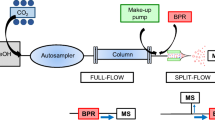
Abstract
Supercritical fluid chromatography (SFC), which employs pressurized carbon dioxide as the major component of the mobile phase, has been known for several decades but has faced a significant resurgence of interest in the recent years, thanks to the development of modern instruments to comply with current expectations in terms of robustness and sensitivity. This review is focused on the recent literature, specifically since the introduction of modern systems but in relation to older literature, to identify the changing trends in application domains. Typically, natural products, bioanalysis, food science, and environmental analyses are all strongly increasing. Together with reduced extra-column volumes in the instruments, the advent of sub-2-μm particles and superficially porous particles in the stationary phases is favoring ultra-high-performance SFC (UHPSFC) allowing for improved resolution and faster analyses, but without the constraints of viscous liquids encountered in ultra-high-performance liquid chromatography (UHPLC). Hyphenation to mass spectrometry is also more frequent and opened the way to new application domains, and raises different issues from liquid chromatography mobile phases, especially due to decompression of carbon dioxide. It is also shown that the frontiers between SFC and HPLC are fading, as switching from one method to the other, even within the course of a single analysis, is facilitated my modern instruments. The present review is not intended to be exhaustive but rather giving a snapshot of recent trends in supercritical fluid chromatography, based on the observation of about 500 papers published in English-written peer-reviewed journals from 2014 to 2018.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As names may be deceiving, modern supercritical fluid chromatography (SFC) does not necessarily employ a supercritical fluid as mobile phase [1]. Early developments in the technique employed several different fluids in their supercritical state (with both pressure and temperature above the critical values) [2], namely, fluorocarbons, ammonia, or carbon dioxide were most often employed. Among all possible fluids, carbon dioxide is the only one to have survived significantly through the ages, for several excellent reasons:
-
(i)
the critical values of pressure and temperature are moderate (7.3 MPa and 31 °C);
-
(ii)
it has interesting features for health and safety, being non-flammable, non-corrosive, and with limited toxicity;
-
(iii)
it is cheap as is obtained as a side-product from many industries; it can be recycled when large amounts are necessary (at preparative scales);
-
(iv)
it is miscible to most organic solvents, allowing for wide possibilities to optimize chromatographic separations and dissolve a wide array of analytes.
Indeed, while early developments principally reported one pure fluid in the supercritical state, modern SFC employs mixtures of carbon dioxide and co-solvents as mobile phase. This mixture is not necessarily a supercritical fluid, as pressure may be above the critical pressure while temperature is often below the critical temperature [3]. This is not causing any issues to the chromatographers, as the advantages of supercritical fluids are retained: the mixed fluid has a lower viscosity than usual liquids employed in high-performance liquid chromatography, which has a number of interesting consequences: high flow rates may be employed as pressure restrictions are less significant than with liquids, and analyte diffusivity is high. These features combined together allow for fast and highly efficient separations.
While most people practicing this technique wish to retain the name of supercritical fluid chromatography, some publications may refer to the same technique with other names (subcritical fluid chromatography, convergence chromatography, etc.). In this review, no distinction will be made among them as there is no fundament to distinguish them.
Recent instrumental developments
The recent resurgence of SFC is majorly due to the introduction of modern instruments. Prior to the years 2010s, most systems available had significant defects that rendered them unfit for current expectations of the analytical scientists. Most importantly, they had limited robustness causing poor reproducibility of the analyses, extra-column volumes were high, yielding significant extra-column band broadening, and baseline noise with UV detectors (most frequently used at that time for all sorts of applications) was too high to reach the low detection limits that are desired for pharmaceutical, clinical, or environmental applications. In chromatography laboratories, where ultra-high-performance liquid chromatography (UHPLC) had rapidly developed in the years 2000s, such defects were strongly felt. Then, a number of major chromatography manufacturers (Agilent, Waters, Jasco, and Shimadzu) released improved systems addressing the above-mentioned defects [4, 5], so much so that the performance of modern SFC is now very near that of UHPLC.
Hyphenation to mass spectrometry (MS) has been largely facilitated (as will be further discussed in the fundamental research section below), so much so that MS is now by far the most employed detection mode, appearing in two thirds of the papers published in the years 2014–2018.
An interesting recent development is the on-line coupling of supercritical fluid extraction (SFE) to SFC. SFE has long been a favorite in the field of natural products [6] but most of the time, the extracts were analyzed with gas chromatography (GC) or UHPLC. However, when a fluid is good for extraction, it seems logical that it should also be good for the separation of the extracted analytes as solubility is ensured. Shimadzu has thus recently released a system allowing for on-line SFE-SFC-MS. Transferring the extracted analytes from the SFE cell to the SFC chromatographic column is achieved with a trap column so as to re-focus the analyte band prior to elution through the chromatographic column. The stationary phase employed in the trap column must be adapted depending on the target analytes. Another possible interface could be based on a split-flow introduction system proposed by Bamba and co-workers [7]. A few papers have been published to illustrate the benefits of the SFE-SFC-MS system in the field of natural products analysis to extract interesting bioactive components from food products [8, 9] or to extract contaminants from soil [10]. Other applications have appeared for bioanalysis, where lyophilized bacteria, dried urine, or dried serum spots were extracted to analyze metabolites or biomarkers [11,12,13]. This original hyphenated system should thus find use in many different application areas in the future.
Besides, the combined use of conventional liquids and carbon dioxide-based mobile phases is especially interesting as reversed-phase HPLC and SFC conducted on polar stationary phases are often found to be highly orthogonal. While several examples exist to illustrate the interest of off-line combination of LC and SFC separations [14, 15], commercial hyphenated systems are desirable that would allow for improved quality of separation. No commercial solution is available yet but a few examples have appeared in the literature to illustrate the benefits and difficulties of such combinations [16,17,18,19,20]. Two-dimensional SFC × SFC would be equally desirable, as changing stationary phases polarity in SFC allows for tremendous changes in separation selectivity; thus, SFC can be said to be orthogonal to itself. However, while neat carbon dioxide offers facilitated solutions (comparable to those employed in GC × GC) [21], practical solutions offering the possibility to use more complex mobile phases would be desirable to extend the application range of such devices [22].
Recent developments in stationary phases
Concomitantly to the development of modern instruments, several column manufacturers producing stationary phases for liquid-phase chromatography started to develop novel stationary phase chemistries designed for SFC use and sometimes improved packing procedures to take account of operating pressures and of the chemical nature of the SFC mobile phase. For instance, in addition to hybrid silica previously developed to sustain ultra-high pressures in liquid chromatography, special bonding chemistry on silica particles was developed by Waters to ensure that retention would not degrade over time with the formation of silyl ethers [23, 24]. Regarding stationary phase chemistries, a large variety of stationary phases, from the most polar silica to the least polar well-endcapped or densely bonded alkyl-bonded silica, is now available [25] allowing for a diversity of selectivities. In the most recent papers (2016–2018), it appears in Fig. 1a that the most frequently cited stationary phase in achiral analytical applications (nearly a quarter of them) is a 2-ethylpyridine-bonded silica phase (2-EP). This stationary phase chemistry was first introduced by Princeton Chromatography in the years 2000s and was always very successful, especially for applications involving the analysis of basic compounds. It was later imitated by other manufacturers. The second most frequently used phase is bare silica (or hybrid silica) in 20% achiral applications, followed by octadecyl-bonded silica (C18). The latter is often employed in the analysis of lipids [26,27,28,29], when intra-class separation is desired (according to carbon chain length and double bonds) but has been found to be useful in many other applications like phthalate esters [30], polyaromatic hydrocarbons [31], pesticides [32], or drug candidates [33]. The fourth most frequently cited stationary phase is a diol-bonded silica, which was, for instance, often selected in the analysis of natural products [34,35,36,37], but also for lipidomics when a separation based on the polar head is desired [38, 39], or in bioanalysis [13, 40]. Other frequently cited phases are 1-aminoanthracene (1-AA) that may be used for fat-soluble vitamins [41]; 2-picolylamine (2-PIC) that is a recent variation of the 2-EP; triacontane-bonded silica (C30), which was often employed for the separation of carotenoid pigments [8]; phenyl-bonded silica; diethylamine-bonded silica (DEA) [42], and pentafluorophenyl-bonded silica (PFP) [43]. Many other stationary phases have been employed, with a short selection of them appearing in Fig. 1 (for instance, aminopropyl-bonded silica [44], cyanopropyl-bonded silica, amide [45] or sulfobetaine [46, 47]). As the screening of stationary phases is usually the first step in developing a method in achiral SFC, diversity of stationary phase chemistry is highly desirable and should still progress in the years to come.
For chiral applications at the analytical scale (Fig. 1b), modified polysaccharide stationary phases are the most often used with amylose tris-(dimethylphenylcarbamate) (ADMPC) being cited in about half the analytical applications. Cellulose tris-(dimethylphenylcarbamate) (CDMPC) and cellulose tris-(3-chloro-4-methylphenylcarbamate) (CClMPC), and several other sorts of modified polysaccharides were employed in recent papers. The Pirkle-type phase WhelkO-1 was also present in recent studies [48], especially as it is now available with sub-2-μm particles.
Similarly to the evolutions seen in high-performance liquid chromatography, smaller particle size (inferior to 2 μm) [49] and superficially porous particles [50,51,52] were both introduced to SFC practice. For chiral stationary phases, the switch to smaller particles is more recent and has thus not been seen in many papers yet. The different trends in particle size stationary phases can be observed in Fig. 2, where it appears that achiral analytical-scale applications are now majorly conducted on sub-2-μm stationary phases (77% in the most recent publications, years 2016–2018) while chiral separations are still essentially conducted on larger particle sizes (2.5, 3, and 5 μm).
The joint use of efficiency-optimized instruments and modern stationary phase technologies is producing ultra-high efficiency separations [53] in what is often termed now “ultra-high-performance supercritical fluid chromatography” (UHPSFC). As mentioned in the “Introduction”, the low viscosity of the fluids employed in SFC causes less pressure issues than the liquids employed in UHPLC. Thus, current SFC systems have pumping pressure limits of 40 to 66 MPa, depending on the manufacturer. For instance, a 40-MPa upper pressure limit allows the use of a 100 × 3.0 mm column packed with 1.7-μm particles at 1–2 mL/min (depending on operating parameters). Obviously, a higher pressure may still be desirable when large proportions of co-solvent are desired, or if higher flow rates or longer column lengths would be necessary for faster analysis or for higher efficiency. Typical column lengths employed are mostly in the 100–150-mm range (about 90% of the analytical applications), with rare examples of shorter columns for faster analysis [54,55,56] (for instance when the SFC separation must be the second dimension of a two-dimensional chromatographic system [17, 18]), or longer columns for higher efficiency [57]. In this respect, superficially porous particles offer the extra advantage over fully porous particles to allow for much longer column lengths without creating so much pressure drop [50, 58].
Who is publishing SFC science?
Reviewing the recent literature, from 2014 to early 2018, about 500 papers, majorly referenced in Scopus database, were examined in preparing this article, taking all sorts of papers into account (fundamental research, applications, or reviews). This is not an exhaustive search, as, for instance, only papers published in English were reviewed. Spanning on a large number of publishers, the observations presented here should however be representative of the overall SFC production. Several features of this production were examined to gain a clear view of current SFC chromatographers.
