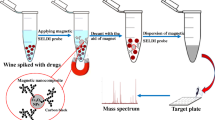
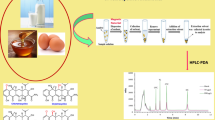
Abstract
In this study, a magnetic molecular sieve material (Fe3O4@MCM-48) was synthesized by a combination of solvothermal and self-assembly methods. The physicochemical properties of the magnetic molecular sieve material were characterized by scanning electron microscopy, energy-dispersive spectroscopy, magnetic hysteresis loop measurements, transmission electron microscopy, powder X-ray diffraction, N2 adsorption–desorption analysis, and Fourier transform infrared spectroscopy. The as-synthesized nanocomposite showed various advantages, including easy magnetic-assisted separation, high specific surface area, and a highly interwoven and branched mesoporous structure. The Fe3O4@MCM-48 nanocomposite was then used as an effective adsorbent material for magnetic solid-phase extraction of fluoroquinolones (FQs) from water samples. The FQs in the extract were determined via liquid chromatography–tandem mass spectrometry. Adsorption and desorption factors that affected the extraction performance were systematically optimized using spiked purified water samples. Good linearity (with R2 > 0.99) was shown by this FQ detection system for FQ concentrations from 5 to 1000 ng L−1. Moreover, low detection limits (0.7–6.0 ng L−1) and quantitation limits (2.5–20.0 ng L−1) and satisfactory repeatability (relative standard deviation < 10%, n = 6) were achieved for water samples. The developed method was also validated for the analysis of FQs in meat and milk samples. Finally, FQs in food and drinking water samples were successfully determined using the developed method.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The misuse and abuse of antibiotics have led to global environmental and human health problems. Moreover, antibiotic residues have become a major public concern in recent years [1]. The main sources of antibiotic residues include hospitals, the pharmaceutical industry, livestock, and fish farms [2]. Trace quantities of antibiotics have started to emerge in various water, soil, and food samples.
Fluoroquinolones (FQs) are a class of synthetic antimicrobial drugs. Owing to their broad-spectrum activity, FQs are licensed in many countries and are widely used in the treatment of human and animal infectious diseases [3, 4]. Due to the emergence of antibiotic-resistant Campylobacter species in chicken and turkey secondary to antimicrobial treatment, the U.S. Food and Drug Administration banned the use of enrofloxacin (ENRO) in poultry in 2005 [5]. Additionally, the European Commission initiated a referral procedure to promote the prudent use of antibiotics across the EU in 2009. China completely banned the use of ofloxacin, norfloxacin (NOR), pefloxacin (PEF), and lomefloxacin (LOM) in livestock in 2015.
Sample pretreatment is a key step when determining the levels of FQ residues in environmental and food samples. FQs should be effectively extracted and purified before instrumental analysis. To date, many sample pretreatment techniques, including solid-phase extraction [6], liquid–liquid extraction [7], matrix solid-phase dispersion [8], solid-phase microextraction [9], magnetic solid-phase extraction (MSPE) [10], and stir bar sorptive extraction [11], have been used to extract FQs from drinking water, food, and environmental samples. Among these extraction techniques, MSPE has attracted special interest in recent years due to its easy separation and quick adsorption. In typical MSPE, these adsorbents are first dispersed in the sample solution. The extraction is usually conducted using vortexing, shaking, or ultrasonication of the dispersion system. After separation by an external magnet, the sorbent is collected and washed. Finally, the analytes adsorbed on the sorbent are eluted for further analysis. This technique has been widely applied in the pretreatment of environmental, biological, and food samples [12, 13].
