Abstract
A unique waxberry-like magnetic hierarchical porous carbon hybrid was synthesized from a nickel based metal–organic framework (Ni-MOF) which, on calcination, produces hierarchical Ni/NiO nanocrystals covered with graphitic carbon (GrC). The material was applied to magnetic solid phase extraction (MSPE) of trace levels of four fluoroquinolones (FQs) from environmental water samples. It is shown to exhibit superior extraction capability for four FQs, because the novel 3D morphology provides an enhanced surface area along with transport channels, while Ni and graphitic carbon as potential adsorption sites are ideal components. The material with inherent magnetism is reusable and displays superb recycling stability for the adsorption and desorption (by using alkaline methanol) of FQs after 6 cycles. The FQs were quantified by HPLC with UV detection, and the limits of detection (at an S/N ratio of 3) range between 7.9 and 57 ng·L−1. Recoveries from spiked environmental water samples are between 88.8 and 103.7%.

A magnetic hierarchical porous carbon hybrid was prepared from a nickel-organic framework and applied to solid phase extraction of trace levels fluoroquinolones from environmental water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoroquinolones (FQs), as a kind of antibiotics, have been extensively used in clinical applications for human and veterinary diseases [1,2,3,4]. They can prevent the synthesis of bacterial DNA, because of the presence of F atom and piperazinyl group [5]. Compared with other antibiotics, FQs have lower solubility, bioavailability, biodegradation rates, and more likely to accumulate in sediment [6], they cannot be efficiently removed from environment water using traditional wastewater treatment processes. Unfortunately, they have been found in various real water samples, such as hospital sewage, treated sewage, and surface water bodies [7, 8]. FQs residues are making their way into food chains and reaching the consumers [9]. Therefore, monitoring FQs at trace levels in a simple and sensitive method is significant.
The environmental monitoring of FQs is a vital task, with regard to water system, usually the low level determination necessitate a pre-concentration process before analysis. Magnetic solid-phase extraction (MSPE), which has captured much attention for trace determination of multifarious analytes in consideration of its reduced consumption of organic solvents, low cost and high enrichment factors [10]. Magnetic enrichment material can be dispersed into the matrix directly and separated from samples by an external magnetic field without extra filtration or centrifugation, which makes the sample pretreatment faster and easier.
Magnetic carbon material, as an extraordinarily wonderful adsorbent with superb adsorption and recyclable properties, has been studied and applied extensively, such as Fe3O4NPs@GO [11], C3N4/Fe3O4 [12], mag-MWCNTs [13, 14], Fe3O4@PVBC@IDA-Ni [15] and Fe3O4@SiO2@C/N [16] were synthesized and used for pre-concentration environmental pollutants. Metal–organic frameworks (MOFs), as newly emerging porous materials with large surface area, low density and ultrahigh porosity, have attracted researchers attention and adopted for widespread applications in many fields, including sensing, luminescence, drug delivery, gas storage/separation and catalysis [17, 18]. In addition, Many efforts have led to the development of MOFs as templates/precursors to construct metal and/or metal oxide nanoparticles embedded in the carbon matrix, such as Fe3O4@MIL-101 [19], Fe@MOF-5 [20] and Fe3O4@MIL-100 [21] were applied to solid-phase extraction of organic contaminants. However, the above MOF-derived porous carbons are non-magnetic, and the magnetic properties (Fe3O4/ Fe) were reinvested by extra synthetic process, which was complex and least environmentally friendly. In this regard, a kind of MOF derived nanoporous carbons with inherent magnetism were synthesized, such as Zn-Co-ZIF nanoparticles for magnetic solid phase extraction of flunitrazepam [22]. As far as we know, Ni-MOF derived waxberry-like magnetic hierarchical porous carbon hybrid with high specific surface area used as adsorbent for solid-phase extraction was not reported.
