Abstract
This study presents a column-switching solid-phase extraction online-coupled to a liquid chromatography/tandem mass spectrometry (SPE-LC-MS/MS) method for simultaneous analysis of 12 antibiotics (7 sulfonamides and 5 fluoroquinolones) and caffeine detected in the sewage and effluent of a pilot anaerobic reactor used in sewage treatment. After acidification and filtration, the samples were directly injected into a simple and conventional LC system. Backflush and foreflush modes were compared based on the theoretical plates and peak asymmetry observed. The method was tested in terms of detection (MDL) and quantification limit (MQL), linearity, relative recovery, and precision intra- and inter-day in lab-made sewage samples. The method presented suitable figures of merit in terms of detection, varying from 8.00 × 10−5 to 6.00 × 10−2 ng (0.800 up to 600 ng L−1; caffeine) with direct injection volume of only 100 μL and 13 min of total analysis time (sample preparation and chromatographic run). When the method was applied in the analysis of sewage and effluent of the anaerobic reactor (n = 15), six antibiotics and caffeine were detected in concentrations ranging from 0.018 to 1097 μg L−1. To guarantee a reliable quantification, standard addition was used to overcome the matrix effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been reported in environmental matrices as a result of their wide use in human and veterinary medicine [1]. Sulfonamides are an important group of antibiotics mainly applied in veterinary medicine to treat urinary tract infections, ear infections, and bronchitis. The co-administration of trimethoprim-sulfamethoxazole enhances the treatment against bacterial infections inhibiting the synthesis of folic acid, essential for bacterial growth [2]. Fluoroquinolones are another important antibiotic group used for human and veterinary medicine. Both antibiotic groups have been found in concentrations of micrograms to nanograms per liter in sewage, wastewater, river water, wastewater treatment plant (WWTP) effluent, and other environmental waters [3–7].
Caffeine is a cardiovascular, cerebral, and respiratory stimulant found in analgesic and headache medication. Moreover, it is a component of a large variety of beverages and numerous food products. Thus, it has been reported in environmental matrices in concentration levels of nanograms to micrograms per liter [8, 9]. Based on these facts, caffeine is considered by researchers as an anthropogenic biomarker [10, 11].
These pharmaceutical compounds reach the sewage through animal or human excreta, disposal of expired or unused medicine, or its direct application into the environment, for example, fish farming [12]. Moreover, WWTPs are not designed for the removal of these compounds, and also around 82 % of Brazilian municipalities discharge sewage directly into the rivers [12–14].
Although sulfonamides and fluoroquinolones occur in low concentrations (ng L−1), their ecotoxicological effects such as genotoxicity and mutagenicity have been described. Moreover, antibiotics in the environment may select resistant bacteria, or even enhance single, cross- and multi-resistance bacteria [15–17].
Both sulfonamides and fluoroquinolones are polar, water soluble, and chemically stable, which contributes to the antibiotic mobility in the environmental matrices and hinders the extraction and chromatographic separation [18, 19].
It is important to develop analytical methods that enable fast, sensitive, and reliable analysis mainly for monitoring antibiotic residues in environmental matrices. Liquid chromatography/tandem mass spectrometry (LC-MS/MS) is the instrumental technique of choice. A large variety of LC-MS/MS methods are available to analyze antibiotics in environmental matrices [6, 20–23]. Currently, priority falls on maximizing the number of antibiotics in a single method, and also on automating sample preparation and decreasing the sample volume used per analysis. Solid-phase extraction (SPE) is a sample preparation technique for environmental water analysis; however, in a conventional offline and manual setup, it is a tedious and time-consuming procedure [24]. On the other hand, an online setup overcomes those limitations, although it might require available dedicated devices or instrumental arrangement with valves and pumps. Recently, online SPE has been applied for the analysis of antibiotics in environmental matrices, although most of these methods only determine antibiotics from the same group, sulfonamides or fluoroquinolones [25–27]. Screening methods for multi-residue analysis of micropollutants using online SPE are also reported applying dedicated instruments for online sample preparation [28–30].