The nature of the sorbent is the most important influence on the performance of MSPE. An ideal MSPE adsorbent should have high-affinity interactions with the target compounds, relatively high magnetic strength, and high specific surface area and pore volume. Many types of nanomaterials, such as nanotubes, graphene [14, 15], molecularly imprinted polymers [16], and metal–organic frameworks [17], have been magnetized by hybridization with Fe3O4 nanoparticles for the determination of FQs. Ordered mesoporous molecular sieves are an attractive candidate sorbent for MSPE as well. MCM-48, a unique mesoporous molecular sieve material, has a highly interwoven and branched structure. The regular pore network of MCM-48 should provide more favorable mass transfer kinetics than the unidirectional pore systems of other molecular sieves. In the work reported in this paper, a magnetic mesoporous nanocomposite (Fe3O4@MCM-48) was synthesized via a combination of solvothermal and self-assembly methods. The synthesized Fe3O4@MCM-48 nanocomposite was then used as a sorbent for MSPE of eight FQs. The adsorption and desorption factors that affected the extraction process were optimized in detail. Finally, an analytical method was developed and validated for quantifying FQs in milk, meat, and water samples.
Experimental
Reagents and materials
Iron(III) chloride hexahydrate (99%) (FeCl3•6H2O), ethylene glycol, ethylalcohol, sodium hydroxide (NaOH), and trifluoroacetic acid (TFA) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium acetate trihydrate (NaAc) and cetyltrimethylammonium bromide (CTAB) were obtained from Kermel Chemical Reagent Company (Tianjin, China). Tetraethyl orthosilicate (TEOS) and sodium laurate (SL) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Deionized water (18.2 MΩ cm−1) obtained from a Millipore Milli-Q system (Millipore, Bedford, MA, USA) was used to prepare aqueous solutions. Eight FQs, including ofloxacin (OFL), norfloxacin (NOR), ciprofloxacin (CIP), enrofloxacin (ENR), difloxacin (DIF), sarafloxacin (SAR), pefloxacin (PEF), and danofloxacin (DAN), were used as target analytes (see Table S1 in the ““Electronic supplementary material,” ESM). Standard solutions of FQs were purchased from AccuStandard (New Haven, CT, USA). All these mixtures were stored in a refrigerator at 4 °C. Methanol, formic acid, and acetonitrile were obtained from Tedia Company Inc. (Fairfield, OH, USA). All reagents were of analytical grade.
Instrumentation
The morphologies of the sorbent materials were observed via scanning electron microscopy (SEM) (SWPRATM55, Carl Zeiss Micro Imaging Co., Ltd., Jena, Germany) and transmission electron microscopy (JEM-2010, JEOL Ltd., Tokyo, Japan). The elemental compositions were recorded via energy-dispersive X-ray spectroscopy (EDS) coupled with SEM. Fourier transform infrared (FTIR) spectra were obtained on a Nicolet Magna 750 FTIR spectrometer (Thermo Scientific, Waltham, MA, USA). Powder X-ray diffraction (PXRD) patterns were acquired at room temperature (298 K) on a SMART APEX CCD-based diffractometer (Bruker, Karlsruhe, Germany). The magnetization curves were obtained on an MPMS-SQUID-VSM (Quantum Design, San Diego, CA, USA). The Brunauer–Emmett–Teller specific surface areas and the pore distributions of the materials were measured using an ASAP 2020 porosimeter (Micromeritics, Norcross, GA, USA). An AB Sciex (Framingham, MA, USA) QTrap®5500 mass spectrometer equipped with a Thermo UltiMate 3000 liquid chromatography system was used to quantify the FQs in extracts.
Synthetic procedure
Synthesis of Fe3O4
The synthetic procedure for the Fe3O4@MCM-48 nanocomposite is shown in Fig. 1. The Fe3O4 magnetic nanoparticles were synthesized by a solvothermal method reported previously [18]. FeCl3•6H2O (1.35 g) was first dissolved in ethylene glycol (50 mL) to form a homogeneous solution under ultrasonication. NaAc (3.6 g) was added subsequently. After being completely dissolved by ultrasonication, the mixture was placed into a stainless steel autoclave and heated at 200 °C for 6 h. After the autoclave had cooled to room temperature, a black product was obtained, which was washed several times with ethanol and ultrapure water and dried at 50 °C for several hours.