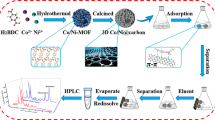
In this paper, a magnetic hierarchical porous carbon hybrid was synthetized from a nickel-based metal-organic framework (Ni-MOF) through pyrolysis process. The resulting hierarchical Ni/NiO nanocrystals are covered with a graphitic carbon (GrC) nanocomposite. Moreover, WMHP-Ni/NiO@GrC with excellent inherent magnetism, can be collected from the matrix by an external magnetic field, economically feasible and more efficient. Remarkably, WMHP-Ni/NiO@GrC nanocomposite as effective adsorbents exhibited satisfactory extraction performance in the simultaneous determination of four FQs by high performance liquid chromatography (HPLC). Furthermore, the MSPE method was successfully used for extraction of FQs residues in environmental water samples.
Experimental
Reagents and chemicals
Fluoroquinolones (FQs), including enrofloxacin (ENR, 98% purity), norfloxacin (NOR, 98% purity), lomefloxacin (LOM, 98% purity), and ciprofloxacin hydrochloride (CIP, 98% purity) were from Tianjin HEOWNS Biochem LLC (China, www.heowns.com), and their chemical structures were shown in Fig. S1. Humic acid (90% purity) were from Shanghai Aladdin Biotechnology Co., (China, www.aladdin-e.com). Trimesic acid (H3BTC, 98% purity), Nickel nitrate hexahydrate (Ni(NO3)2 •6H2O, 98% purity), polyvinylpyrrolidone K-30 (PVP), HPLC grade acetonitrile (ACN), N,N-dimethylformamide (DMF) and Other chemicals were analytical reagent and from Sinopharm Company (China, www.sinoreagent.com).
Instruments
Micro-structure of materials was investigated in a field emission scanning electron microscope (SEM, HITACHI SU8000, Japan). The crystalline phases in the composite were confirmed by X-ray diffraction (XRD, Siemens D5000, Germany). The BET surface area was estimated by N2 adsorption/desorption isotherm in a Micromeritics Tristar 3020 (Norcross, GA). Electronic state of ions of WMHP-Ni/NiO@GrC were analysed by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, USA). Raman spectras of composite were measured by Laser Confocal Micro-Raman Spectroscope (LabRAM XploRA, HORIBAJOBINYVONS.A.S, France). The FT-IR spectra were acquired on a Nicolet 330 FT-IR (Nicolet, USA). The zeta potential of adsorbent was taken with a zetasizer Nano-ZS90 apparatus (Malvern.UK). Vibrating sample magnetometer (VSM, Lakeshore 7407, USA) was used to measure the magnetic prosperities of WMHP-Ni/NiO@GrC in the field of ±20 KOe at room temperature. Quantitative analysis were completed using high performance liquid chromatography (HPLC, Simadzu Instruments Manufacturing, Co., Ltd., Japan). The pH values of solutions were measured by PHSJ-4F Model pH meter (Shanghai instrument electric science instrument, China).
Preparation of Ni-MOF precursor and WMHP-Ni/NiO@GrC
The Ni-MOF precursor was synthesized by hydrothermal method [23]. Firstly, 0.864 g Ni(NO3)2·6H2O, 0.3 g H3BTC, and 3 g PVP were dissolved in 60 mL of mixture solution (double-distilled water: ethanol: DMF = 1:1:1 v/v/v) and stirring constantly for 30 min. Secondly, the homogeneous suspension was poured into a 100 mL Teflon-lined autoclave and reaction for 10 h at 150 °C. At last the pale green products were washed with ethanol and dried in an oven for 12 h at 60 °C.
The pale green Ni-MOF precursor powders were heated to 450 °C with a ramp-rate of 1 °C·min−1 and maintained 30 min. The whole annealing process was carried out in a tube furnace at N2 atmosphere. The resulting black powders were WMHP-Ni/NiO@GrC nanocomposite. The synthesis processes was illustrated in Scheme 1.
Sample preparation
For evaluate the applicability of the method, two kinds of environment water samples were analyzed. River water sample was collected from Xinkai River (Shengyang) and Seawater sample was collected from Bayuquan Bay, these samples were stored below 4 °C after filtered through 0.45 μm nylon for later use.