First, the geographical origin of the corresponding author was noted. A heat map representing the proportion of papers published per country is presented in Fig. 3. It appears that the USA and China are the two major sources of SFC science, with about 23 and 18% papers reviewed respectively. China would probably have a higher share if papers published in Chinese had also been counted. This observation is especially interesting when related to the fields of applications, as will be further discussed below. From Asian countries, another significant part of the production is issuing from Japan (5% of the total) and, to a lesser extent, from India (2%). Europe is actually the first major region for SFC-related articles, with 45% papers originating from this area. In Europe, most active countries are France (10%), Belgium (6%), Sweden, and Switzerland (5% each). Not far behind is Czech Republic (4%), followed by Germany, Austria, and Italy (about 2% each).
Secondly, another feature of the authors was observed; this time taking all authors into account, the academics, industry users, or manufacturers were observed (Fig. 4). Not surprisingly, academics make up the largest part of the production, with 71% papers being produced solely by academic institutions. Industry users are thus still present, with 17% papers produced solely by industry chromatographers. Eight percent of the papers were produced by academics and industry chromatographers working together. Finally, manufacturers were little present with 1% papers produced by manufacturers only, and 3% by collaborations between academics and manufacturers. Possibly, taking account of application notes would increase the share of manufacturers, but as these are mostly published on the manufacturer’s websites and not in peer-reviewed journals, they are not referenced by major bibliographic search engines and were not counted to prepare this review. These proportions have changed over time. Understandably, in the early years of SFC developments, in the 1980s and 1990s, academics were the major producers of SFC research. But in the years 2000s, when university researchers had deserted the field, the portion of industry papers was more significant, reaching as high as 35% contribution. It may be taken as a good sign that university chromatographers are again investing the field, as the fundamental understanding of the technique still requires some work.
Fundamental studies
From the beginnings of SFC to the years 2000s, the part taken by fundamental papers was essentially decreasing, which seems rather logical as early developments of a technique require fundamental investigations prior to clever applications. The renewal of the technology has brought a surge of new fundamental studies. They now make up about 20–25% of the articles published in the years 2014–2018. Note that the frontier between fundamentals and applications is not always easy to trace, hence the imprecision in the statistics.
Apart from instrument developments described above, fundamental studies are mainly related to the following topics.
-
(i)
Efficiency issues [59,60,61,62,63] and the best means to evaluate efficiency were mostly investigated in the years 2012–2014, when new instrument technologies were released, but are now less explored. Extra-column band broadening is however still a concern, and should be addressed by further improvements in systems [53, 62, 64, 65].
-
(ii)
Injection and dilution solvent effects at the analytical scale [62, 66, 67] or preparative scale [68] are a concern to avoid peak deformation that may be due to limited miscibility of the diluent solvent and the mobile phase (especially at the beginning of a gradient elution program where carbon dioxide proportion may be high) and to avoid viscosity mismatch causing viscous fingering. While this was long a concern at the preparative scale, it has most recently been explored also at the analytical scale.
-
(iii)
Several mobile phase-related issues have been explored, namely, the adsorption of mobile phase components on stationary phase [69] and its effect on peak shapes [70,71,72,73]; the effects of additives (acids, bases, salts) on chromatographic quality in achiral or chiral modes [74,75,76,77]; and the acidity caused by carbon dioxide coming into contact with water or alcohols [78, 79].
-
(iv)
Stationary phases have been explored for their durability [23, 80] and selectivity (achiral or chiral separations) [24, 25, 81], and have been compared to liquid-phase behavior [82, 83]. The interest of combining two stationary phase in tandem column systems was also investigated [84, 85].
-
(v)
Retention behaviors in SFC, both in achiral [86] and chiral [77, 87, 88] modes, are still a matter of interest. In addition, retention modeling [89,90,91] is desirable to facilitate method development with computer-assisted processes and compound identification in complex samples. As tendency curves relating retention to mobile phase composition or operating parameters (temperature, pressure, flow rate) are usually not linear over a large range of conditions (and sometimes not even monotonous), modeling retention is not as straightforward as it may be with GC or reversed-phase HPLC.
-
(vi)
Preparative scale has been the major topic of a series of papers from the group of Fornstedt [92, 93], inspired by a seminal paper from Guiochon and Tarafder [94]. In particular, transferring methods between analytical and preparative scale [95,96,97] is also setting some additional problems as compared to liquid-phase scaling up, due to compressibility of the fluid.
Finally, the hyphenation to mass spectrometry (MS) has been the topic of many recent studies. First, the ways of hyphenating the chromatographic system to the mass spectrometer have experienced many changes over the years [98, 99], partly due to the fact that the preferred ionization source has changed (from atmospheric pressure chemical ionization APCI to electrospray ionization ESI) [100]. Most recently, a new design of atmospheric pressure ionization source was introduced called UniSpray and was evaluated for the analysis of 120 natural compounds [101]. This source was found to provide improved sensitivity to certain classes of analytes, and decreased sensitivity for others, when compared to ESI. Secondly, it was shown on several occasions that the MS response may vary depending on the SFC mobile phase composition, even though a make-up fluid is most often introduced prior to the MS [74, 102, 103]. Akbal and Hopfgartner thoroughly investigated the effect of post-column addition on 20 analytes belonging to different classes (beta-blockers, HIV protease inhibitors, steroids, and polar metabolites), with variations of make-up flow rate, solvent composition, pH level, and buffer concentration. They concluded that the introduction of ammonia in the make-up fluid was useful, especially when it was not previously introduced in the chromatographic mobile phase. Ammonia would perhaps counteract the acidification of the mobile phase by carbon dioxide. In addition, dimethylsulfoxide (DMSO) was found to be particularly useful in enhancing SFC-ESI-MS response. The post-column addition may also be advantageously employed to favor ionization of certain species, as was demonstrated by Touboul and co-workers with the use of lithium iodide to favor the detection of acetogenins [104]. Finally, matrix effects are a very significant topic, especially when analysis of complex biofluids (urine, serum etc.) is concerned. In particular, Haglind et al. [105] indicated that the abundant presence of alkali ions (sodium and potassium) in biological fluids (plasma and urine) was causing major ion suppression for the analytes co-eluting with them. Sample preparation to get rid of these ions, or adjustment of chromatographic conditions could both be successful strategies to avoid these complications. Comparing matrix effects between SFC-MS and GC-MS [55] is generally to the advantage of the former, while comparing SFC-MS and LC-MS shows that both of them may be positively or negatively impacted by matrix effects [106,107,108].
Application domains
While the number of fundamental studies has increased only moderately in the recent years, application papers have faced a tremendous increase. Meanwhile, the trends in application domains have greatly changed. Figure 5 presents the most significant application domains. In addition to the expansion of SFC technology, the increased accessibility to mass spectrometers has favored SFC-MS application to new domains, where simpler, less informative detections modes like UV are not considered sufficient. Therefore, while SFC-MS studies in 2014 represented about 35% of analytical applications, they have reached 75% in 2017.
Only 10 years ago, about 75% of the papers related to SFC concerned the pharmaceutical industry, either for analysis or purification of synthetic drugs [109, 110], mostly in chiral separations. In 2014, still 40% of the analytical applications were related to this topic. Considering the 2014–2018 years, the analysis of chemical pharmaceuticals (as opposed to natural products) is now only the third application domain in terms of number of papers published (Fig. 5). Possibly, because pharmaceutical analysis with SFC has been explored for a long time, there are perhaps less expectations in this area than in other, less investigated domains. However, there are still interesting features to explore with modern SFC of pharmaceutical products. Most importantly, the capability of analytical SFC or UHPSFC to comply with the requirements of good manufacturing practice (GMP) [111] and to obtain methods that can be validated [42, 112] according to the recommendations of the International Conference of Harmonization (ICH) are the topic of several recent studies. This is especially important in order for the technique to spread in quality control laboratories. Other concerns in this field are related to the determination of generic conditions to achieve analytical separations of chemical or chiral impurities with minimal effort [33, 74, 113] or to the acceleration of analysis, either through ultra-fast separations [114,115,116,117] or through multiple injections within a single run [118], in cases when large numbers of samples must be analyzed within a short time. Apart from active pharmaceutical ingredients and impurities, other components of drug formulations or contaminants have been analyzed like polymers [119] and plasticizers issuing from plastic medical devices [120]. In the future, it may be expected that the analysis of full drug formulations will be seen, because SFC is equally capable to handle polar, non-polar, ionic [121], small and large chemical species.
While chemical and enantiomeric analyses of drug compounds are not as significant as they used to be, chemical pharmaceuticals still explore in pharmacokinetic studies, in urine, or in plasma samples. Thanks to SFC-MS expansion [122], the years 2014–2018 have seen a strong increase in bioanalysis applications [123], which are now the second most important application domain for SFC. Pharmacokinetic studies constitute about a half of these bioanalysis studies. The other half comprises forensic applications (doping agents [40, 108] or drugs of abuse [124,125,126]), and a significant portion of lipidomics [127,128,129,130,131].
Another portion of pharmaceutical applications may be found in the “natural products” section. As pointed out above, China is now one of the most productive countries regarding SFC publications. However, traditional Chinese medicine (TCM) is based on natural products. China has started the huge project of investigating these natural products in more details to improve knowledge on the bioactive species. This task requires several high-performance separation methods with orthogonal selectivities in order to achieve the broadest picture of these TCM. In this respect, SFC has a natural place next to reversed-phase liquid chromatography, of which it is generally found to be complementary [56, 132]. Also for quality control of TCM, modern SFC has found its place [133]. Similarly, natural products are being (re-)examined in occidental medicine and their SFC analysis has produced several publications [134]. The variety of structural families having been explored in this field is an excellent example of the versatility of the technique. Indeed, while it had been known for a long time that lipids [29, 50, 135] or carotenoid pigments [136,137,138,139,140,141] were well soluble in pressurized carbon dioxide mobile phases, other molecular families have been observed in recent papers such as alkaloids [37, 132, 142, 143], anthraquinones [144, 145], coumarins [146, 147], furostanol saponins [36], triterpenoid saponins [148], flavonoids [43, 149], carbohydrates [45, 150, 151], or nucleobases and nucleosides [152]. Some papers related to medicinal cannabis have also shown that SFC can be an interesting complement to UHPLC for this fast rising application field [48]. The variety of possibilities is now making natural products the first application domain for analytical SFC (Fig. 5). Natural products have also been a significantly growing field at the preparative scale [35, 37, 153,154,155,156,157,158,159,160,161,162], not only at the analytical scale. In addition to the above examples of structural families, purification of lignans [14] and polyphenols [163] with preparative SFC has been demonstrated.
Natural products are also a part of the food science application domain, which appears as the fourth most significant at the analytical scale. Most papers in this field relate the analysis of lipids (not only in vegetable oils but also in milk products [164, 165]), carotenoids and lipophilic vitamins [41, 166].
The food industry is increasingly concerned with contaminants, which may issue from packaging [167], drug residues from contaminated irrigation or, most frequently, pesticides. Here again, China has been the most productive in the investigation of the latter.
Pesticides (along with other contaminants) may also be looked for in soils [168,169,170,171,172,173,174], water [46], or wastewater [175, 176], thereby belonging to the field of environment applications. Other environmental applications were observed with the analysis of emerging contaminants in aqueous ecosystems [177], allergenic textile dyes [178, 179], flame retardants [180], and halogenated pollutants [181, 182].