Synthesis of Fe3O4@MCM-48
The Fe3O4@MCM-48 nanocomposite was prepared using self-assembly technology [19]. Fe3O4 nanoparticles (0.1 g) and ultrapure water (45 mL) were added to a 100-mL round-bottom flask. Following the complete dissolution of the mixture under ultrasonication, a mechanical agitator was started. CTAB (2.35 g), SL (0.19 g), and NaOH (0.86 g) were added to the solution under vigorous mechanical stirring. After the mixture had been stirred for 15 min, TEOS (8 mL) was added slowly. Afterward, the mixture was transferred to a 100-mL Teflon-lined steel autoclave and heated at 100 °C for 72 h in an oven. The obtained product was washed several times with ethanol and dried at 60 °C for several hours. Finally, the dry product was calcined at 400 °C for 2 h under a nitrogen atmosphere.
Extraction procedure
A 20-mL aliquot of the sample solution (or diluted extract) was placed into a 50-mL plastic tube. Next, 30 mg of sorbent were directly added to the sample solution. The tube was then put into an ultrasonic bath and ultrasonicated at 30 °C for 20 min. The sorbent was collected by placing an external magnet on the side of the tube. After removing the aqueous solution, 2 mL of 5% formic acid–methanol were added to the tube as eluent. The tube was placed in an ultrasonic bath under ultrasonication for 5 min. The eluent was concentrated to near-dryness using a gentle stream of nitrogen at 25 °C. The obtained residue was redissolved in 200 μL, and 10.0 μL were used for HPLC-MS/MS analysis. Sorbent regeneration was achieved by sequentially washing in 5% formic acid–methanol and water under ultrasonication (2 mL) for 5 min before the next MSPE application.
Real sample preparation
Drinking water samples were obtained from purified drinking water. Milk and meat samples were purchased from a local supermarket in Jinan, Shandong Province. Pre-extraction of FQs from milk and meat samples was carried out according to previously reported methods [15, 20]. One milliliter of milk (spiked) was accurately measured into the centrifuge tube. TFA (1.0 mL) was then added to the milk sample for protein precipitation and fat removal. The mixed solution was vortexed for 2.0 min and subsequently centrifuged at 8000 rpm for 5 min. Finally, the supernatant was transferred to a 50-mL tube and diluted with phosphate-buffered saline solution (PBS, pH 6.0) to 20 mL for MSPE use. Each pork or fish muscle sample was first minced and homogenized; a 1-g sample was then accurately weighed into a 50-mL plastic centrifuge tube. The FQs in the samples were extracted under ultrasonication for 30 min with 2 mL ACN in an ultrasonic bath and then centrifuged at 8000 rpm for 10 min. The supernatant was collected and passed through a 0.45-μm nylon filter for cleanup. The eluate was concentrated to dryness using a stream of N2 at 25 °C and dissolved to 20 mL with deionized water for MSPE use.
HPLC–MS/MS analysis
The target FQs were separated by a Unitary C18 column (150 mm × 2.1 mm, i.d. 5 μm, Acchrom, Beijing, China). The binary mobile phases were acetonitrile (A) and 0.1% formic acid in water (B). The gradient elution mode was as follows: 0–3 min = 80% B; 3–6 min = 80% B to 10% B; 6–8 min = 10% B to 80% B; and 8–13 min = 80% B. The column temperature was 35 °C. The flow rate was set at 0.3 mL min−1. The injection volume was 10 μL. The mass spectrometer was operated in positive electrospray ionization (ESI) and multiple reaction monitoring (MRM) modes. The MRM transition (precursor ions → product ions) and collision energy are presented in Table S2 (see the ESM). The source temperature was 550 °C and the ESI voltage was 5500 V. Nitrogen was used as ion source nebulizing gas (gas 1, 45 psi) and auxiliary gas (gas 2, 50 psi).