MSPE procedures
The MSPE procedure was carried out as follows: 25 mL working standard solution with 25 mg WMHP-Ni/NiO@GrC were placed on a shaker at a stirring rate of 200 rpm and allowed to equilibrate for 10 min. After extraction, the WMHP-Ni/NiO@GrC was isolated from the solution using an external magnet. The target compounds were ultrasonically eluted with 1.0 mL of methanol/NaOH (1 mol· L−1) (4/6, v/v) for 10 min. Eluent was separated from the WMHP-Ni/NiO@GrC by a magnet and filtered through a 0.45 μm PTFE filter membrane for HPLC analysis. Each stock solution of four FQs were obtained by dissolving 0.5 g of drug into 0.1 mol·L−1 HCl (0.5 mL), then diluting to 500 mL with double-distilled water and refrigerated at 5 °C, 2 ppm of four FQs mixed solution were prepared as working standard solution. The extraction process was shown in Scheme 2.
Chromatography
The HPLC analyses were performed by a Shimadzu LC-16 HPLC system equipped with SPD-16 UV detector. Chromatographic separation of four FQs was performed on an ODS-SP-C18 (150 mm × 4.6 mm, 5 μm) column (Simadzu, Japan) and the injection volume was 20 μL. The mobile phase was a mixture of triethylamine phosphate (1.5‰, pH 3 ± 0.1)–acetonitrile (83/17, v/v) delivered at a flow rate of 1.0 mL·min−1, and the detection wavelength was 276 nm, column temperature was 30 °C.
Results and discussion
Characterization of adsorbent
SEM analysis was carried out to observe the morphological characteristics of the Ni-MOF precursor and WMHP-Ni/NiO@GrC. As shown in Fig. 1a, the Ni-MOF precursor exhibits obvious 3D waxberry-like structure with a submicrometer size of 3 μm, magnification of the Ni-MOF precursor was shown in Fig. 1b, the spinals grown on the surface of the sphere with an average diameter of less than 100 nm. After calcination treatment, the organic linker in the MOF scaffold would be decomposition and carbonization to produce the corresponding carbons, along with hierarchical NiO/Ni nanocrystals produced and covered with the carbons shell, and the final WMHP-Ni/NiO@GrC composites maintained the original micro-spherical hierarchical structure of the Ni-MOFs precursor (Fig. 1c). In addition, as shown in Fig. 1d, the magnetic properties of WMHP-Ni/NiO@GrC analyzed by room temperature VSM with −20.0 kOe≤H≤20.0 kOe field. The value of saturation magnetization (Ms) is about 33.71 emu·g−1 (inset: the photo of magnetic separation), which was enough for magnetic separation.
The phase purity of Ni-MOF precursor and WMHP-Ni/NiO@GrC were studied using X-ray diffraction (XRD) patterns. As shown in Fig. 2a, the Ni-MOF precursor is still retains the original structure. After calcination, the organic linker undergo decomposition and carbonization, the metal ions (Ni2+) transform to NiOx or Ni. WMHP-Ni/NiO@GrC display obvious XRD diffraction patterns, the peaks at the 2θ values of 44.88° and 52.16° are correspond to (111) and (200) crystal planes of cubic Ni (JCPDS 4–0850), and the peaks at 44.53° and 76.63° are correspond to (200) and (220) crystal planes of NiO (JCPDS No. 78–0429), which are in agreement with other references of Ni-MOF [23]. Furthermore, an additional broad peak appeared in WMHP-Ni/NiO@GrC at nearly 25°, which can explain the presence of graphitic carbon [24]. No other phases appeared at XRD diffraction pattern, demonstrates the high purity of the sample.
XPS was used to further investigate the chemical compositions and the bonding states of the WMHP-Ni/NiO@GrC. The results prove the presence of C, O, and Ni elements. As shown in C 1 s spectra (Fig.S2A), a dominant peak at 284.6 eV ascribe to C-C bonds and relate to the sp2 hybridized graphite-like carbon atom in graphene [17], several weak peaks are assigned to C-O (286.9 eV), and C = O (288.5 eV). The O 1 s spectra (Fig.S2B) show that the peak at 533.8 eV is assigned to C-O bond, the binding energy peak at 532.4 eV and 530.1 eV are contributed to the presence of C=O bond, and metal–oxygen (Ni-O) bond, also suggests that the presence of lattice oxygen and oxygen-containing groups in WMHP-Ni/NiO@GrC. Likewise, the Ni 2p spectra [25] (Fig.S2C), reveal two major peaks centred at around 870.1 and 852.8 eV with a spin-energy separation of 17.3 eV, contribute to the presence of Ni 2p1/2 and Ni 2p3/2, which are characteristic peaks of Ni2+, other two peaks centred at around 874.3 and 856.8 eV corresponded to Ni NPs, which are in good agreement with previous XRD result.