Petroleum products have been a classical application domain for SFC for a long time [183]. As petroleum components have limited polarity, it is feasible to analyze them with neat carbon dioxide and thus maintaining compatibility to GC-like detectors (typically flame ionization detector). However, the field of energy is changing with the growing introduction of biomass-related products. Biodiesel is a new type of energy source requiring deep investigation. In conjunction with other chromatographic methods, SFC may furnish interesting information in the composition of such samples [184,185,186].
A small portion of papers related to cosmetic applications is emerging [187, 188]. The cosmetic industry is currently highly demanding of natural ingredients produced with environmentally friendly solvents and processes, which should naturally place SFC as a favorite method in this field.
Finally, a word should be said regarding the relative proportions of achiral and chiral separations. While chiral separations used to be the major application field for SFC, it is no longer the case, with only 20% of the analytical applications related to chiral separations in the years 2014–2018. However, analytical chiral SFC is still in use especially to measure the enantiomeric excess of synthetic compounds [189, 190], in pharmacokinetic studies [191, 192], to assess the fate of chiral pesticides [168, 170, 173, 193, 194] and chiral drugs in the environment [195] and as a first step of method development prior to purification with preparative-scale chiral SFC [196,197,198,199]. At the preparative scale, chiral separations still make up about 60% of the publications.
Trends in method development
The use of design of experiments (DoE) to develop analytical SFC methods is increasing. Most often, an initial screening of stationary phase with either isocratic or gradient conditions is done, and then an experimental design is proposed to optimize the conditions of mobile phase composition, temperature, pressure, or even flow rate [42, 73, 91, 200, 201]. The importance of measuring the exact conditions inside the system rather than relying on instrument settings was pointed out in several occasions [93, 202,203,204]. Considering most recent papers (years 2016–2018), methanol is still the most frequently employed co-solvent in achiral analytical applications as can be seen in Fig. 6a. Acetonitrile, which was rarely employed in the past, is progressing, especially in mixtures with an alcohol. For chiral applications, methanol is also dominant but isopropanol is also very frequently used. The use of water in ternary mobile phase compositions, along with carbon dioxide and a major co-solvent (alcohol or acetonitrile), has been noted in many recent achiral applications (about 17% of the recent papers). A large portion of the applications (50 and 60% of the achiral and chiral applications respectively) is based on the sole use of such simple mobile phase compositions, namely carbon dioxide, major co-solvent, and sometimes water. Other applications mention the additional use of acids, bases, or salts to favor the elution with satisfactory peak shapes or to improve resolution. For achiral applications, the recent years have seen increasing use of ammonium formate, ammonium acetate, and ammonium hydroxide, along with formic acid. All of them are usually observed to favor chromatographic quality in addition to mass spectrometric response with electrospray ionization.
The advantages of the so-called enhanced-fluidity liquid chromatography (EFLC) region (when carbon dioxide is the minor component of the mobile phase, as opposed to SFC where it is the major component) have long been known and explored, principally by Olesik and co-workers [45, 205, 206]. The interest in introducing a small portion of carbon dioxide in an aqueous reversed-phase HPLC system was to improve efficiency and reduce analysis time through reduced mobile phase viscosity. This is also allowing to elute analytes with higher polarities than normally encountered in “more classical” SFC conditions with a larger portion of carbon dioxide. A most innovative paper from the team of Bamba [207] has demonstrated the SFC-MS analysis of a mixture of lipophilic and hydrophilic vitamins in a single run, with a wide elution gradient starting in classical SFC conditions (large proportion of CO2, low proportion of co-solvent), moving to EFLC and finishing with a pure liquid (methanol-water 95:5 v/v) mobile phase. This was definitely breaking the imaginary barrier between SFC and HPLC. Since this fundamental paper, other research have been produced to investigate in details the “transition” from HPLC to SFC [208]. More research is expected in the near future, which will show that the former views on operating conditions are no longer valid and that using the full gradient range from 0 to 100%, co-solvent should open new application possibilities, especially for samples containing a wide diversity of analyte polarities.
Outlook
Supercritical fluid chromatography is clearly facing changing trends in many respects: less chiral but more achiral applications; less preparative-scale but more analytical-scale applications; less pharmaceutics applications but more natural products, food science, bioanalysis, and environmental applications. Many of these application domains have benefited of the increasing availability of mass spectrometry. Also, the way of developing methods is changing, with less trial-and-error and more design of experiment processes. Still, some improvement in retention modeling is desirable to help predict the outcome of a separation and favor analyte identification in complex mixtures. The improvements in instruments and developments of innovative stationary phases have both been great contributors to these changes but further improvement, especially in reducing extra-column volumes and expanding stationary phase diversity, should be observed in the near future. The frontiers between SFC and HPLC are fading and more versatile methods, moving from one to the other within the course of a single analysis, should be seen in future papers to expand the range of analyte polarities eluted within one experiment. SFC research is very active in many geographical regions of the world and it is hoped that it will further develop in the years to come.
References
Tarafder A. Metamorphosis of supercritical fluid chromatography to SFC: an overview. TrAC Trends in Anal Chem. 2016;81:3–10. https://doi.org/10.1016/j.trac.2016.01.002.
Lesellier E, West C. The many faces of packed column supercritical fluid chromatography - a critical review. J Chromatogr A. 2015;1382:2–46. https://doi.org/10.1016/j.chroma.2014.12.083.
Nováková L, Grand-Guillaume Perrenoud A, Francois I, West C, Lesellier E, Guillarme D. Modern analytical supercritical fluid chromatography using columns packed with sub-2 μm particles: a tutorial. Anal Chim Acta. 2014;824:18–35. https://doi.org/10.1016/j.aca.2014.03.034.
Berger TA, Fogleman K. Improving signal-to-noise ratio and dynamic range in supercritical fluid chromatography with UV detection. The Peak E-Zine. 2009.
Berger TA, Berger BK. Minimizing UV noise in supercritical fluid chromatography. I. Improving back pressure regulator pressure noise. J Chromatogr A. 2011;1218:2320–6. https://doi.org/10.1016/j.chroma.2011.02.030.
Herrero M, Cifuentes A, Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem. 2006;98:136–48. https://doi.org/10.1016/j.foodchem.2005.05.058.
Sakai M, Hayakawa Y, Funada Y, Ando T, Fukusaki E, Bamba T. Development of a split-flow system for high precision variable sample introduction in supercritical fluid chromatography. J Chromatogr A. 2017;1515:218–31. https://doi.org/10.1016/j.chroma.2017.07.077.
Giuffrida D, Zoccali M, Arigò A, Cacciola F, Roa CO, Dugo P, et al. Comparison of different analytical techniques for the analysis of carotenoids in tamarillo (Solanum betaceum Cav.). Arch Biochem Biophys. 2018;646:161–7. https://doi.org/10.1016/j.abb.2018.03.011.
Zoccali M, Giuffrida D, Dugo P, Mondello L. Direct online extraction and determination by supercritical fluid extraction with chromatography and mass spectrometry of targeted carotenoids from red habanero peppers (Capsicum chinense Jacq.). J Sep Sci. 2017;40:3905–13. https://doi.org/10.1002/jssc.201700669.
Wicker AP, Carlton DD, Tanaka K, Nishimura M, Chen V, Ogura T, et al. On-line supercritical fluid extraction—supercritical fluid chromatography-mass spectrometry of polycyclic aromatic hydrocarbons in soil. J Chromatogr B. 2018;1086:82–8. https://doi.org/10.1016/j.jchromb.2018.04.014.
Matsubara A, Harada K, Hirata K, Fukusaki E, Bamba T. High-accuracy analysis system for the redox status of coenzyme Q10 by online supercritical fluid extraction–supercritical fluid chromatography/mass spectrometry. J Chromatogr A. 2012;1250:76–9. https://doi.org/10.1016/j.chroma.2012.05.009.
Hofstetter R, Fassauer GM, Link A. Supercritical fluid extraction (SFE) of ketamine metabolites from dried urine and on-line quantification by supercritical fluid chromatography and single mass detection (on-line SFE–SFC–MS). J Chromatogr B. 2018;1076:77–83. https://doi.org/10.1016/j.jchromb.2018.01.024.
Suzuki M, Nishiumi S, Kobayashi T, Sakai A, Iwata Y, Uchikata T, et al. Use of on-line supercritical fluid extraction-supercritical fluid chromatography/tandem mass spectrometry to analyze disease biomarkers in dried serum spots compared with serum analysis using liquid chromatography/tandem mass spectrometry: SFE-SFC/MS/MS to analyze disease biomarkers in dried serum spots. Rapid Commun Mass Spectrom. 2017;31:886–94. https://doi.org/10.1002/rcm.7857.
Yang B, Xin H, Wang F, Cai J, Liu Y, Fu Q, et al. Purification of lignans from Fructus Arctii using off-line two-dimensional supercritical fluid chromatography/reversed-phase liquid chromatography. J Sep Sci. 2017;40:3231–8. https://doi.org/10.1002/jssc.201700139.
Li K, Fu Q, Xin H, Ke Y, Jin Y, Liang X. Alkaloids analysis using off-line two-dimensional supercritical fluid chromatography × ultra-high performance liquid chromatography. Analyst. 2014;139:3577–87. https://doi.org/10.1039/C4AN00438H.
Sarrut M, Corgier A, Crétier G, Le Masle A, Dubant S, Heinisch S. Potential and limitations of on-line comprehensive reversed phase liquid chromatography×supercritical fluid chromatography for the separation of neutral compounds: an approach to separate an aqueous extract of bio-oil. J Chromatogr A. 2015;1402:124–33. https://doi.org/10.1016/j.chroma.2015.05.005.
Sun M, Sandahl M, Turner C. Comprehensive on-line two-dimensional liquid chromatography × supercritical fluid chromatography with trapping column-assisted modulation for depolymerised lignin analysis. J Chromatogr A. 2018;1541:21–30. https://doi.org/10.1016/j.chroma.2018.02.008.
Venkatramani CJ, Al-Sayah M, Li G, Goel M, Girotti J, Zang L, et al. Simultaneous achiral-chiral analysis of pharmaceutical compounds using two-dimensional reversed phase liquid chromatography-supercritical fluid chromatography. Talanta. 2016;148:548–55. https://doi.org/10.1016/j.talanta.2015.10.054.
Goel M, Larson E, Venkatramani CJ, Al-Sayah MA. Optimization of a two-dimensional liquid chromatography-supercritical fluid chromatography-mass spectrometry (2D-LC-SFS-MS) system to assess “in-vivo” inter-conversion of chiral drug molecules. J Chromatogr B. 2018;1084:89–95. https://doi.org/10.1016/j.jchromb.2018.03.029.
Stevenson PG, Tarafder A, Guiochon G. Comprehensive two-dimensional chromatography with coupling of reversed phase high performance liquid chromatography and supercritical fluid chromatography. J Chromatogr A. 2012;1220:175–8. https://doi.org/10.1016/j.chroma.2011.11.020.
Petkovic O, Guibal P, Sassiat P, Vial J, Thiébaut D. Active modulation in neat carbon dioxide packed column comprehensive two-dimensional supercritical fluid chromatography. J Chromatogr A. 2018;1536:176–84. https://doi.org/10.1016/j.chroma.2017.08.063.