Regarding wastewater treatment techniques, new techniques are under development such as Fenton-like degradation using Fe3+ and H2O2, photo-Fenton mediated by Fe3+ and oxalate, nanofiltration, electrochemical remediation, membrane bioreactor, and anaerobic reactors to evaluate the removal efficiency of pharmaceutical compounds [31–36]. The advancement of analytical instruments to determine compounds in sub-nanogram-per-liter level with minimum sample handling is essential to evaluate those new treatment technologies.
This study offers several advantages; firstly, the minimization of sample handling by using online column-switching sample preparation. Secondly, the injection of low sample volume of 100 μL was enough to perform the total analysis. Thirdly, a low nanogram-per-liter level was reached for simultaneous analysis of sulfonamides, fluoroquinolones, and caffeine (major compounds found in environmental matrices), using only injection volume of 100 μL applied for sample pre-concentration. Fourthly, the method development, validation, and sample analysis was carried out in a conventional high-performance liquid chromatography (HPLC) system (with autosampler and six-port valves) capable of column switching, not requiring a dedicated device to perform online sample preparation. Only 13 min were necessary for sample preparation and chromatographic run to analyze 13 environmental-relevant compounds.
Finally, the method developed was successfully applied in the analyses of antibiotics found in the sewage and pilot-scale anaerobic reactor effluent. To the author’s knowledge, this is the first published report of effluent analysis from vertical structured-bed reactor under intermittent aeration, a new reactor setup and the first publication of this online automated setup for this group of compounds in complex environmental liquid samples as sewage and wastewater effluent (see Electronic supplementary material (ESM) Table S1). There were seven compounds detected in all samples analyzed.
Materials and methods
Chemicals and reagents
Purified water was produced in the laboratory by a Milli-Q Plus Ultra (Billerica, MA) purification station.
All drug standards used were of high purity grade (>98 %). Sulfamethazine (SMZ), sulfamethoxazole (SMTX), sulfamerazine (SMER), sulfadiazine (SDIZ), sulfacetamide (SAC), sulfadimethoxine (SDMX), trimethoprim (TMP), norfloxacin (NOR), ofloxacin (OFLO), ciprofloxacin (CIP), pefloxacin (PEF), enrofloxacin (ENRO), and caffeine (CAF) were obtained from Sigma-Aldrich (St. Louis, MO). Isotopically labeled compounds used as internal standard (IS) 13C-SMTX, 13C-SDMX, 13C-SMZ, TMP-D9, ENRO-D5, CIP-D8, and NOR-D5 were also acquired from Sigma-Aldrich. Individual sulfonamide stock solutions at 500 mg L−1 were prepared in acetonitrile (Panreac, Barcelona), and 0.1 % of phosphoric acid or formic acid (Panreac) was added to improve dissolution. Stock solution of 500 mg L−1 of fluoroquinolones and CAF were prepared in methanol (Panreac).
Instrumentation
Chromatographic system
An Agilent 1200 LC series (Palo Alto, CA) was used in all analysis. This system consisted of one binary LC pumps; autosampler ALS 1200 with injection volume capacity from 0.1 to 100 μL; thermostatted column compartment (TCC) 1200, and diode array detector (DAD) Infinity 1290. An additional LC pump, model LC-10 AVP from Shimadzu (Kyoto, Japan) was also used for sample loading.
ESI-MS/MS
A hybrid triple quadrupole-linear ion trap mass spectrometer QTRAP 5500 (AB SCIEX, Foster, CA) with turbo ion spray source was coupled to the LC system described above. Source-dependent parameters were optimized by flow injection analysis as follow: curtain gas (CUR) of 20 V, nitrogen collision gas (CAD) medium, source temperature (TEM) of 650 °C, ion spray voltage of 5500 V, and ion source gases GS1 and GS2 at 50 psi.