Results and discussion
Interactions between the FQs and Fe3O4@MCM-48
The physicochemical properties of the magnetic molecular sieve material (Fe3O4@MCM-48) were characterized by scanning electron microscopy, energy-dispersive spectroscopy, magnetic hysteresis loop measurements, transmission electron microscopy, powder X-ray diffraction, N2 adsorption–desorption analysis, and Fourier transform infrared spectroscopy (see Fig. S1 in the ESM). The nanocomposite has advantages that include easy separation with a magnetic core, high specific surface area, and mesoporous structure, which fulfill the requirements of a candidate material for MSPE. As shown in Fig. 2, multiple types of interactions, including π–π, hydrophobic, and hydrogen-bonding interactions, should occur between the FQs and Fe3O4@MCM-48. The adsorption of FQs onto the Fe3O4@MCM-48 may occur on the outer surface or within the channels of the sorbent. The carboxyl groups of FQ molecules may form hydrogen-bonding interactions with hydroxyl groups that are exposed on the surface of the Fe3O4@MCM-48. The molecular sieve shell MCM-48 has a multi-mesoporous structure with an average pore diameter of approximately 4.1 nm, which is larger than the molecular sizes of FQs, meaning that they can easily enter the inner channels of the sorbent. The π electrons in the molecular sieve shell can form a conjugated π–π network with the aromatic rings and heterocyclic rings of FQs. FQs contain a hydrophilic piperazine ring and hydrophobic fluorine atoms, meaning that there are also hydrophobic interactions at the surface that improve the FQ affinity of the material.
Parameter optimization
The effects of MSPE parameters such as the extraction temperature, extraction time, and pH of the sample solution were systematically investigated. Experiments were carried out using a FQ-spiked purified water sample. MSPE was used to extract trace-level target analytes from the sample solution. Therefore, the concentration of FQs was maintained constant at 0.1 μg L−1 during the optimization process. The initial elution utilized 5 mL of a formic acid–methanol mixture (5:95, v:v) under ultrasonication for 5 min. The adsorption capacity of the sorbent was kept constant. Therefore, the amount of sorbent was optimized separately to minimize the consumption of sorbent and avoid a long sorbent collection time. The recoveries of FQs exceeded 90% when the amount of sorbent was 30 mg (Fig. 3a). Therefore, 30 mg sorbent were selected for use in subsequent experiments.
a Effect of the amount of sorbent on the adsorption of eight FQs. Initial elution was performed using 5 mL of a formic acid–methanol mixture (5:95, v:v) under ultrasonication for 5 min. b Estimated response surface from a Box–Behnken design for the mean peak area of eight FQs, as obtained by plotting extraction temperature versus extraction time. Initial elution was carried out with 5 mL of a formic acid–methanol mixture (5:95, v:v) under ultrasonication for 5 min. c Effects of different desorption solvents on the extraction efficiencies of selected FQs in water. d Effect of the volume of desorption solvent on the extraction efficiencies of selected FQs in water
The other parameters, including extraction temperature, extraction time, and the pH of the sample solution, were further optimized by a response surface methodology using a Box–Behnken design [20]. The initial elution was carried out using 5 mL of a formic acid–methanol mixture (5:95, v:v) under ultrasonication for 5 min. The experimental matrix is listed in Table S3 (see the ESM). The peak area of the eight FQs was selected as the response, and Fig. 3b shows the 3D response surface graph. An extraction time in the range of 10–60 min was considered. The response of the FQs increased with time until 20 min, after which there was no apparent variation. Therefore, an extraction time of 20 min was sufficient to ensure a high extraction efficiency. The response to the FQs continued to increase with extraction temperature up to 30 °C, above which it declined. Thus, we selected 30 °C as the optimum extraction temperature. The optimal pH of the solution was approximately 6, and the peak area increased as the pH decreased below 6. However, when the pH was above 6, the peak area decreased with increasing pH (see Fig. S2 in the ESM). This behavior can be explained by considering the interaction between the magnetic sorbent and the analytical targets. The FQs have pKa values in the range 5.66–8.56 [21,22,23], indicating that the FQs were protonated at low pH values (< 5.66) and were negatively charged when the pH exceeded 8.56. Under alkaline conditions, the hydroxyl groups on the sorbent and carboxyl groups in the FQ molecules may have been deprotonated. Additionally, the surfaces of the sorbent and the target analytes were negatively charged. Electrostatic repulsion between the adsorbate and sorbent resulted in decreased extraction efficiency. At the optimal pH value of approximately 6, the FQs achieved an overall neutral state in the zwitterionic form, which may have maximized the interactions between the FQs and Fe3O4@MCM-48, optimizing the extraction efficiency.