The graphitic structures of Ni-MOF precursor and WMHP-Ni/NiO@GrC were further examined by Raman spectroscopy. As shown in Fig. 2b, the Ni-MOF precursor is not display obvious, intense characteristic Raman band. In turn, WMHP-Ni/NiO@GrC show two characteristic Raman bands belong to graphitic carbon: D band and G band. The result is consistent with XRD diffraction patterns [26].
According to the nitrogen adsorption − desorption isotherm measurements (Fig.S3), the surface area of WMHP-Ni/NiO@GrC is 110 cm2·g−1, and pore-size is mainly distributed around 12 nm. The large surface area, and abundant porosity, enhances the contact area and channel between adsorbent and the analytes, which are favorable for the extraction.
Optimization of method
For achieving the satisfied extraction efficiency, the following parameters were optimized: (a) Sample pH value; (b) Absorbent amount; (c) ionic strength (d) shake time. Respective data and Figures are given in the Electronic Supporting Material. We found the following experimental conditions to give best results: (a) A sample pH value of pH 6.0 (Fig.S4A); (b) WMHP-Ni/NiO@GrC amount of 25 mg (Fig.S4B); (c) no NaCl was added (Fig.S4C); (d) shake time of 10 min (Fig.S4D).
Interaction mechanism for the adsorption of FQs on WMHP-Ni/NiO@GrC
FQs are ampholyte compounds, the pKa values of the four FQs ranging from 8.30 to 8.89 was for the piperazinyl group and from 5.90 to 6.23 was for the carboxylic functions [27], the existing forms of FQs were influenced by the pH value of solution. When solution pH was below 6.0, FQs existed as a cation, at pH between 6.2 and 8.8, existed primarily in intermediate forms no net electrical charge and anion from solution pH greater than 8.8. Combining the study of zeta potential, as shown in Fig. 3a, the isoelectric point of WMHP-Ni/NiO@GrC is 4.0. WMHP-Ni/NiO@GrC present negative charges while pH > pHpzc, FQs exist in cationic forms while the pH value from 2.0 to 6.0 [28]. Therefore, it would result in electrostatic interactions between cationic forms FQs with negative charge on the surface of WMHP-Ni/NiO@GrC. In order to further investigate the interaction mechanism between FQs and WMHP-Ni/NiO@GrC, FT-IR spectra was studied. As shown in Fig. 3b, the new peak on curve c at 1715 cm−1 is assigned to carbonyl group of ketone and peaks from 2800 to 2975 cm−1 are assigned to C-H band stretching vibration of alkane, indicating that WMHP-Ni/NiO@GrC effectively adsorbed FQs. The characteristic peak of -OH is broadened and weaken, and exhibited shifts from 3442 to 3426 cm−1, suggested that FQs was attached to WMHP-Ni/NiO@GrC through H-bonding. The bands assigned to vibrational stretching frequencies of C = C at 1637 and 1618 cm−1 are shifted to 1627 and 1605 cm−1 [29], which demonstrated the π-π conjugate effect between FQs and WMHP-Ni/NiO@GrC are presented in the adsorption process and influenced the electronic cloud distribution of the molecule. In addition, FQs contained hydroxyl functional groups and amide groups, WMHP-Ni/NiO@GrC contained abundant Ni (II) sites, the characteristic peak of N-H at 3417 cm−1 on curve c disappeared confirmed that the interactions between FQs and WMHP-Ni/NiO@GrC become possible through the coordinated covalent bonds [30]. Therefore, it can be deduced the interaction mechanism between FQs and WMHP-Ni/NiO@GrC was hydrogen bond, complexation, π–π interaction and electrostatic interaction.