Zeng L, Xu R, Zhang Y, Kassel DB. Two-dimensional supercritical fluid chromatography/mass spectrometry for the enantiomeric analysis and purification of pharmaceutical samples. J Chromatogr A. 2011;1218:3080–8. https://doi.org/10.1016/j.chroma.2011.03.041.
Fairchild JN, Brousmiche DW, Hill JF, Morris MF, Boissel CA, Wyndham KD. Chromatographic evidence of silyl ether formation (SEF) in supercritical fluid chromatography. Anal Chem. 2015;87:1735–42. https://doi.org/10.1021/ac5035709.
Desfontaine V, Veuthey J-L, Guillarme D. Evaluation of innovative stationary phase ligand chemistries and analytical conditions for the analysis of basic drugs by supercritical fluid chromatography. J Chromatogr A. n.d. https://doi.org/10.1016/j.chroma.2016.02.029.
West C, Lemasson E, Bertin S, Hennig P, Lesellier E. An improved classification of stationary phases for ultra-high performance supercritical fluid chromatography. J Chromatogr A. 2016;1440:212–28. https://doi.org/10.1016/j.chroma.2016.02.052.
Jones JW, Carter CL, Li F, Yu J, Pierzchalski K, Jackson IL, et al. Ultraperformance convergence chromatography-high resolution tandem mass spectrometry for lipid biomarker profiling and identification: UPCC-HRMS for lipid biomarker profiling and ID. Biomed Chromatogr. 2017;31:e3822. https://doi.org/10.1002/bmc.3822.
de Souza GL, Jardim ICSF, de Melo LV, Beppu MM, Breitkreitz MC, Santana CC. Study of the effect of the operating parameters on the separation of bioactive compounds of palm oil by ultra-high performance supercritical fluid chromatography using a design of experiments approach. Can J Chem Eng. 2017;95:2306–14. https://doi.org/10.1002/cjce.22969.
Gao B, Luo Y, Lu W, Liu J, Zhang Y, Yu L(L). Triacylglycerol compositions of sunflower, corn and soybean oils examined with supercritical CO2 ultra-performance convergence chromatography combined with quadrupole time-of-flight mass spectrometry. Food Chem. 2017;218:569–74. https://doi.org/10.1016/j.foodchem.2016.09.099.
Duval J, Colas C, Pecher V, Poujol M, Tranchant J-F, Lesellier É. Contribution of supercritical fluid chromatography coupled to high resolution mass spectrometry and UV detections for the analysis of a complex vegetable oil – application for characterization of a Kniphofia uvaria extract. Comptes Rendus Chimie. 2016;19:1113–23. https://doi.org/10.1016/j.crci.2015.11.022.
Lou C, Guo D, Zhang K, Wu C, Zhang P, Zhu Y. Simultaneous determination of 11 phthalate esters in bottled beverages by graphene oxide coated hollow fiber membrane extraction coupled with supercritical fluid chromatography. Anal Chim Acta. 2018;1007:71–9. https://doi.org/10.1016/j.aca.2017.12.018.
Yoshioka T, Nagatomi Y, Harayama K, Bamba T. Development of an analytical method for polycyclic aromatic hydrocarbons in coffee beverages and dark beer using novel high-sensitivity technique of supercritical fluid chromatography/mass spectrometry. J Biosci Bioeng. 2018. https://doi.org/10.1016/j.jbiosc.2018.01.014.
Cutillas V, Galera MM, Rajski Ł, Fernández-Alba AR. Evaluation of supercritical fluid chromatography coupled to tandem mass spectrometry for pesticide residues in food. J Chromatogr A. 2018;1545:67–74. https://doi.org/10.1016/j.chroma.2018.02.048.
Lemasson E, Bertin S, Hennig P, Boiteux H, Lesellier E, West C. Development of an achiral supercritical fluid chromatography method with ultraviolet absorbance and mass spectrometric detection for impurity profiling of drug candidates. Part II. Selection of an orthogonal set of stationary phases. J Chromatogr A. 2015;1408:227–35. https://doi.org/10.1016/j.chroma.2015.07.035.
Prothmann J, Sun M, Spégel P, Sandahl M, Turner C. Ultra-high-performance supercritical fluid chromatography with quadrupole-time-of-flight mass spectrometry (UHPSFC/QTOF-MS) for analysis of lignin-derived monomeric compounds in processed lignin samples. Anal Bioanal Chem. 2017. https://doi.org/10.1007/s00216-017-0663-5.
Qin X, Wang Y, Li A, Sun A, Yu L, Liu R. Separation and purification of six components from the roots of Rheum officinale Baill. by supercritical fluid chromatography. J Liq Chromatogr Relat Technol. 2017;40:156–64. https://doi.org/10.1080/10826076.2017.1295389.
Yang J, Zhu L, Zhao Y, Xu Y, Sun Q, Liu S, et al. Separation of furostanol saponins by supercritical fluid chromatography. J Pharm Biomed Anal. 2017;145:71–8. https://doi.org/10.1016/j.jpba.2017.05.023.
Yang W, Zhang Y, Pan H, Yao C, Hou J, Yao S, et al. Supercritical fluid chromatography for separation and preparation of tautomeric 7-epimeric spiro oxindole alkaloids from Uncaria macrophylla. J Pharm Biomed Anal. 2017;134:352–60. https://doi.org/10.1016/j.jpba.2016.10.021.
Al Hamimi S, Sandahl M, Armeni M, Turner C, Spégel P. Screening of stationary phase selectivities for global lipid profiling by ultrahigh performance supercritical fluid chromatography. J Chromatogr A. 2018;1548:76–82. https://doi.org/10.1016/j.chroma.2018.03.024.
Teubel J, Wüst B, Schipke CG, Peters O, Parr MK. Methods in endogenous steroid profiling – a comparison of gas chromatography mass spectrometry (GC–MS) with supercritical fluid chromatography tandem mass spectrometry (SFC-MS/MS). J Chromatogr A. 2018;1554:101–16. https://doi.org/10.1016/j.chroma.2018.04.035.
Nováková L, Desfontaine V, Ponzetto F, Nicoli R, Saugy M, Veuthey J-L, et al. Fast and sensitive supercritical fluid chromatography – tandem mass spectrometry multi-class screening method for the determination of doping agents in urine. Anal Chim Acta. 2016;915:102–10. https://doi.org/10.1016/j.aca.2016.02.010.
Oberson J-M, Campos-Giménez E, Rivière J, Martin F. Application of supercritical fluid chromatography coupled to mass spectrometry to the determination of fat-soluble vitamins in selected food products. J Chromatogr B. 2018;1086:118–29. https://doi.org/10.1016/j.jchromb.2018.04.017.
Dispas A, Desfontaine V, Andri B, Lebrun P, Kotoni D, Clarke A, et al. Quantitative determination of salbutamol sulfate impurities using achiral supercritical fluid chromatography. J Pharm Biomed Anal. 2017;134:170–80. https://doi.org/10.1016/j.jpba.2016.11.039.
Wang B, Liu X, Zhou W, Hong Y, Feng S. Fast separation of flavonoids by supercritical fluid chromatography using a column packed with a sub-2 μm particle stationary phase. J Sep Sci. 2017;40:1410–20. https://doi.org/10.1002/jssc.201601021.
Muscat Galea C, Didion D, Clicq D, Mangelings D, Vander HY. Method optimization for drug impurity profiling in SFC: application to a pharmaceutical mixture. J Chromatogr A. n.d. https://doi.org/10.1016/j.chroma.2017.10.036.
Bennett R, Olesik SV. Enhanced fluidity liquid chromatography of inulin fructans using ternary solvent strength and selectivity gradients. Anal Chim Acta. 2018;999:161–8. https://doi.org/10.1016/j.aca.2017.10.036.
Bieber S, Greco G, Grosse S, Letzel T. RPLC-HILIC and SFC with mass spectrometry: polarity-extended organic molecule screening in environmental (water) samples. Anal Chem. 2017;89:7907–14. https://doi.org/10.1021/acs.analchem.7b00859.
Lemasson E, Bertin S, Hennig P, Lesellier E, West C. Comparison of ultra-high performance methods in liquid and supercritical fluid chromatography coupled to electrospray ionization – mass spectrometry for impurity profiling of drug candidates. J Chromatogr A. 2016;1472:117–28. https://doi.org/10.1016/j.chroma.2016.10.045.
Mazzoccanti G, Ismail OH, D’Acquarica I, Villani C, Manzo C, Wilcox M, et al. Cannabis through the looking glass: chemo- and enantio-selective separation of phytocannabinoids by enantioselective ultra high performance supercritical fluid chromatography. Chem Commun. 2017;53:12262–5. https://doi.org/10.1039/C7CC06999E.
Khater S, West C, Lesellier E. Characterization of five chemistries and three particle sizes of stationary phases used in supercritical fluid chromatography. J Chromatogr A. 2013;1319:148–59. https://doi.org/10.1016/j.chroma.2013.10.037.
Lesellier E, Latos A, de Oliveira AL. Ultra high efficiency/low pressure supercritical fluid chromatography with superficially porous particles for triglyceride separation. J Chromatogr A. 2014;1327:141–8. https://doi.org/10.1016/j.chroma.2013.12.046.
Lesellier E. Efficiency in supercritical fluid chromatography with different superficially porous and fully porous particles ODS bonded phases. J Chromatogr A. 2012;1228:89–98. https://doi.org/10.1016/j.chroma.2011.11.058.
Grand-Guillaume Perrenoud AG-G, Farrell WP, Aurigemma CM, Aurigemma NC, Fekete S, Guillarme D. Evaluation of stationary phases packed with superficially porous particles for the analysis of pharmaceutical compounds using supercritical fluid chromatography. J Chromatogr A. 2014;1360:275–87. https://doi.org/10.1016/j.chroma.2014.07.078.
Berger TA. Instrument modifications that produced reduced plate heights <2 with sub-2μm particles and 95% of theoretical efficiency at k=2 in supercritical fluid chromatography. J Chromatogr A. 2016;1444:129–44. https://doi.org/10.1016/j.chroma.2016.03.021.
Végh K, Riethmüller E, Tóth A, Alberti Á, Béni S, Balla J, et al. Convergence chromatographic determination of camphor in the essential oil of Tanacetum parthenium L.: convergence chromatographic determination of camphor in essential oil. Biomed Chromatogr. 2016;30:2031–7. https://doi.org/10.1002/bmc.3781.
Desfontaine V, Nováková L, Ponzetto F, Nicoli R, Saugy M, Veuthey J-L, et al. Liquid chromatography and supercritical fluid chromatography as alternative techniques to gas chromatography for the rapid screening of anabolic agents in urine. J Chromatogr A. 2016;1451:145–55. https://doi.org/10.1016/j.chroma.2016.05.004.
Zhu L, Zhao Y, Xu Y, Sun Q, Sun X, Kang L, et al. Comparison of ultra-high performance supercritical fluid chromatography and ultra-high performance liquid chromatography for the separation of spirostanol saponins. J Pharm Biomed Anal. 2016;120:72–8. https://doi.org/10.1016/j.jpba.2015.12.002.
Hori K, Hori-Koriyama N, Tsumura K, Fukusaki E, Bamba T. Insights into the formation mechanism of chloropropanol fatty acid esters under laboratory-scale deodorization conditions. J Biosci Bioeng. 2016;122:246–51. https://doi.org/10.1016/j.jbiosc.2015.12.018.