Compound-dependent MS parameters (declustering potential (DP), collision energy (CE), and collision cell exit potential (CXP)) were optimized by direct infusion of standard solution of each compound at concentration ranging from 10 to 50 μg L−1. Entrance potential (EP) was set at 10 V.
QTRAP was operated in positive ionization mode with selected reaction monitoring (SRM) using a dwell time of 20 ms. For quantitative purposes, three different SRM transitions were monitored for each compound, except for OFLO and NOR which presented two intense and stable transitions. SRM transitions and other MS parameters are shown in Table 1. Resolution at the first and third quadrupole (Q1 and Q3) was set to unitary, and inter-scanning delay was 5 ms. All data was collected and processed in Analyst 1.6.1 software.
Column switching
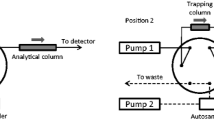
A column-switching setup was used in the sample preparation procedure. The system consisted of two LC pumps, pump A for sample loading and pump B for gradient elution. Column switching was performed using a two-position six-port valve from Rheodyne (Rhonert Park, CA).
A SPE column with hydrophilic-lipophilic balanced polymer Oasis HLB (cartridge column, 2.1 × 20 mm, 25 μm) was acquired from Waters (Milford, MA) and applied in the online sample preparation. The column-switching procedure was evaluated in forwardflush and backflush modes, as shown in Fig. 1.
In backflush mode, the sample was introduced in the SPE column by an aqueous mobile phase from pump A (position A). Simultaneously, the analytical column (Agilent Poroshel EC18 column 50 × 2.1 mm × 2.7 μm) was conditioned by the mobile phase flushed by that present in pump B. At 3.01 min, the valve switched to position B, allowing the pre-concentrated analytes to elute from the SPE column on the opposite direction of sample loading, towards to the analytical column and detector. Similarly, in forwardflush mode the sample was injected in the SPE column (position A), and after switching the valve to position B, the analytes were eluted towards the analytical column by pump B mobile phase but in the same flow direction as the sample loading.
Chromatographic separation and method development
The SPE column retention factor (k) was evaluated for each compound using DAD detector monitoring at 254 and 270 nm wavelengths using different mixtures of mobile phase ranging from 2 to 50 % of B.
The mobile phase used in the binary pump B was a mixture of ultra-pure water (A) containing 0.1 % of formic acid and acetonitrile (B) also with 0.1 % of formic acid at a flow rate of 0.6 mL min−1.
A gradient elution was programmed in pump B, starting with isocratic 95 % of A for 3.1 min. From 3.1 to 9 min, the mobile phase changed linearly down to 35 % of A and, then, changed linearly down to 5 % of A between 10 and 14 min. The injection valve was switched from positions A to B after 3.0 min, returning to its former position at 11.4 min, thereafter; it was submitted to 3 cycles of switching to avoid any carryover effect. The analytical column was kept at 20 °C at TCC compartment.
For sample loading, the mobile phase consisted of ultra-pure water at flow rate of 1.0 mL min−1.
A rheodyne diverter valve was used to introduce mobile phase into MS detector ion source only after 3.0 min. It was programmed to deliver mobile phase for MS analysis for 10 min, as mentioned for the column-switching valve, this valve was also cycled to avoid carryover.
Backflush and foreflush were compared based on asymmetry (As) and number of theoretical plates (N).
Validation procedure
Linearity
For validation, injection volume of 100 μL and backflush mode was chosen. The method was validated considering international accepted criteria [37, 38]. From the stock solutions, two different dilutions of 1000 and 1 μg L−1 of each analyte were prepared in water. Thus, working solutions containing all compounds were prepared and used to spike lab-made sewage samples at seven different concentration levels to evaluate method linearity (matrix-matched calibration).
Table 2 shows the lab-made sewage composition. Before sample analysis, pH was adjusted to 3.0 with formic acid and filtered using 0.22 μm acetate cellulose membrane.
Calibration curves were obtained using external calibration and IS calibration. The sewage and anaerobic reactor effluent samples were quantified using both calibration methods.