Desorption conditions
The type of eluent directly affects the recovery of analytes. In order to obtain higher recovery, several eluents (methanol, acetonitrile, ethanol, acetone, methanol/5% NH3·OH, methanol/NaOH (1 mol·L−1) (6/4, v/v), methanol/HCOOH (9/1, v/v), methanol/HCl (1 mol·L−1) (5/5, v/v)) were investigateds. As shown in Fig. 4A, methanol/NaOH (1 mol·L−1) (6/4, v/v) exhibited the best desorption efficiency, which were in accordance with the pH result. Hence, methanol/NaOH (1 mol·L−1) (6/4, v/v) was chosen as the eluent.
a Effect of the desorption solvent on the recoveries of four FQs (absorbent amount: 25 mg; FQs solution concentrations: 2 mg·L−1; solution volume: 25 mL; extraction time: 10 min). (b) HPLC-UV Chromatograms obtained at 276 nm. (a) Blank river water sample after pretreatment by MSPE; (b) The spiked river water sample (50.0 μg·L−1) after pretreatment by MSPE. (WMHP-Ni/NiO@GrC: 25 mg; FQs concentrations: 2 mg·L−1; sample volume: 25 mL)
In order to totally desorbing the FQs from the adsorbent and achieve satisfactory recoveries, the influence of desorption time and eluent volume on desorption efficiency were investigated. The volume of eluent was investigated ranging from 0.5 to 5.0 mL. Results showed most of the FQs can be desorbed from the sorbent with 1 mL of eluent. The influence of desorption time was investigated, and the recoveries of the FQs evidently increased with increasing desorption time from 5 to 10 min, while maintained with time elapsing. Thus, 1 mL methanol/NaOH (1 mol·L−1) (6/4, v/v) eluent and 10 min of desorption time were chosen for the further experiments.
Interference and regeneration studies
In order to evaluate the influence of co-existing ions and organic matter, Cl−, HCO3 −, PO4 3−, NO3−, Mg2+, Cu2+, Cu2+, Zn2+, Ni2+, Pb2+, Na+, K+, and humic acid were selected as possible interfering ions. The results indicated that no obvious changes in recoveries of four FQs were found after added the interfering ions (0–10 mg·L−1) and humic acid (3 mg·L−1) into the samples, proving that the potential application of WMHP-Ni/NiO@GrC as adsorbents for magnetic solid-phase extraction of four FQs in environment water samples.
To test the regeneration of WMHP-Ni/NiO@GrC, six times adsorption/desorption cycles were carried out. The result illustrated the WMHP-Ni/NiO@GrC can be used for six cycles with lost less than 9.8% of its recovery on average, indicated that this magnetic adsorbent was stable and durable during the MSPE procedure.
Method validation
Enrichment factor (EF) is an important indicator to measure the sensitivity of method, and it can be calculated by the following equations:
where c a and c s are the concentrations of the target compounds in eluent and sample solutions, respectively.
The effect of sample volume on quantitative analysis of the four FQs was investigated in the range from 25 to 250 mL with the constant amount of each target analytes (20 μg). Then, the MSPE procedure was performed under the optimum conditions, and the extraction efficiencies of four FQs ranged from 86.1 to 95.5% with the sample volumes up to 200 mL. When the sample volume was above 200 mL, the extraction efficiency was reduced to less than 80%. Combining on the result of eluent volume experimentation, it was found that the EF is about 200 under optimal conditions, shown excellent pre-concentration ability.
The linearity and intra-day and inter-day precision of the method were evaluated under the optimal conditions. The calibration curves were obtained by plotting the peak area of FQs spiked in aqueous solutions versus the concentrations (NOR: y = 7,303,760x + 30,365 (r = 0.9928); CIP: y = 9,175,330x + 32,429 (r = 0.9986); LOM: y = 5,189,720x + 8489 (r = 0.9983); ENR: y = 8,139,230x + 51,669 (r = 0.9977)), and other results were summarized in Table 1. Satisfying linearity was got in the range of 0.10–500.00 μg·L−1 for four FQs, and the RSDs were more than 0.9928. The limit of detection (LOD) (S/N = 3) and limit of quantification (LOQ) (S/N = 10), were in the range of 7.9–57 ng·L−1 and 26–189 ng·L−1, respectively.