Duval J, Colas C, Pecher V, Poujol M, Tranchant J-F, Lesellier E. Hyphenation of ultra high performance supercritical fluid chromatography with atmospheric pressure chemical ionisation high resolution mass spectrometry: Part 1. Study of the coupling parameters for the analysis of natural non-polar compounds. J Chromatogr A. 2017;1509:132–40. https://doi.org/10.1016/j.chroma.2017.06.016.
Gritti F. Unexpected retention and efficiency behaviors in supercritical fluid chromatography: a thermodynamic interpretation. J Chromatogr A. 2016;1468:209–16. https://doi.org/10.1016/j.chroma.2016.09.020.
Helmueller SC, Poe DP. Efficiency studies in supercritical fluid chromatography: importance of thermal diffusivity near the critical point. Biophys J. 2014;106:622a. https://doi.org/10.1016/j.bpj.2013.11.3440.
Delahaye S, Broeckhoven K, Desmet G, Lynen F. Application of the isopycnic kinetic plot method for elucidating the potential of sub-2 μm and core–shell particles in SFC. Talanta. 2013;116:1105–12. https://doi.org/10.1016/j.talanta.2013.08.023.
De Pauw R, Shoykhet (C)K, Desmet G, Broeckhoven K. Understanding and diminishing the extra-column band broadening effects in supercritical fluid chromatography. J Chromatogr A. 2015;1403:132–7. https://doi.org/10.1016/j.chroma.2015.05.017.
De Pauw R, Choikhet K, Desmet G, Broeckhoven K. Temperature effects in supercritical fluid chromatography: a trade-off between viscous heating and decompression cooling. J Chromatogr A. 2014;1365:212–8. https://doi.org/10.1016/j.chroma.2014.09.022.
Gritti F, Fogwill M, Gilar M, Jarrell JA. Maximizing performance in supercritical fluid chromatography using low-density mobile phases. J Chromatogr A. 2016;1468:217–27. https://doi.org/10.1016/j.chroma.2016.09.024.
De Pauw R, Shoykhet (C)K, Desmet G, Broeckhoven K. Effect of reference conditions on flow rate, modifier fraction and retention in supercritical fluid chromatography. J Chromatogr A. 2016;1459:129–35. https://doi.org/10.1016/j.chroma.2016.06.040.
Desfontaine V, Tarafder A, Hill J, Fairchild J, Grand-Guillaume Perrenoud A, Veuthey J-L, et al. A systematic investigation of sample diluents in modern supercritical fluid chromatography. J Chromatogr A. 2017;1511:122–31. https://doi.org/10.1016/j.chroma.2017.06.075.
Fairchild JN, Hill JF, Iraneta PC. Influence of sample solvent composition for SFC separations. LCGC N Am. 2013;31:326–33.
Enmark M, Åsberg D, Shalliker A, Samuelsson J, Fornstedt T. A closer study of peak distortions in supercritical fluid chromatography as generated by the injection. J Chromatogr A. 2015;1400:131–9. https://doi.org/10.1016/j.chroma.2015.04.059.
Siders PD. Simulated molecular-scale interaction of supercritical fluid mobile and stationary phases. J Chromatogr A. 2017;1527:97–104. https://doi.org/10.1016/j.chroma.2017.10.056.
Glenne E, Leek H, Klarqvist M, Samuelsson J, Fornstedt T. Systematic investigations of peak deformations due to co-solvent adsorption in preparative supercritical fluid chromatography. J Chromatogr A. 2017;1496:141–9. https://doi.org/10.1016/j.chroma.2017.03.053.
Glenne E, Öhlén K, Leek H, Klarqvist M, Samuelsson J, Fornstedt T. A closer study of methanol adsorption and its impact on solute retentions in supercritical fluid chromatography. J Chromatogr A. 2016;1442:129–39. https://doi.org/10.1016/j.chroma.2016.03.006.
Glenne E, Leek H, Klarqvist M, Samuelsson J, Fornstedt T. Peak deformations in preparative supercritical fluid chromatography due to co-solvent adsorption. J Chromatogr A. 2016;1468:200–8. https://doi.org/10.1016/j.chroma.2016.09.019.
Muscat Galea C, Mangelings D, Vander Heyden Y. Investigation of the effect of mobile phase composition on selectivity using a solvent-triangle based approach in achiral SFC. J Pharm Biomed Anal. 2017;132:247–57. https://doi.org/10.1016/j.jpba.2016.10.016.
Lemasson E, Bertin S, Hennig P, Boiteux H, Lesellier E, West C. Development of an achiral supercritical fluid chromatography method with ultraviolet absorbance and mass spectrometric detection for impurity profiling of drug candidates. Part I: optimization of mobile phase composition. J Chromatogr A. 2015;1408:217–26. https://doi.org/10.1016/j.chroma.2015.07.037.
Speybrouck D, Doublet C, Cardinael P, Fiol-Petit C, Corens D. The effect of high concentration additive on chiral separations in supercritical fluid chromatography. J Chromatogr A. 2017;1510:89–99. https://doi.org/10.1016/j.chroma.2017.06.049.
Geryk R, Kalíková K, Schmid MG, Tesařová E. Enantioselective separation of biologically active basic compounds in ultra-performance supercritical fluid chromatography. Anal Chim Acta. 2016;932:98–105. https://doi.org/10.1016/j.aca.2016.04.044.
Aranyi A, Ilisz I, Péter A, Fülöp F, West C. Exploring the enantioseparation of amino-naphthol analogues by supercritical fluid chromatography. J Chromatogr A. 2015;1387:123–33. https://doi.org/10.1016/j.chroma.2015.01.084.
West C, Melin J, Ansouri H, Mengue Metogo M. Unravelling the effects of mobile phase additives in supercritical fluid chromatography. Part I: polarity and acidity of the mobile phase. J Chromatogr A. 2017;1492:136–43. https://doi.org/10.1016/j.chroma.2017.02.066.
Andersson M, Rodriguez-Meizoso I, Turner C, Hjort K, Klintberg L. Dynamic pH determination at high pressure of aqueous additive mixtures in contact with dense CO 2. J Supercrit Fluids. 2018;136:95–101. https://doi.org/10.1016/j.supflu.2018.02.012.
Ebinger K, Weller HN. Comparative assessment of achiral stationary phases for high throughput analysis in supercritical fluid chromatography. J Chromatogr A. 2014;1332:73–81. https://doi.org/10.1016/j.chroma.2014.01.060.
Delahaye S, Lynen F. Implementing stationary-phase optimized selectivity in supercritical fluid chromatography. Anal Chem. 2014;86:12220–8. https://doi.org/10.1021/ac503313j.
Vera CM, Shock D, Dennis GR, Samuelsson J, Enmark M, Fornstedt T, et al. Contrasting selectivity between HPLC and SFC using phenyl-type stationary phases: a study on linear polynuclear aromatic hydrocarbons. Microchem J. 2015;119:40–3. https://doi.org/10.1016/j.microc.2014.10.008.
Wolrab D, Macíková P, Boras M, Kohout M, Lindner W. Strong cation exchange chiral stationary phase—a comparative study in high-performance liquid chromatography and subcritical fluid chromatography. J Chromatogr A. 2013;1317:59–66. https://doi.org/10.1016/j.chroma.2013.08.037.
West C, Lemasson E, Bertin S, Hennig P, Lesellier E. Interest of achiral-achiral tandem columns for impurity profiling of synthetic drugs with supercritical fluid chromatography. J Chromatogr A. 2018;1534:161–9. https://doi.org/10.1016/j.chroma.2017.12.061.
Wang C, Tymiak AA, Zhang Y. Optimization and simulation of tandem column supercritical fluid chromatography separations using column back pressure as a unique parameter. Anal Chem. 2014;86:4033–40. https://doi.org/10.1021/ac500530n.
Pokrovskiy OI, Ustinovich KB, Usovich OI, Parenago OO, Lunin VV, Ovchinnikov DV, et al. A case of Z/E-isomers elution order inversion caused by cosolvent percentage change in supercritical fluid chromatography. J Chromatogr A. 2017;1479:177–84. https://doi.org/10.1016/j.chroma.2016.11.037.
West C, Konjaria M-L, Shashviashvili N, Lemasson E, Bonnet P, Kakava R, et al. Enantioseparation of novel chiral sulfoxides on chlorinated polysaccharide stationary phases in supercritical fluid chromatography. J Chromatogr A. n.d. https://doi.org/10.1016/j.chroma.2017.03.089.
Khater S, Canault B, Azzimani T, Bonnet P, West C. Thermodynamic enantioseparation behavior of phenylthiohydantoin-amino acid derivatives in supercritical fluid chromatography on polysaccharide chiral stationary phases. J Sep Sci. 2018;41:1450–9. https://doi.org/10.1002/jssc.201701196.
Tyteca E, Desfontaine V, Desmet G, Guillarme D. Possibilities of retention modeling and computer assisted method development in supercritical fluid chromatography. J Chromatogr A. 2015;1381:219–28. https://doi.org/10.1016/j.chroma.2014.12.077.
Galea C, West C, Mangelings D, Vander Heyden Y. Is the solvation parameter model or its adaptations adequate to account for ionic interactions when characterizing stationary phases for drug impurity profiling with supercritical fluid chromatography? Anal Chim Acta. 2016;924:9–20. https://doi.org/10.1016/j.aca.2016.04.014.
Andri B, Dispas A, Marini RD, Hubert P, Sassiat P, Al Bakain R, et al. Combination of partial least squares regression and design of experiments to model the retention of pharmaceutical compounds in supercritical fluid chromatography. J Chromatogr A. 2017;1491:182–94. https://doi.org/10.1016/j.chroma.2017.02.030.
Enmark M, Samuelsson J, Forss E, Forssén P, Fornstedt T. Investigation of plateau methods for adsorption isotherm determination in supercritical fluid chromatography. J Chromatogr A. 2014;1354:129–38. https://doi.org/10.1016/j.chroma.2014.05.070.
Enmark M, Forssén P, Samuelsson J, Fornstedt T. Determination of adsorption isotherms in supercritical fluid chromatography. J Chromatogr A. 2013;1312:124–33. https://doi.org/10.1016/j.chroma.2013.09.007.
Guiochon G, Tarafder A. Fundamental challenges and opportunities for preparative supercritical fluid chromatography. J Chromatogr A. 2011;1218:1037–114. https://doi.org/10.1016/j.chroma.2010.12.047.
Tarafder A, Hudalla C, Iraneta P, Fountain KJ. A scaling rule in supercritical fluid chromatography. I. Theory for isocratic systems. J Chromatogr A. 2014;1362:278–93. https://doi.org/10.1016/j.chroma.2014.08.009.
Tarafder A, Hill JF. Scaling rule in SFC. II. A practical rule for isocratic systems. J Chromatogr A. 2017;1482:65–75. https://doi.org/10.1016/j.chroma.2016.12.044.
Enmark M, Åsberg D, Leek H, Öhlén K, Klarqvist M, Samuelsson J, et al. Evaluation of scale-up from analytical to preparative supercritical fluid chromatography. J Chromatogr A. 2015;1425:280–6. https://doi.org/10.1016/j.chroma.2015.11.001.
