J.C., Veríssimo Correia, F.: Lethal and sub-lethal evaluation of Indigo Carmine dye and byproducts after TiO2 photocatalysis in the immune system of Eisenia andrei earthworms. Ecotoxicol. Environ. Saf. 143, 275–282 (2017). https://doi.org/10.1016/j.ecoenv.2017.05.043
Ghaedi, M., Azad, F.N., Dashtian, K., Hajati, S., Goudarzi, A., Soylak, M.: Central composite design and genetic algorithm applied for the optimization of ultrasonic-assisted removal of malachite Green by ZnO nanorod-loaded activated carbon. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 167, 157–164 (2016)
Gharaghani, F.M., Akhond, M., Hemmateenejad, B.: A three-dimensional origami microfluidic device for paper chromatography: Application to quantification of Tartrazine and Indigo carmine in food samples. J. Chromatogr. A 1621, 461049 (2020). https://doi.org/10.1016/j.chroma.2020.461049
Gholami, Z., Marhamatizadeh, M., Yousefinejad, S., Rashedinia, M., Mazloomi, S.: Vortex-assisted dispersive liquid-liquid microextraction based on hydrophobic deep eutectic solvent for the simultaneous identification of eight synthetic dyes in jellies and drinks using HPLC-PDA. Microchem. J. 170, 106671 (2021). https://doi.org/10.1016/j.microc.2021.106671
Gokul Eswaran, S., Shahid Afridi, P., Vasimalai, N.: Efective multi toxic dyes degradation using bio fabricated silver nanoparticles as a green catalyst. Appl. Biochem. Biotechnol. 195, 3872–3887 (2022). https://doi.org/10.1007/s12010-022-03902-y
Gupta, V.K., Suhas: Application of low-cost adsorbents for dye removal—a review. J. Environ. Manag. 90(8), 2313–2342 (2009). https://doi.org/10.1016/j.jenvman.2008.11.017
Güray, T.: A novel method for simultaneous analysis of tartrazine and indigo carmine by cloud point extraction using spectrophotometric technique. Int. J. Chem. Stud. 7(6), 17–23 (2019)
Hashemi, B., Zohrabi, P., Dehdashtian, S.: Application of green solvents as sorbent modifiers in sorptive-based extraction techniques for extraction of environmental pollutants. TrAC Trends Anal. Chem. 109, 50–61 (2018). https://doi.org/10.1016/j.trac.2018.09.026
Heydari, R., Hosseini, M., Zarabi, S.: A simple method for determination of carmine in food samples based on cloud point extraction and spectrophotometric detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 150, 786–791 (2015). https://doi.org/10.1016/j.saa.2015.06.032
Hoseinzadeh, A., Heidari, H., Matin, A.A., Soylak, M.: Multi-response optimization of a deep eutectic solvent-based microextraction method for the simultaneous extraction of twenty organochlorine pesticides for monitoring in various water samples. Microchem. J. 194, 109226 (2023). https://doi.org/10.1016/j.microc.2023.109226
Iammarino, M., Mentana, A., Centonze, D., Palermo, C., Mangiacotti, M., Chiaravalle, A.E.: Chromatographic determination of 12 dyes in meat products by HPLC-UV-DIODE array detection. MethodsX 6, 856–861 (2019). https://doi.org/10.1016/j.mex.2019.04.018
Jagirani, M.S., Soylak, M.: Review: microextraction technique based new trends in food analysis. Crit. Rev. Anal. Chem. 52, 968–999 (2022)
Karapanagiotis, I., de Villemereuil, V., Magiatis, P., Polychronopoulos, P., Vougogiannopoulou, K., Skaltsounis, A.: Identification of the coloring constituents of four natural indigoid dyes. J. Liq. Chromatogr. Related Technol. 29(10), 1491–1502 (2006). https://doi.org/10.1080/10826070600674935
Kavieva, L., Ziyatdinova, G.: Voltammetric sensor based on SeO2 nanoparticles and surfactants for indigo carmine determination. Sensors 22(9), 3224 (2022). https://doi.org/10.3390/s22093224
Lakshmi, U.R., Srivastava, V.C., Mall, I.D., Lataye, D.H.: Rice husk ash as an efective adsorbent: evaluation of adsorptive characteristics for Indigo Carmine dye. J. Environ. Manag. 90(2), 710–720 (2009). https://doi.org/10.1016/j.jenvman.2008.01.002
Li, G., Row, K.: Utilization of deep eutectic solvents in dispersive liquid-liquid microextraction. Trends Anal. Chem. 120, 115651 (2019). https://doi.org/10.1016/j.trac.2019.115651
Martins, M.A.R., Pinho, S.P., Coutinho, J.A.P.: Insights into the nature of eutectic and deep eutectic mixtures. J. Solut. Chem. 48, 962–982 (2019). https://doi.org/10.1007/s10953-018-0793-1
Maugeri, Z., Domínguez de María, P.: Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols. RSC Adv. 2(2), 421–425 (2012). https://doi.org/10.1039/C1RA00630D
Minioti, K.S., Sakellariou, C.F., Thomaidis, N.S.: Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 583(1), 103–110 (2007). https://doi.org/10.1016/j.aca.2006.10.002
Mittal, A., Mittal, J., Kurup, L.: Batch and bulk removal of hazardous dye, indigo carmine from wastewater through adsorption. J. Hazard. Mater. 137, 591–602 (2006). https://doi.org/10.1016/j.jhazmat.2006.02.047
Mo, S., Na, J., Mo, H., Chen, L.: Voltammetric determination of indigo carmine and amaranth on a silver-based mercury film electrode. Anal. Lett. 25(5), 899–909 (1992). https://doi.org/10.1080/00032719208020044