The intra-day and inter-day precisions of the MSPE method were evaluated by measuring different concentrations of FQs at 50, 100, and 300 μg·L−1. Six-parallel experiments in a day obtained the intra-day RSDs, while the inter-day RSDs were obtained by the detection during three consecutive days. The results indicated that the intra-day and inter-day RSDs were below 7.92 and 9.47%, indicating the satisfying repeatability was obtained.
Sample analysis
The method was further used for the analysis of FQs in real samples (river water and sea water). As listed in Table 2, the average recoveries were 88.8–103.7% with the RSD less than 5.63. Figure 4B shows the chromatograms of samples without or with spiked standard solution, no significant interference peaks are found at the retention time of FQs.
Comparison with other reported method
The comparative study result of this work to other works was listed in Table 3. It indicated that the present method showed high sensitivity, wide linearity range and satisfactory recoveries. In addition, this work showed small solvent consumption, eco-friendly, and low cost. Thus, the MSPE method is better for the extraction and determination of FQs in real samples.
Conclusion
In this study, novel Waxberry-like magnetic hierarchical porous carbon hybrid (WMHP-Ni/NiO@GrC) was synthesized and used as MSPE adsorbent for the simultaneous determination of four FQs by HPLC with UV detection. The MSPE method achieved effective extraction efficiency, short extraction times, high enrichment effects, and low detection limits. It has been successfully applied for the analysis of trace FQs in environmental water samples, demonstrates a fantastic application potential for the determination of FQs, and the feasibility of using this procedure for determination FQs in other complex sample is still underway.
References
Marouzi S, Sharifi Rad A, Beigoli S, Teimoori Baghaee P, Assaran Darban R, Chamani J (2017) Study on effect of lomefloxacin on human holo-transferrin in the presence of essential and nonessential amino acids: Spectroscopic and molecular modeling approaches. Int J Biol Macromol 97:688–699. doi:10.1016/j.ijbiomac.2017.01.047
Kamble R, Sharma S, Mehta P (2017) Norfloxacin mixed solvency based solid dispersions: In-vitro and In-vivo investigation. J Taibah Univ Sci. doi:10.1016/j.jtusci.2016.11.003
Jiang WT, Chang PH, Wang YS, Tsai Y, Jean JS, Li Z, Krukowski K (2013) Removal of ciprofloxacin from water by birnessite. J Hazard Mater 250:362–369. doi:10.1016/j.jhazmat.2013.02.015
Ahn Y, Linder SW, Veach BT, Steve Yan S, Haydee Fernandez A, Pineiro SA, Cerniglia CE (2012) In vitro enrofloxacin binding in human fecal slurries. Regul Toxicol Pharmacol: RTP 62(1):74–84. doi:10.1016/j.yrtph.2011.11.013
Fengxian C, Reti H (2017) Analysis of positions and substituents on genotoxicity of fluoroquinolones with quantitative structure-activity relationship and 3D Pharmacophore model. Ecotoxicol Environ Saf 136:111–118. doi:10.1016/j.ecoenv.2016.10.036
Liu X, Liu Y, Xu J-R, Ren K-J, Meng X-Z (2016) Tracking aquaculture-derived fluoroquinolones in a mangrove wetland, South China. Environ Pollut 219:916–923. doi:10.1016/j.envpol.2016.09.011
Golet EM, Alder AC, Hartmann A, Ternes TA, Giger W (2001) Trace determination of fluoroquinolone antibacterial agents in urban wastewater by solid-phase extraction and liquid chromatography with fluorescence detection. Anal Chem 73(15):3632–3638. doi:10.1021/ac0015265
Poliwoda A, Krzyzak M, Wieczorek PP (2010) Supported liquid membrane extraction with single hollow fiber for the analysis of fluoroquinolones from environmental surface water samples. J Chromatogr A 1217(22):3590–3597. doi:10.1016/j.chroma.2010.03.051