Guillarme D, Desfontaine V, Heinisch S, Veuthey J-L. What are the current solutions for interfacing supercritical fluid chromatography and mass spectrometry? J Chromatogr B. 2018;1083:160–70. https://doi.org/10.1016/j.jchromb.2018.03.010.
Tarafder A. Designs and methods for interfacing SFC with MS. J Chromatogr B. 2018;1091:1–13. https://doi.org/10.1016/j.jchromb.2018.05.003.
Wolrab D, Frühauf P, Gerner C. Direct coupling of supercritical fluid chromatography with tandem mass spectrometry for the analysis of amino acids and related compounds: comparing electrospray ionization and atmospheric pressure chemical ionization. Anal Chim Acta. 2017;981:106–15. https://doi.org/10.1016/j.aca.2017.05.005.
Ciclet O, Barron D, Bajic S, Veuthey J-L, Guillarme D, Grand-Guillaume PA. Natural compounds analysis using liquid and supercritical fluid chromatography hyphenated to mass spectrometry: evaluation of a new design of atmospheric pressure ionization source. J Chromatogr B. 2018;1083:1–11. https://doi.org/10.1016/j.jchromb.2018.02.037.
Fujito Y, Hayakawa Y, Izumi Y, Bamba T. Importance of optimizing chromatographic conditions and mass spectrometric parameters for supercritical fluid chromatography/mass spectrometry. J Chromatogr A. 2017;1508:138–47. https://doi.org/10.1016/j.chroma.2017.05.071.
Nováková L, Grand-Guillaume Perrenoud A, Nicoli R, Saugy M, Veuthey J-L, Guillarme D. Ultra high performance supercritical fluid chromatography coupled with tandem mass spectrometry for screening of doping agents. I: investigation of mobile phase and MS conditions. Anal Chim Acta. 2015;853:637–46. https://doi.org/10.1016/j.aca.2014.10.004.
Laboureur L, Bonneau N, Champy P, Brunelle A, Touboul D. Structural characterisation of acetogenins from Annona muricata by supercritical fluid chromatography coupled to high-resolution tandem mass spectrometry: structural characterization of acetogenins by SFC-HRMS/MS. Phytochem Anal. 2017. https://doi.org/10.1002/pca.2700.
Haglind A, Hedeland M, Arvidsson T, Pettersson CE. Major signal suppression from metal ion clusters in SFC/ESI-MS-cause and effects. J Chromatogr B. 2018;1084:96–105. https://doi.org/10.1016/j.jchromb.2018.03.024.
Desfontaine V, Capetti F, Nicoli R, Kuuranne T, Veuthey J-L, Guillarme D. Systematic evaluation of matrix effects in supercritical fluid chromatography versus liquid chromatography coupled to mass spectrometry for biological samples. J Chromatogr B. 2018;1079:51–61. https://doi.org/10.1016/j.jchromb.2018.01.037.
Svan A, Hedeland M, Arvidsson T, Pettersson CE. The differences in matrix effect between supercritical fluid chromatography and reversed phase liquid chromatography coupled to ESI/MS. Anal Chim Acta. 2018;1000:163–71. https://doi.org/10.1016/j.aca.2017.10.014.
Nováková L, Rentsch M, Grand-Guillaume Perrenoud A, Nicoli R, Saugy M, Veuthey J, et al. Ultra high performance supercritical fluid chromatography coupled with tandem mass spectrometry for screening of doping agents. II: analysis of biological samples. Anal Chim Acta. 2015;853:647–59. https://doi.org/10.1016/j.aca.2014.10.007.
Desfontaine V, Guillarme D, Francotte E, Nováková L. Supercritical fluid chromatography in pharmaceutical analysis. J Pharm Biomed Anal. n.d. https://doi.org/10.1016/j.jpba.2015.03.007.
Lemasson E, Bertin S, West C. Use and practice of achiral and chiral supercritical fluid chromatography in pharmaceutical analysis and purification. J Sep Sci. 2016;39:212–33. https://doi.org/10.1002/jssc.201501062.
Hicks MB, Regalado EL, Tan F, Gong X, Welch CJ. Supercritical fluid chromatography for GMP analysis in support of pharmaceutical development and manufacturing activities. J Pharm Biomed Anal. 2016;117:316–24. https://doi.org/10.1016/j.jpba.2015.09.014.
Dispas A, Lebrun P, Ziemons E, Marini R, Rozet E, Hubert P. Evaluation of the quantitative performances of supercritical fluid chromatography: from method development to validation. J Chromatogr A. 2014;1353:78–88. https://doi.org/10.1016/j.chroma.2014.01.046.
De Klerck K, Vander Heyden Y, Mangelings D. Generic chiral method development in supercritical fluid chromatography and ultra-performance supercritical fluid chromatography. J Chromatogr A. 2014;1363:311–22. https://doi.org/10.1016/j.chroma.2014.06.011.
Ismail OH, Ciogli A, Villani C, De Martino M, Pierini M, Cavazzini A, et al. Ultra-fast high-efficiency enantioseparations by means of a teicoplanin-based chiral stationary phase made on sub-2μm totally porous silica particles of narrow size distribution. J Chromatogr A. 2016;1427:55–68. https://doi.org/10.1016/j.chroma.2015.11.071.
Barhate CL, Joyce LA, Makarov AA, Zawatzky K, Bernardoni F, Schafer WA, et al. Ultrafast chiral separations for high throughput enantiopurity analysis. Chem Commun. 2017;53:509–12. https://doi.org/10.1039/C6CC08512A.
Welch CJ, Regalado EL. Estimating optimal time for fast chromatographic separations. J Sep Sci. 2014;37:2552–8. https://doi.org/10.1002/jssc.201400508.
Regalado EL, Welch CJ. Pushing the speed limit in enantioselective supercritical fluid chromatography: liquid chromatography. J Sep Sci. 2015;38:2826–32. https://doi.org/10.1002/jssc.201500270.
Zawatzky K, Biba M, Regalado EL, Welch CJ. MISER chiral supercritical fluid chromatography for high throughput analysis of enantiopurity. J Chromatogr A. 2016;1429:374–9. https://doi.org/10.1016/j.chroma.2015.12.057.
Schou-Pedersen AMV, Østergaard J, Johansson M, Dubant S, Frederiksen RB, Hansen SH. Evaluation of supercritical fluid chromatography for testing of PEG adducts in pharmaceuticals. J Pharm Biomed Anal. 2014;88:256–61. https://doi.org/10.1016/j.jpba.2013.08.039.
Lecoeur M, Decaudin B, Guillotin Y, Sautou V, Vaccher C. Comparison of high-performance liquid chromatography and supercritical fluid chromatography using evaporative light scattering detection for the determination of plasticizers in medical devices. J Chromatogr A. 2015;1417:104–15. https://doi.org/10.1016/j.chroma.2015.09.026.
Foulon C, Di Giulio P, Lecoeur M. Simultaneous determination of inorganic anions and cations by supercritical fluid chromatography using evaporative light scattering detection. J Chromatogr A. 2018;1534:139–49. https://doi.org/10.1016/j.chroma.2017.12.047.
Desfontaine V, Nováková L, Guillarme D. SFC–MS versus RPLC–MS for drug analysis in biological samples. Bioanalysis. 2015;7:1193–5. https://doi.org/10.4155/bio.15.41.
Dispas A, Jambo H, André S, Tyteca E, Hubert P. Supercritical fluid chromatography: a promising alternative to current bioanalytical techniques. Bioanalysis. 2018;10:107–24. https://doi.org/10.4155/bio-2017-0211.
Wang M, Wang Y-H, Avula B, Radwan MM, Wanas AS, Mehmedic Z, et al. Quantitative determination of cannabinoids in Cannabis and Cannabis products using ultra-high-performance supercritical fluid chromatography and diode array/mass spectrometric detection. J Forensic Sci. 2017;62:602–11. https://doi.org/10.1111/1556-4029.13341.
Segawa H, Iwata YT, Yamamuro T, Kuwayama K, Tsujikawa K, Kanamori T, et al. Enantioseparation of methamphetamine by supercritical fluid chromatography with cellulose-based packed column. Forensic Sci Int. 2017;273:39–44. https://doi.org/10.1016/j.forsciint.2017.01.025.
Li L. Direct enantiomer determination of methorphan by HPLC-MS and SFC-MS. Forensic Chem. 2016;2:82–5. https://doi.org/10.1016/j.forc.2016.10.004.
Tamura S, Koike Y, Takeda H, Koike T, Izumi Y, Nagasaka R, et al. Ameliorating effects of D-47, a newly developed compound, on lipid metabolism in an animal model of familial hypercholesterolemia (WHHLMI rabbits). Eur J Pharmacol. 2018;822:147–53. https://doi.org/10.1016/j.ejphar.2018.01.013.
Takeda H, Koike T, Izumi Y, Yamada T, Yoshida M, Shiomi M, et al. Lipidomic analysis of plasma lipoprotein fractions in myocardial infarction-prone rabbits. J Biosci Bioeng. 2015;120:476–82. https://doi.org/10.1016/j.jbiosc.2015.02.015.
Yamada T, Uchikata T, Sakamoto S, Yokoi Y, Nishiumi S, Yoshida M, et al. Supercritical fluid chromatography/Orbitrap mass spectrometry based lipidomics platform coupled with automated lipid identification software for accurate lipid profiling. J Chromatogr A. 2013;1301:237–42. https://doi.org/10.1016/j.chroma.2013.05.057.
Montenegro-Burke JR, Sutton JA, Rogers LM, Milne GL, McLean JA, Aronoff DM. Lipid profiling of polarized human monocyte-derived macrophages. Prostaglandins & Other Lipid Mediators. 2016;127:1–8. https://doi.org/10.1016/j.prostaglandins.2016.11.002.
Lísa M, Cífková E, Khalikova M, Ovčačíková M, Holčapek M. Lipidomic analysis of biological samples: comparison of liquid chromatography, supercritical fluid chromatography and direct infusion mass spectrometry methods. J Chromatogr A. 2017;1525:96–108. https://doi.org/10.1016/j.chroma.2017.10.022.
Wang M, Carrell EJ, Ali Z, Avula B, Avonto C, Parcher JF, et al. Comparison of three chromatographic techniques for the detection of mitragynine and other indole and oxindole alkaloids in Mitragyna speciosa (kratom) plants: liquid chromatography. J Sep Sci. 2014;37:1411–8. https://doi.org/10.1002/jssc.201301389.
Huang Y, Tang G, Zhang T, Fillet M, Crommen J, Jiang Z. Supercritical fluid chromatography in traditional Chinese medicine analysis. J Pharm Biomed Anal. 2018;147:65–80. https://doi.org/10.1016/j.jpba.2017.08.021.
Eisath NG, Sturm S, Stuppner H. Supercritical fluid chromatography in natural product analysis–an update. Planta Med. 2018;84:361–71. https://doi.org/10.1055/s-0037-1599461.
Lee JW, Nagai T, Gotoh N, Fukusaki E, Bamba T. Profiling of regioisomeric triacylglycerols in edible oils by supercritical fluid chromatography/tandem mass spectrometry. J Chromatogr B. 2014;966:193–9. https://doi.org/10.1016/j.jchromb.2014.01.040.