Mortada, W.I., Zedan, H.E., Khalifa, M.E.: Spectrophotometric determination of trace vanadium in fresh fruit juice samples by ion pair-based surfactant-assisted microextraction procedure with solidification of floating organic drop. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 302, 123107 (2023). https://doi.org/10.1016/j.saa.2023.123107
Nakamura, T., Hirata, M., Kawasaki, N., Tanada, S., Tamura, T., Nakahori, Y.: Decolorization of indigo carmine by charcoal from extracted residue of coffee beans. J. Environ. Sci. Health Part A 38(3), 555–562 (2003). https://doi.org/10.1081/ESE-120016917
Omar, K., Sadeghi, R.: Physicochemical properties of deep eutectic solvents: a review. J. Mol. Liq. 360, 119524 (2022). https://doi.org/10.1016/j.molliq.2022.119524
Otterstatter, G.: Coloring of Food, Drugs and Cosmetics, 1st edn., pp. 385–386. Taylor and Francis Group, New York (1999). https://doi.org/10.1201/9781482270082
Pan, G., Jing, X., Ding, X., Shen, Y., Xu, S., Miao, W.: Synergistic effects of photocatalytic and electrocatalytic oxidation based on a three-dimensional electrode reactor toward degradation of dyes in wastewater. J. Alloys Compd. 809, 151749 (2019). https://doi.org/10.1016/j.jallcom.2019.151749
Quintero, L., Cardona, S.: Technologies for the decolorization of dyes: Indigo and indigo carmine. DYNA 77, 371–386 (2010)
Rodríguez-Ramos, R., Santana-Mayor, Á., Socas-Rodríguez, B., Rodríguez-Delgado, M.: Recent applications of deep eutectic solvents in environmental analysis. Appl. Sci. 11(11), 4779 (2021). https://doi.org/10.3390/app11114779
Rub, C., Konig, B.: Low melting mixtures in organic synthesis-an alternative to ionic liquids. Green Chem. 14, 2969–2982 (2012). https://doi.org/10.1039/C2GC36005E
Sajid, M., Alhooshani, K.: Dispersive liquid-liquid microextraction based binary extraction techniques for chromatographic analysis: a review. TrAC Trends Anal. Chem. 108, 167–182 (2018). https://doi.org/10.1016/j.trac.2018.08.016
Santana-Mayor, Á., Rodríguez-Ramos, R., Herrera-Herrera, A.V., Socas-Rodríguez, B., Rodríguez-Delgado, M.Á.: Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 134, 116108 (2021). https://doi.org/10.1016/j.trac.2020.116108
Shishov, A., Volodina, N., Nechaeva, D., Gagarinova, S., Bulatov, A.: An automated homogeneous liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of caffeine in beverages. Microchem. J. 144, 469–473 (2019). https://doi.org/10.1016/j.microc.2018.10.014
Soylak, M., Koksal, M.: Deep eutectic solvent microextraction of Lead(II), Cobalt(II), Nickel(II) and Manganese(II) ions for the separation and preconcentration in some oil samples from turkey prior to their microsampling flame atomic absorption spectrometric determination. Microchem. J. 147, 832–837 (2019)
Tabti, S., Benchettara, A., Smaili, F., Benchettara, A., Berrabah, S.: Electrodeposition of lead dioxide on Fe electrode: application to the degradation of Indigo Carmine dye. J. Appl. Electrochem. 52(8), 1207–1217 (2022). https://doi.org/10.1007/s10800-022-01709-7
Tang, B., Row, K.: Recent developments in deep eutectic solvents in chemical sciences. Monatshefte Für Chemie Chem. Mon. 144(10), 1427–1454 (2013). https://doi.org/10.1007/s00706-013-1050-3
Tsai, C.F., Kuo, C.H., Shih, D.Y.C.: Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 23(3), 453–462 (2015). https://doi.org/10.1016/j.jfda.2014.09.003
Üstün Özgür, M., Delmidan, M.: Development and validation of an effective spectrophotometric method for simultaneous determination of synthetic colorants after cloud point extraction and comparision with new green HPLC method. J. AOAC Int. 102(4), 1241–1252 (2019). https://doi.org/10.5740/jaoacint.18-0229
Yazdi, M.G., Ivanic, M., Mohamed, A., Uheida, A.: Surface modified composite nanofibers for the removal of indigo carmine dye from polluted water. RSC Adv. 8, 24588–24598 (2018). https://doi.org/10.1039/c8ra02463d
Zhan, W., Du, Y., Lan, J., Lei, R., Li, R., Du, D., Zhang, T.: Electrochemical degradation of indigo carmine by low voltage pulse electrolysis. J. Mol. Liq. 348, 118006 (2022). https://doi.org/10.1016/j.molliq.2021.118006
Zhang, Q., De Oliveira Vigier, K., Royer, S., Jérôme, F.: Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 41(21), 7108–7146 (2012). https://doi.org/10.1039/C2CS35178A
Funding
There is no funding received.
Author information
Authors and Affiliations
Contributions
Authors are equally conducting and co-writing the paper.
Corresponding author
Ethics declarations
Conflict of interest
We declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical approval
This work is not applicable for both human and/ or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kizil, N., Erkmen Erbilgin, D., Yola, M.L. et al. An environmentally friendly hydrophobic deep eutectic solvent dispersive liquid liquid microextraction for spectrophotometric analysis of indigo carmine (E132). Opt Quant Electron 56, 341 (2024). https://doi.org/10.1007/s11082-023-05964-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05964-6