Sun X, He J, Cai G, Lin A, Zheng W, Liu X, Chen L, He X, Zhang Y (2010) Room temperature ionic liquid-mediated molecularly imprinted polymer monolith for the selective recognition of quinolones in pork samples. J Sep Sci 33:3786–3793. doi:10.1002/jssc.201000337
Marazuela MD, Bogialli S (2009) A review of novel strategies of sample preparation for the determination of antibacterial residues in foodstuffs using liquid chromatography-based analytical methods. Anal Chim Acta 645(1–2):5–17. doi:10.1016/j.aca.2009.04.031
Nasiri J, Naghavi MR, Motamedi E, Alizadeh H, Moghadam MRF, Nabizadeh M, Mashouf A (2017) Carbonaceous sorbents alongside an optimized magnetic solid phase extraction (MSPE) towards enrichment of crude Paclitaxel extracts from callus cultures of Taxus baccata. J Chromatogr B 1043:96–106. doi:10.1016/j.jchromb.2016.10.029
Fan S, Zhu J, Ren L, Wang M, Bi W, Li H, Huang X, Chen DD (2017) Co-solvent enhanced adsorption with magnetic velvet-like carbon nitride for high efficiency solid phase extraction. Anal Chim Acta 960:63–71. doi:10.1016/j.aca.2017.01.020
Hekmatara H, Seifi M, Forooraghi K (2013) Microwave absorption property of aligned MWCNT/Fe3O4. J Magn Magn Mater 346:186–191. doi:10.1016/j.jmmm.2013.06.032
Ma J, Jiang L, Wu G, Xia Y, Lu W, Li J, Chen L (2016) Determination of six sulfonylurea herbicides in environmental water samples by magnetic solid-phase extraction using multi-walled carbon nanotubes as adsorbents coupled with high-performance liquid chromatography. J Chromatogr A 1466:12–20. doi:10.1016/j.chroma
Cao J, Zhang X, He X, Chen L, Zhang Y (2013) Facile synthesis of a Ni(II)-immobilized core–shell magnetic nanocomposite as an efficient affinity adsorbent for the depletion of abundant proteins from bovine blood. J Mater Chem B 1:3619–3718. doi:10.1039/c3tb20573h
Zhang Y, Zhang M, Yang J, Ding L, Zheng J, Xu J, Xiong S (2016) Formation of Fe3O4@SiO2@C/Ni hybrids with enhanced catalytic activity and histidine-rich protein separation. Nanoscale 8:15978–15988. doi:10.1039/C6NR05078F
Tang B, Song W-C, Yang E-C, Zhao X-J (2017) MOF-derived Ni-based nanocomposites as robust catalysts for chemoselective hydrogenation of functionalized nitro compounds. RSC Adv 7(3):1531–1539. doi:10.1039/c6ra26699a
Xu X, Nosheen F, Wang X (2016) Ni-decorated molybdenum carbide hollow structure derived from carbon-coated metal–organic framework for electrocatalytic hydrogen evolution reaction. Chem Mater 28(17):6313–6320. doi:10.1021/acs.chemmater.6b02586
Ma J, Yao Z, Hou L, Lu W, Yang Q, Li J, Chen L (2016) Metal organic frameworks (MOFs) for magnetic solid-phase extraction of pyrazole/pyrrole pesticides in environmental water samples followed by HPLC-DAD determination. Talanta 161:686–692. doi:10.1016/j.talanta.2016.09.035
Zhou Q, Lei M, Li J, Liu Y, Zhao K, Zhao D (2017) Magnetic solid phase extraction of N- and S-containing polycyclic aromatic hydrocarbons at ppb levels by using a zerovalent ironnanoscale material modified with a metal organic framework of type Fe@MOF-5, and their determination by HPLC. Microchim Acta 184:1029–1036. doi:10.1007/s00604-017-2094-6
Chen X, Ding N, Zang H, Yeung H, Zhao R-S, Cheng C, Liu J, Chan TWD (2013) Fe3O4@MOF core–shell magnetic microspheres for magnetic solid-phase extraction of polychlorinated biphenyls from environmental water samples. J Chromatogr A 1304:241–245. doi:10.1016/j.chroma.2013.06.053
Wu Q, Cheng S, Wang C, Li X, Li Z, Hao C (2016) Magnetic porous carbon derived from a zinc-cobaltmetal-organic framework: a adsorbent for magnetic solid phase extraction of flunitrazepam. Microchim Acta 183:3009–3017. doi:10.1007/s00604-016-1948-7
Zou F, Chen YM, Liu K, Yu Z, Liang W, Bhaway SM, Gao M, Zhu Y (2016) Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 10(1):377–386. doi:10.1021/acsnano.5b05041