Rathi D-N, Liew CY, Fairulnizal MNM, Isameyah D, Barknowitz G. Fat-soluble vitamin and carotenoid analysis in cooking oils by ultra-performance convergence chromatography. Food Anal Methods. 2017;10:1087–96. https://doi.org/10.1007/s12161-016-0661-9.
Giuffrida D, Zoccali M, Giofrè SV, Dugo P, Mondello L. Apocarotenoids determination in Capsicum chinense Jacq. cv. Habanero, by supercritical fluid chromatography-triple-quadrupole/mass spectrometry. Food Chem. 2017;231:316–23. https://doi.org/10.1016/j.foodchem.2017.03.145.
Jumaah F, Plaza M, Abrahamsson V, Turner C, Sandahl M. A fast and sensitive method for the separation of carotenoids using ultra-high performance supercritical fluid chromatography-mass spectrometry. Anal Bioanal Chem. 2016;408:5883–94. https://doi.org/10.1007/s00216-016-9707-5.
Li B, Zhao H, Liu J, Liu W, Fan S, Wu G, et al. Application of ultra-high performance supercritical fluid chromatography for the determination of carotenoids in dietary supplements. J Chromatogr A. 2015;1425:287–92. https://doi.org/10.1016/j.chroma.2015.11.029.
Abrahamsson V, Rodriguez-Meizoso I, Turner C. Determination of carotenoids in microalgae using supercritical fluid extraction and chromatography. J Chromatogr A. 2012;1250:63–8. https://doi.org/10.1016/j.chroma.2012.05.069.
Matsubara A, Bamba T, Ishida H, Fukusaki E, Hirata K. Highly sensitive and accurate profiling of carotenoids by supercritical fluid chromatography coupled with mass spectrometry. J Sep Sci. 2009;32:1459–64. https://doi.org/10.1002/jssc.200800699.
Murauer A, Ganzera M. Quantitative determination of major alkaloids in Cinchona bark by supercritical fluid chromatography. J Chromatogr A. 2018. https://doi.org/10.1016/j.chroma.2018.04.038.
Fu Q, Li Z, Sun C, Xin H, Ke Y, Jin Y, et al. Rapid and simultaneous analysis of sesquiterpene pyridine alkaloids from Tripterygium wilfordii Hook. f. Using supercritical fluid chromatography-diode array detector-tandem mass spectrometry. J Supercrit Fluids. 2015;104:85–93. https://doi.org/10.1016/j.supflu.2015.05.006.
Aichner D, Ganzera M. Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta. 2015;144:1239–44. https://doi.org/10.1016/j.talanta.2015.08.011.
Duval J, Destandau E, Pecher V, Poujol M, Tranchant J-F, Lesellier E. Selective enrichment in bioactive compound from Kniphofia uvaria by super/subcritical fluid extraction and centrifugal partition chromatography. J Chromatogr A. 2016;1447:26–38. https://doi.org/10.1016/j.chroma.2016.04.029.
Winderl B, Schwaiger S, Ganzera M. Fast and improved separation of major coumarins in Ammi visnaga (L.) Lam. by supercritical fluid chromatography: other techniques. J Sep Sci. 2016;39:4042–8. https://doi.org/10.1002/jssc.201600734.
Pfeifer I, Murauer A, Ganzera M. Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J Pharm Biomed Anal. 2016;129:246–51. https://doi.org/10.1016/j.jpba.2016.07.014.
Huang Y, Zhang T, Zhou H, Feng Y, Fan C, Chen W, et al. Fast separation of triterpenoid saponins using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J Pharm Biomed Anal. 2016;121:22–9. https://doi.org/10.1016/j.jpba.2015.12.056.
Huang Y, Feng Y, Tang G, Li M, Zhang T, Fillet M, et al. Development and validation of a fast SFC method for the analysis of flavonoids in plant extracts. J Pharm Biomed Anal. 2017;140:384–91. https://doi.org/10.1016/j.jpba.2017.03.012.
Qiao X, Song W, Wang Q, Liu K, Zhang Z, Bo T, et al. Comprehensive chemical analysis of triterpenoids and polysaccharides in the medicinal mushroom Antrodia cinnamomea. RSC Adv. 2015;5:47040–52. https://doi.org/10.1039/C5RA04327A.
Pauk V, Pluháček T, Havlíček V, Lemr K. Ultra-high performance supercritical fluid chromatography-mass spectrometry procedure for analysis of monosaccharides from plant gum binders. Anal Chim Acta. 2017. https://doi.org/10.1016/j.aca.2017.07.036.
Huang Y, Zhang T, Zhao Y, Zhou H, Tang G, Fillet M, et al. Simultaneous analysis of nucleobases, nucleosides and ginsenosides in ginseng extracts using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J Pharm Biomed Anal. 2017;144:213–9. https://doi.org/10.1016/j.jpba.2017.03.059.
Xin H, Dai Z, Cai J, Ke Y, Shi H, Fu Q, et al. Rapid purification of diastereoisomers from Piper kadsura using supercritical fluid chromatography with chiral stationary phases. J Chromatogr A. 2017;1509:141–6. https://doi.org/10.1016/j.chroma.2017.06.020.
Zhang L, Sun A, Li A, Kang J, Wang Y, Liu R. Isolation and purification of osthole and imperatorin from Fructus Cnidii by semi-preparative supercritical fluid chromatography. J Liq Chromatogr Relat Technol. 2017;40:407–14. https://doi.org/10.1080/10826076.2017.1315723.
Nothias L-F, Boutet-Mercey S, Cachet X, De La Torre E, Laboureur L, Gallard J-F, et al. Environmentally friendly procedure based on supercritical fluid chromatography and tandem mass spectrometry molecular networking for the discovery of potent antiviral compounds from Euphorbia semiperfoliata. J Nat Prod. 2017;80:2620–9. https://doi.org/10.1021/acs.jnatprod.7b00113.
Scheuba J, Wronski V-K, Rollinger J, Grienke U. Fast and green – CO2 based extraction, isolation, and quantification of phenolic Styrax constituents. Planta Med. 2017;83:1068–75. https://doi.org/10.1055/s-0043-105499.
Nie L, Dai Z, Ma S. Improved chiral separation of (R,S)-goitrin by SFC: an application in traditional Chinese medicine. J Anal Meth Chem. 2016;2016:1–5. https://doi.org/10.1155/2016/5782942.
Ashraf-Khorassani M, Coleman WM, Dube MF, Isaac G, Taylor LT. Synthesis, purification, and quantification of fatty acid ethyl esters after trans-esterification of large batches of tobacco seed oil. Contrib Tobacco Res. 2015;26 https://doi.org/10.1515/cttr-2015-0008.
Han NM, Choo MY. Enhancing the separation and purification efficiency of palm oil carotenes using supercritical fluid chromatography. J Palm Oil Res. 2015;27:387–92.
Song W, Qiao X, Liang W, Ji S, Yang L, Wang Y, et al. Efficient separation of curcumin, demethoxycurcumin, and bisdemethoxycurcumin from turmeric using supercritical fluid chromatography: from analytical to preparative scale: sample preparation. J Sep Sci. 2015;38:3450–3. https://doi.org/10.1002/jssc.201500686.
Krief A, Dunkle M, Bahar M, Bultinck P, Herrebout W, Sandra P. Elucidation of the absolute configuration of rhizopine by chiral supercritical fluid chromatography and vibrational circular dichroism: other techniques. J Sep Sci. 2015;38:2545–50. https://doi.org/10.1002/jssc.201500138.
Samimi R, Xu WZ, Alsharari Q, Charpentier PA. Supercritical fluid chromatography of North American ginseng extract. J Supercrit Fluids. 2014;86:115–23. https://doi.org/10.1016/j.supflu.2013.12.004.
Fernández-Ponce MT, Casas L, Mantell C, Martínez de la Ossa E. Fractionation of Mangifera indica Linn polyphenols by reverse phase supercritical fluid chromatography (RP-SFC) at pilot plant scale. J Supercrit Fluids. 2014;95:444–56. https://doi.org/10.1016/j.supflu.2014.10.005.
Zhou Q, Gao B, Zhang X, Xu Y, Shi H, Yu L(L). Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra-performance convergence chromatography combined with a quadrupole time-of-flight mass spectrometry. Food Chem. 2014;143:199–204. https://doi.org/10.1016/j.foodchem.2013.07.114.
Tu A, Ma Q, Bai H, Du Z. A comparative study of triacylglycerol composition in Chinese human milk within different lactation stages and imported infant formula by SFC coupled with Q-TOF-MS. Food Chem. 2017;221:555–67. https://doi.org/10.1016/j.foodchem.2016.11.139.
Qi N, Gong X, Feng C, Wang X, Xu Y, Lin L. Simultaneous analysis of eight vitamin E isomers in Moringa oleifera Lam. leaves by ultra performance convergence chromatography. Food Chem. 2016;207:157–61. https://doi.org/10.1016/j.foodchem.2016.03.089.
Zhang Y, Du Z, Xia X, Guo Q, Wu H, Yu W. Evaluation of the migration of UV-ink photoinitiators from polyethylene food packaging by supercritical fluid chromatography combined with photodiode array detector and tandem mass spectrometry. Polym Test. 2016;53:276–82. https://doi.org/10.1016/j.polymertesting.2016.06.008.
Tao Y, Zheng Z, Yu Y, Xu J, Liu X, Wu X, et al. Supercritical fluid chromatography–tandem mass spectrometry-assisted methodology for rapid enantiomeric analysis of fenbuconazole and its chiral metabolites in fruits, vegetables, cereals, and soil. Food Chem. 2018;241:32–9. https://doi.org/10.1016/j.foodchem.2017.08.038.
Li R, Chen Z, Dong F, Xu J, Liu X, Wu X, et al. Supercritical fluid chromatographic-tandem mass spectrometry method for monitoring dissipation of thiacloprid in greenhouse vegetables and soil under different application modes. J Chromatogr B. 2018;1081–1082:25–32. https://doi.org/10.1016/j.jchromb.2018.02.021.
Cheng Y, Zheng Y, Dong F, Li J, Zhang Y, Sun S, et al. Stereoselective analysis and dissipation of propiconazole in wheat, grapes, and soil by supercritical fluid chromatography–tandem mass spectrometry. J Agric Food Chem. 2017;65:234–43. https://doi.org/10.1021/acs.jafc.6b04623.
Pan X, Dong F, Xu J, Liu X, Chen Z, Zheng Y. Stereoselective analysis of novel chiral fungicide pyrisoxazole in cucumber, tomato and soil under different application methods with supercritical fluid chromatography/tandem mass spectrometry. J Hazard Mater. 2016;311:115–24. https://doi.org/10.1016/j.jhazmat.2016.03.005.
Liu N, Dong F, Xu J, Liu X, Chen Z, Pan X, et al. Enantioselective separation and pharmacokinetic dissipation of cyflumetofen in field soil by ultra-performance convergence chromatography with tandem mass spectrometry. J Sep Sci. 2016;39:1363–70. https://doi.org/10.1002/jssc.201501123.
Chen X, Dong F, Xu J, Liu X, Chen Z, Liu N, et al. Enantioseparation and determination of isofenphos-methyl enantiomers in wheat, corn, peanut and soil with supercritical fluid chromatography/tandem mass spectrometric method. J Chromatogr B. 2016;1015–1016:13–21. https://doi.org/10.1016/j.jchromb.2016.02.003.