The elution conditions, including the type and volume of solvent, were studied. Methanol, acetone, acetonitrile, and dichloromethane as well as ammonium–methanol (5:95, v:v) and formic acid–methanol (5:95, v:v) mixtures were used as elution solvents. Moreover, the tube was placed in an ultrasonic bath for 5 min. As shown in Fig. 3c, the formic acid–methanol mixture gave the best desorption performance. Eluent volumes of 0.5-4 mL were evaluated to determine the minimum volume of solvent that could be used without negatively impacting the desorption performance. The FQs were almost completely desorbed by 2 mL of the 5% formic acid–methanol mixture (Fig. 3d).
In summary, the optimal working parameters and conditions were as follows: extraction time, 20 min; extraction temperature, 30 °C; solution pH, 6; amount of sorbent, 30 mg; and elution using 2 mL of the formic acid–methanol mixture (5:95, v:v) under ultrasonication for 5 min.
Method evaluation
Under the optimal conditions, the analytical methodology was established and evaluated using spiked purified water samples. The analytical performance of the Fe3O4@MCM-48-based MSPE/HPLC-MS/MS method is summarized in Table 1. The limits of detection (LODs, S/N = 3) and limits of quantification (LOQs, S/N = 10) for the eight FQs were in the ranges 0.7–6.0 ng L−1 and 2.5–20 ng L−1, respectively. A five-point external calibration method (5.0, 10, 50, 200, and 1000 ng L−1) was used. The linear ranges varied from 5–1000 ng L−1 to 20–1000 ng L−1, with correlation coefficients (r) of > 0.99 for the selected FQs. The precision of the method was evaluated based on the intraday (n = 6) and interday (3 days, n = 6) reproducibilities; the relative standard deviations (RSDs, n = 6) were in the ranges 2.9–5.8% and 3.2–8.2%, respectively. The analytical method was also validated for real samples, including fish, pork, milk, and drinking water samples. Matrix-matched calibration curves (five points) were derived by spiking real sample extracts at relevant FQ concentrations. Linearities, LODs, LOQs, and precision data were also obtained and are shown in Table 2. Good linearity (r > 0.99) and satisfactory precision (RSD% < 10%) were observed for all the FQs. The regeneration and reusability of the sorbent were also tested by performing continuous MSPE cycles from spiked water samples. The sorbent could be reused at least five times with a tolerable loss (5%) of extraction performance for purified water samples. However, it is important to note that the reusability of the sorbent also strongly depends on the complexity of the matrix to which it was applied. The reusability of the sorbent should be carefully retested to avoid carryover effects. Typical extracted ion chromatograms of FQs in drinking water samples are shown in Fig. 4. The absence of a matrix interference peak in the chromatogram demonstrates the specificity of the proposed methodology.