Carrasco JA, Romero J, Abellan G, Hernandez-Saz J, Molina SI, Marti-Gastaldo C, Coronado E (2016) Small-pore driven high capacitance in a hierarchical carbon via carbonization of Ni-MOF-74 at low temperatures. Chem Commun 52(58):9141–9144. doi:10.1039/c6cc02252a
Mukoyoshi M, Kobayashi H, Kusada K, Hayashi M, Yamada T, Maesato M, Taylor JM, Kubota Y, Kato K, Takata M, Yamamoto T, Matsumura S, Kitagawa H (2015) Hybrid materials of Ni NP@MOF prepared by a simple synthetic method. Chem Commun 51(62):12463–12466. doi:10.1039/c5cc04663g
Lee G, Lee C, Yoon CM, Kim M, Jang J (2017) High-performance three-dimensional mesoporous graphene electrode for supercapacitors using lyophilization and plasma reduction. ACS Appl Mater Interfaces 9(6):5222–5230. doi:10.1021/acsami.6b13050
Wagil M, Kumirska J, Stolte S, Puckowski A, Maszkowska J, Stepnowski P, Bialk-Bielinska A (2014) Development of sensitive and reliable LC-MS/MS methods for the determination of three fluoroquinolones in water and fish tissue samples and preliminary environmental risk assessment of their presence in two rivers in northern Poland. Sci Total Environ 493:1006–1013. doi:10.1016/j.scitotenv.2014.06.082
Tang Y, Guo H, Xiao L, Yu S, Gao N, Wang Y (2013) Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf A Physicochem Eng Asp 424:74–80. doi:10.1016/j.colsurfa.2013.02.030
Zhou Q, Zhu L, Xia X, Tang H (2016) The water – resistant zeolite imidazolate framework 67 is a viable solid phase sorbent for fluoroquinolones while efficiently excluding macromolecules. Microchim Acta 183(6):1839–1846. doi:10.1007/s00604-016-1814-7
Wu X, Huang M, Zhou T, Mao J (2016) Recognizing removal of norfloxacin by novel magnetic molecular imprinted chitosan/γ-Fe2O3 composites: Selective adsorption mechanisms, practical application and regeneration. Sep Purif Technol 165:92–100. doi:10.1016/j.seppur.20 16.03.04
Andrea S, Michela S, Federica M, Elettra M, Dhanalakshmi V, Daniele D, Antonella P (2016) Preparation of silica-supported carbon by Kraft lignin pyrolysis, and its use in solid-phase extraction of fluoroquinolone from environmental waters. Microchim Acta 183:2241–2249. doi:10.1007/s00604-016-1859-7
Wang R, Yuan Y, Yang X, Han Y, Yan H (2015) Polymethacrylate microparticles covalently functionalized with an ionic liquid for solid-phase extraction of fluoroquinolone antibiotics. Microchim Acta 182:2201–2208. doi:10.1007/s00604-015-1544-2
Parirokh L, Noor M, Mohammad R, Khalil A, Seyed M (2017) Colorimetric aptamer based assay for the determination of fluoroquinolones by triggering the reduction-catalyzing activity of gold nanoparticles. Microchim Acta 184:2039–2045. doi:10.1007/s00604-017-2213-4
Acknowledgements
This project was supported by the National Nature Science Foundation of China (NSFC51672116), Science and technology foundation of ocean and fisheries of Liaoning province (201408, 201406), General project of scientific research of the education department of Liaoning province (L2015206), Liaoning scientific instruments service sharing information platform ability construction funds (201507A003), Liaoning provincial department of education innovation team projects (LT2015012) and the foundation of 211 project for innovative talent training, Liaoning university. The authors also thank their colleagues and other students who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 730 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Wang, Y., Zhang, Z. et al. Waxberry-like magnetic porous carbon composites prepared from a nickel-organic framework for solid-phase extraction of fluoroquinolones. Microchim Acta 184, 4107–4115 (2017). https://doi.org/10.1007/s00604-017-2438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2438-2