Tao Y, Dong F, Xu J, Liu X, Cheng Y, Liu N, et al. Green and sensitive supercritical fluid chromatographic–tandem mass spectrometric method for the separation and determination of flutriafol enantiomers in vegetables, fruits, and soil. J Agric Food Chem. 2014;62:11457–64. https://doi.org/10.1021/jf504324t.
Lou C, Wu C, Zhang K, Guo D, Jiang L, Lu Y, et al. Graphene-coated polystyrene-divinylbenzene dispersive solid-phase extraction coupled with supercritical fluid chromatography for the rapid determination of 10 allergenic disperse dyes in industrial wastewater samples. J Chromatogr A. 2018;1550:45–56. https://doi.org/10.1016/j.chroma.2018.03.040.
González-Mariño I, Thomas KV, Reid MJ. Determination of cannabinoid and synthetic cannabinoid metabolites in wastewater by liquid-liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry: cannabinoid derivatives in wastewater by UHPSFC-MS/MS. Drug Test Anal. 2018;10:222–8. https://doi.org/10.1002/dta.2199.
Lorenzo M, Campo J, Picó Y. Analytical challenges to determine emerging persistent organic pollutants in aquatic ecosystems. TrAC Trends in Anal Chem. 2018;103:137–55. https://doi.org/10.1016/j.trac.2018.04.003.
Zhou Y, Du Z, Zhang Y. Simultaneous determination of 17 disperse dyes in textile by ultra-high performance supercritical fluid chromatography combined with tandem mass spectrometry. Talanta. 2014;127:108–15. https://doi.org/10.1016/j.talanta.2014.03.055.
Khalikova MA, Šatínský D, Solich P, Nováková L. Development and validation of ultra-high performance supercritical fluid chromatography method for determination of illegal dyes and comparison to ultra-high performance liquid chromatography method. Anal Chim Acta. 2015;874:84–96. https://doi.org/10.1016/j.aca.2015.03.003.
Riddell N, van Bavel B, Ericson Jogsten I, McCrindle R, McAlees A, Chittim B. Examination of technical mixtures of halogen-free phosphorus based flame retardants using multiple analytical techniques. Chemosphere. 2017;176:333–41. https://doi.org/10.1016/j.chemosphere.2017.02.129.
Riddell N, van Bavel B, Ericson Jogsten I, McCrindle R, McAlees A, Chittim B. Coupling supercritical fluid chromatography to positive ion atmospheric pressure ionization mass spectrometry: ionization optimization of halogenated environmental contaminants. Int J Mass Spectrom. 2017;421:156–63. https://doi.org/10.1016/j.ijms.2017.07.005.
Gross MS, Olivos HJ, Butryn DM, Olson JR, Aga DS. Analysis of hydroxylated polybrominated diphenyl ethers (OH-BDEs) by supercritical fluid chromatography/mass spectrometry. Talanta. 2016;161:122–9. https://doi.org/10.1016/j.talanta.2016.08.013.
Thiébaut D. Separations of petroleum products involving supercritical fluid chromatography. J Chromatogr A. 2012;1252:177–88. https://doi.org/10.1016/j.chroma.2012.06.074.
Crepier J, Le Masle A, Charon N, Albrieux F, Heinisch S. Development of a supercritical fluid chromatography method with ultraviolet and mass spectrometry detection for the characterization of biomass fast pyrolysis bio oils. J Chromatogr A. 2017;1510:73–81. https://doi.org/10.1016/j.chroma.2017.06.003.
Crepier J, Le Masle A, Charon N, Albrieux F, Duchene P, Heinisch S. Ultra-high performance supercritical fluid chromatography hyphenated to atmospheric pressure chemical ionization high resolution mass spectrometry for the characterization of fast pyrolysis bio-oils. J Chromatogr B. 2018;1086:38–46. https://doi.org/10.1016/j.jchromb.2018.04.005.
Ashraf-Khorassani M, Yang J, Rainville P, Jones MD, Fountain KJ, Isaac G, et al. Ultrahigh performance supercritical fluid chromatography of lipophilic compounds with application to synthetic and commercial biodiesel. J Chromatogr B. 2015;983–984:94–100. https://doi.org/10.1016/j.jchromb.2014.12.012.
Lesellier E, Mith D, Dubrulle I. Method developments approaches in supercritical fluid chromatography applied to the analysis of cosmetics. J Chromatogr A. 2015;1423:158–68. https://doi.org/10.1016/j.chroma.2015.10.053.
Khater S, West C. Development and validation of a supercritical fluid chromatography method for the direct determination of enantiomeric purity of provitamin B5 in cosmetic formulations with mass spectrometric detection. J Pharm Biomed Anal. 2015;102:321–5. https://doi.org/10.1016/j.jpba.2014.09.036.
Yamamoto E, Hilton MJ, Orlandi M, Saini V, Toste FD, Sigman MS. Development and analysis of a Pd(0)-catalyzed enantioselective 1,1-diarylation of acrylates enabled by chiral anion phase transfer. J Am Chem Soc. 2016;138:15877–80. https://doi.org/10.1021/jacs.6b11367.
Krupkova S, Aguete GP, Kocmanova L, Volna T, Grepl M, Novakova L, et al. Solid-phase synthesis of ɤ-lactone and 1,2-oxazine derivatives and their efficient chiral analysis. PLoS One. 2016;11:e0166558. https://doi.org/10.1371/journal.pone.0166558.
Fassauer GM, Hofstetter R, Hasan M, Oswald S, Modeß C, Siegmund W, et al. Ketamine metabolites with antidepressant effects: fast, economical, and eco-friendly enantioselective separation based on supercritical-fluid chromatography (SFC) and single quadrupole MS detection. J Pharm Biomed Anal. 2017;146:410–9. https://doi.org/10.1016/j.jpba.2017.09.007.
Su C, Yang H, Meng X, Fawcett JP, Cao J, Yang Y, et al. Determination of rabeprazole enantiomers in dog plasma by supercritical fluid chromatography tandem mass spectrometry and its application to a pharmacokinetic study. J Sep Sci. 2017;40:1010–6. https://doi.org/10.1002/jssc.201601232.
Tan Q, Fan J, Gao R, He R, Wang T, Zhang Y, et al. Stereoselective quantification of triticonazole in vegetables by supercritical fluid chromatography. Talanta. 2017;164:362–7. https://doi.org/10.1016/j.talanta.2016.08.077.
Chen Z, Dong F, Pan X, Xu J, Liu X, Wu X, et al. Influence of uptake pathways on the stereoselective dissipation of chiral neonicotinoid sulfoxaflor in greenhouse vegetables. J Agric Food Chem. 2016;64:2655–60. https://doi.org/10.1021/acs.jafc.5b05940.
Camacho-Muñoz D, Kasprzyk-Hordern B, Thomas KV. Enantioselective simultaneous analysis of selected pharmaceuticals in environmental samples by ultrahigh performance supercritical fluid based chromatography tandem mass spectrometry. Anal Chim Acta. 2016;934:239–51. https://doi.org/10.1016/j.aca.2016.05.051.
Storch J, Kalíková K, Tesařová E, Maier V, Vacek J. Development of separation methods for the chiral resolution of hexahelicenes. J Chromatogr A. 2016;1476:130–4. https://doi.org/10.1016/j.chroma.2016.10.083.
Hoguet V, Charton J, Hecquet P-E, Lakhmi C, Lipka E. Supercritical fluid chromatography versus high performance liquid chromatography for enantiomeric and diastereoisomeric separations on coated polysaccharides-based stationary phases: application to dihydropyridone derivatives. J Chromatogr A. 2018;1549:39–50. https://doi.org/10.1016/j.chroma.2018.03.035.
Leek H, Thunberg L, Jonson AC, Öhlén K, Klarqvist M. Strategy for large-scale isolation of enantiomers in drug discovery. Drug Discov Today. 2017;22:133–9. https://doi.org/10.1016/j.drudis.2016.09.018.
Dai J, Wang C, Traeger SC, Discenza L, Obermeier MT, Tymiak AA, et al. The role of chromatographic and chiroptical spectroscopic techniques and methodologies in support of drug discovery for atropisomeric drug inhibitors of Bruton’s tyrosine kinase. J Chromatogr A. 2017;1487:116–28. https://doi.org/10.1016/j.chroma.2017.01.016.
Muscat Galea C, Slosse A, Mangelings D, Vander Heyden Y. Investigation of the effect of column temperature and back-pressure in achiral supercritical fluid chromatography within the context of drug impurity profiling. J Chromatogr A. 2017;1518:78–88. https://doi.org/10.1016/j.chroma.2017.08.008.
Muscat Galea C, Didion D, Clicq D, Mangelings D, Vander Heyden Y. Method optimization for drug impurity profiling in supercritical fluid chromatography: application to a pharmaceutical mixture. J Chromatogr A. 2017;1526:128–36. https://doi.org/10.1016/j.chroma.2017.10.036.
Tarafder A, Vajda P, Guiochon G. Accurate on-line mass flow measurements in supercritical fluid chromatography. J Chromatogr A. 2013;1320:130–7. https://doi.org/10.1016/j.chroma.2013.10.041.
Forss E, Haupt D, Stålberg O, Enmark M, Samuelsson J, Fornstedt T. Chemometric evaluation of the combined effect of temperature, pressure, and co-solvent fractions on the chiral separation of basic pharmaceuticals using actual vs set operational conditions. J Chromatogr A. 2017;1499:165–73. https://doi.org/10.1016/j.chroma.2017.03.077.
Åsberg D, Enmark M, Samuelsson J, Fornstedt T. Evaluation of co-solvent fraction, pressure and temperature effects in analytical and preparative supercritical fluid chromatography. J Chromatogr A. 2014;1374:254–60. https://doi.org/10.1016/j.chroma.2014.11.045.
Beres Martin J, Olesik Susan V. Enhanced-fluidity liquid chromatography using mixed-mode hydrophilic interaction liquid chromatography/strong cation-exchange retention mechanisms. J Sep Sci. 2015;38:3119–29. https://doi.org/10.1002/jssc.201500454.
Treadway JW, Philibert GS, Olesik SV. Enhanced fluidity liquid chromatography for hydrophilic interaction separation of nucleosides. J Chromatogr A. 2011;1218:5897–902. https://doi.org/10.1016/j.chroma.2010.12.059.
Taguchi K, Fukusaki E, Bamba T. Simultaneous analysis for water- and fat-soluble vitamins by a novel single chromatography technique unifying supercritical fluid chromatography and liquid chromatography. J Chromatogr A. 2014;1362:270–7. https://doi.org/10.1016/j.chroma.2014.08.003.
Wolrab D, Frühauf P, Gerner C, Kohout M, Lindner W. Consequences of transition from liquid chromatography to supercritical fluid chromatography on the overall performance of a chiral zwitterionic ion-exchanger. J Chromatogr A. 2017;1517:165–75. https://doi.org/10.1016/j.chroma.2017.08.022.
Funding
Caroline West is grateful for the support received by the Institut Universitaire de France (IUF), of which she is a Junior Member.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Caroline West has received support in her research from Waters Corporation through the Centers of Innovation Program.
Rights and permissions
About this article
Cite this article
West, C. Current trends in supercritical fluid chromatography. Anal Bioanal Chem 410, 6441–6457 (2018). https://doi.org/10.1007/s00216-018-1267-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1267-4