In principle, the presence of matrix components in the final extract should affect the electrospray ionization process for the analytes as compared to when the process is carried out for the analytes in pure solvent. To evaluate matrix effects, five concentration levels (20, 50, 100, 500, and 1000 ng L−1) of FQs were analyzed in the extracts of blank food samples (milk, fish, and pork meat) and in pure solvent. Slope ratios for the eight FQs were obtained by comparing the calibration slopes obtained with the matched matrix with the calibration slopes obtained with the pure solvent. Matrix effects were evaluated for the food samples. For the milk samples, the slope ratios were 0.86, 0.96, 0.87, 0.97, 0.91, 0.98, 0.95, and 0.92 for OFL, NOR, CIP, ENR, DIF, SAR, PEF, and DNA, respectively. For the fish samples, the slope ratios were 0.90, 0.85, 0.84, 0.86, 0.97, 1.03, 0.88, and 0.91 for OFL, NOR, CIP, ENR, DIF, SAR, PEF, and DNA, respectively. For the pork meat samples, the slope ratios were 0.81, 0.84, 0.83, 0.83, 0.89, 0.87, 0.83, and 0.85 for OFL, NOR, CIP, ENR, DIF, SAR, PEF, and DNA, respectively. Based on a previous report, signal suppression or enhancement effects are tolerable if the slope ratio is between 0.8 and 1.2 [24]. For the drinking water samples, the analytical parameters had similar values to those obtained with purified water. Therefore, no significant matrix effects for the eight FQs were observed in this study, which confirms the efficacy of the MSPE sorbent.
Other sorbent materials, including MIPs [5, 8], polymerized ionic liquid [25], oxidized MWCNTs [14], graphene [15], and phenyl-C8 [17] have been used as sorbents for the MSPE of FQs from various matrices. A comparison of the developed analytical method with other reported methods is shown in Table S4 (see the ESM). For the water samples, the sensitivity of the developed method was similar to or higher than those of the other methods. The FQ recoveries obtained using Fe3O4@MCM-48 were generally higher than those obtained using MIPs and graphene nanocomposites. The precisions (RSD, %) of the methods were lower than 13%. As shown in Table S4 of the ESM, in terms of sensitivity, precision, and accuracy, the analytical performance of the method developed in this work is comparable or superior to some of the reported methods.
Application to real samples
The method was further applied to the detection of FQs in drinking water and food samples. Food samples (e.g., milk, pork, and fish) that possibly contained residues of quinolone antibiotics were selected and analyzed using the developed method. The analytical results for these real samples are listed in Table 3. No FQs were detected in the drinking water and milk samples. The spiked recoveries were in the ranges 82.5–112.5% and 75.0–88.3% for drinking water and milk samples, respectively. OFL and ENR were detected at concentrations of 1.43 and 8.60 μg kg−1, respectively, in the pork samples and 0.72 and 2.50 μg kg−1, respectively, in the fish samples. These values are far below the maximum residue limits (MRLs) defined in the Official Journal of the European Union, which states that the MRL of ENR is 100 μg kg−1 in animal muscle. The recoveries obtained from the spiked meat samples ranged from 75.3% to 104.7%. These satisfactory results demonstrate that the proposed method of sample pretreatment and analysis is suitable for determining FQs in real food samples.
Conclusions
In this work, a magnetic mesoporous nanocomposite, Fe3O4@MCM-48, was successfully synthesized and applied as an adsorbent material for the high-efficiency MSPE of FQs from water and food samples. By applying this adsorbent in combination with HPLC-MS/MS, an analytical method was developed and evaluated for the analysis of FQs in water and food samples. Moreover, the MSPE performance of the magnetic molecular sieve sorbent was compared with those of other sorbent materials. The developed method is simple, rapid, efficient, and inexpensive. The magnetic molecular sieve adsorbent has the potential for broad application in the extraction of antibiotics from complex matrices.
Change history
07 January 2021
A Correction to this paper has been published: <ExternalRef><RefSource>https://doi.org/10.1007/s00216-020-03139-w</RefSource><RefTarget Address="10.1007/s00216-020-03139-w" TargetType="DOI"/></ExternalRef>
References
Gouvêa R, Santos FD, Aquino MD, Pereira VDA. Fluoroquinolones in industrial poultry production, bacterial resistance and food residues: a review. Rev Bras Cienc Avic. 2015;17:1–10.
Riaz L, Mahmood T, Khalid A, Rashid A. Fluoroquinolones (FQs) in the environment: a review on their abundance, sorption and toxicity in soil. Chemosphere. 2018;191:704–20.
Amorim CL, Maia AS, Mesquita RBR. Performance of aerobic granular sludge in a sequencing batch bioreactor exposed to ofloxacin, norfloxacin and ciprofloxacin. Water Res. 2014;50:101–13.
Pan Z, Peng J, Chen Y, Zang X. Simultaneous determination of five fluoroquinolones by the selective high performance liquid chromatography associating with sensitive resonance light scattering and mechanism study. Microchem J. 2018;136:71–9.
Urraca JL, Castellari M, Barrios CA, Moreno-Bondi MC. Multiresidue analysis of fluoroquinolone antimicrobials in chicken meat by molecularly imprinted solid-phase extraction and high performance liquid chromatography. J Chromatogr A. 2014;1343:1–9.
Batt AL, Aga DS. Simultaneous analysis of multiple classes of antibiotics by ion trap LC/MS/MS for assessing surface water and groundwater contamination. Anal Chem. 2005;77:2940–7.
Yang ZJ, Qin WD. Separation of fluoroquinolones in acidic buffer by capillary electrophoresis with contactless conductivity detection. J Chromatogr A. 2009;1216:5327–32.
Sun X, Wang J, Li Y, Yang J, Jin J. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J Chromatogr A. 2014;1359:1–7.
Liu X, Wang XC, Tan F, Zhao HX, Quan X, Chen JW, et al. An electrochemically enhanced solid-phase microextraction approach based on molecularly imprinted polypyrrole/multi-walled carbon nanotubes composite coating for selective extraction of fluoroquinolones in aqueous samples. Anal Chim Acta. 2012;727:26–33.
Wang Q, Wang Y, Zhang ZZ, Tong Y, Zhang L. Waxberry-like magnetic porous carbon composites prepared from a nickel-organic framework for solid-phase extraction of fluoroquinolones. Microchim Acta. 2017;184:4107–15.
Fan WY, He M, Wu XR, Chen BB, Hu B. Graphene oxide/polyethyleneglycol composite coated stir bar for sorptive extraction of fluoroquinolones from chicken muscle and liver. J Chromatogr A. 2015;1418:36–44.
Huo SH, Yan XP. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst. 2012;137:3445–51.
Gu ZY, Chen JQ, Jiang JQ, Yan XP. Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Chem Commun. 2011;47:4787–9.
Herrera-Herrera AV, Ravelo-Pérez LM, Hernández-Borges J, Afonso MM, Palenzuela JA. Oxidized multi-walled carbon nanotubes for the dispersive solid-phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J Chromatogr A. 2011;1218:5352–61.
He X, Wang GN, Yang K, Liu HZ, Wu XJ. Magnetic graphene dispersive solid phase extraction combining high performance liquid chromatography for determination of fluoroquinolones in foods. Food Chem. 2017;221:1226–31.
Rodríguez E, Navarro-Villoslada F, Benito-Peña E, Marazuela MD, Moreno-Bondi MC. Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography. Anal Chem. 2011;83:2046–55.
Ibarra IS, Rodriguez JA, Páez-Hernández ME, Santos EM, Miranda JM. Determination of quinolones in milk samples using a combination of magnetic solid-phase extraction and capillary electrophoresis. Electrophoresis. 2012;33:2041–8.
Anbia M, Khoshbooei S. Functionalized magnetic MCM-48 nanoporous silica by cyanuric chloride for removal of chlorophenol and bromophenol from aqueous media. JNSC. 2015;5:139–46.
Qiang ZM, Bao XL, Ben WW. MCM-48 modified magnetic mesoporous nanocomposite as an attractive adsorbent for the removal of sulfamethazine from water. Water Res. 2013;47:4107–41147.
Mei M, Huang X. Determination of fluoroquinolones in environmental water and milk samples treated with stir cake sorptive extraction based on a boron-rich monolith. J Sep Sci. 2016;39:1908–18.
Khodadoust S, Ghaedi M. Application of response surface methodology for determination of methyl red in water samples by spectrophotometry method. Spectrochim Acta A. 2014;133:87–92.
Ferdig M, Kaleta A, Vo TDT, Buchberger W. Improved capillary electrophoretic separation of nine (fluoro)quinolones with fluorescence detection for biological and environmental samples. J Chromatogr A. 2004;1047:305–11.
Jiménez-Lozano E, Marqués I, Barrón D, Beltrán JL, Barbosa J. Determination of pKa values of quinolones from mobility and spectroscopic data obtained by capillary electrophoresis and a diode array detector. Anal Chim Acta. 2002;464:37–45.
Frenich AG, Romero-González R, Gómez-Pérez ML. Martinez Vidal JL. Multi-mycotoxin analysis in eggs using a QuEChERS-based extraction procedure and ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. J Chromatogr A. 2011;1218:4349–56.
Wang N, Wang YF, Omer AM, Ouyang XK. Fabrication of novel surface-imprinted magnetic grapheme oxide-grafted cellulose nanocrystals for selective extraction and fast adsorption of fluoroquinolones from water. Anal Bioanal Chem. 2017;409:6643–53.
Su SC, Chang MH, Chang CL, Chang PC, Chou SS. Simultaneous determination of quinolones in livestock and marine products by high performance liquid chromatography. J Food Drug Anal. 2003;11:114–27.
Chen L, Zhang X, Xu Y, Du X, Sun X, Sun L, et al. Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2010;662:31–8.
Wu H, Shi Y, Guo X, Zhao S, Du J. Determination and removal of sulfonamides and quinolones from environmental water samples using magnetic adsorbents. J Sep Sci. 2016;39:4398–407.
Liu C, Liao Y, Huang X. Preparation of a boronic acid functionalized magnetic adsorbent for sensitive analysis of fluoroquinolones in environmental water samples. Anal Methods. 2016;8:4744–54.
Zheng HB, Mo JZ, Zhang Y, Gao Q, Ding J. Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A. 2014;1329:17–23.
Jin T, Wu H, Gao N, Chen X, Lai H. Extraction of quinolones from milk samples using bentonite/magnetite nanoparticles before determination by high-performance liquid chromatography with fluorimetric detection. J Sep Sci. 2016;39:545–51.
Kantiani L, Farré M, Barceló D. Rapid residue analysis of fluoroquinolones in raw bovine milk by online solid phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A. 2000;1218:9019–27.
Barreto F, Ribeiro CBD, Hoff RB, Costa TD. Development and validation of a high-throughput method for determination of nine fluoroquinolones residues in muscle of different animal species by liquid chromatography coupled to tandem mass spectrometry with low temperature clean up. J Chromatogr A. 2017;1521:131–9.
Acknowledgements
Financial support from the Natural Science Foundation of Shandong Province (ZR2017MB011), the Research Grant Council of the Hong Kong Special Administrative Region (Research Grant Direct Allocation refs. 3132667 & 4053152), and the Key R&D Program of Shandong Province (2017CXGC0223) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in regard to this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 574 kb)
Rights and permissions
About this article
Cite this article
Yu, H., Jia, Y., Wu, R. et al. Determination of fluoroquinolones in food samples by magnetic solid-phase extraction based on a magnetic molecular sieve nanocomposite prior to high-performance liquid chromatography and tandem mass spectrometry. Anal Bioanal Chem 411, 2817–2826 (2019). https://doi.org/10.1007/s00216-019-01726-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01726-0