The linear model was obtained using a weighted least-squares linear regression (WLSLR). Empirical weights of 1/x, 1/x 2, 1/x 0.5, and 1/y 2 were evaluated in order to determine the best weighting model. Linearity lack of fit was evaluated by analysis of variance (ANOVA).
Precision, limit of detection and quantification, relative recovery, and stability
Inter-day precision was evaluated at one level (medium level) while intra-day precision was measured at three concentration levels (low, medium, and high) also in a lab-made sewage matrix. Method detection limit (MDL) and method quantification limit (MQL) were determined as the concentration resulting in signal/noise (S/N) ratio of 3 and 10, respectively, after the same analytical procedure applied for real samples.
Relative recovery (RR) was measured by comparing the absolute area of each compound. Analytes were spiked into ultra-pure water and lab-made sewage samples at the concentration levels. Stability was evaluated in three different processes related to analysis, autosampler stability, frozen-thaw cycle storage at 4 °C. The lab-made sewage sample was fortified and injected (t 0), the area values obtained were compared with sample aliquots analyzed after 2, 4, 6, 8, 24, 48, 120, and 504 h (3 weeks) for both studies.
Analysis of sewage and anaerobic reactor effluent
Samples of domestic sewage were collected in São Carlos, SP, Brazil. To remove nitrogen, carbon, and organic matter present in the sewage, a pilot-scale anaerobic reactor was applied. A vertical structured-bed reactor under intermittent aeration (volume of 650 L) described by Moura et al. [39] is promising technology for sewage treatment.
About 20 mL of sewage and bioreactor effluent were collected in 50 mL polypropylene sample bottles. The samples were submitted to a simple pre-treatment based on pH adjustment to 3.0 followed by filtration using 0.22 μm acetate cellulose membrane. Finally, the samples were analyzed using the SPE-LC-MS/MS system and quantified by standard addition method to better overcome the matrix effect. Standard addition was done at five different concentration levels spiking only the seven compounds detected in the samples. The first level refers to the sample without sample addition; the second level is the concentration which presented a signal 3.0 times higher than observed previously. Further levels were spiked taking into account a concentration ratio from the second level of 2.8 (third), 1.7 (fourth), and 1.2 (fifth).
Results and discussion
MS optimization
Antibiotics and caffeine were analyzed in positive-ion mode (ESI+) using formic acid as additive, while the selected reaction monitoring (SRM) mode was carried out for acquisition.
For fluoroquinolones, precursor [M+H]+ and product ion were selected according to the highest signal obtained in the SRM optimization. A neutral loss of CO2 ([M+H-44]+) as well as related fragments of 3-carboxyl group ring [M+H-74]+ and piperazinic ring, [M+H-87]+, [M+H-101]+, [M+H-115]+, and [M+H-129]+ were selected. Also, loss of H20 ([M+H-18]+) common for this antibiotics was avoided, only monitored for NOR. All those SRM transitions were used in a previous publication [40].
ESI+ also was used for sulfonamides, and product ions of m/z 186, 156, 108, and 92 were selected. Product ion at m/z 186 correspond to [C6H8N3O2S]+, m/z 156 to [C6H6NO2S]+, m/z 108 to [C6H6NO]+, and m/z 92 to [C6H6N]+ as observed in previous studies [41–43].
For CAF, the product ions selected were at m/z 138 ([C6H8N3O]+), 123 ([C6H7N2O)]+), and 83 ([C4H7N2]+); m/z of 261 ([C13H17N4O2]+), 230 ([C12H14N4O].+), and 123 ([C8H11O]+) were used for TMP as previously reported by Brenner et al. [44].
Column-switching online SPE
In order to avoid sample handling, cross contamination, tedious and time-consuming procedure, column-switching online SPE was selected as the sample preparation technique. In this technique, minimal procedures are necessary for sample analysis.
In current analytical market, there are different setups and column phases available. A column switching is a simple technique which requires a six-port valve found in many typical HPLC instruments. In previous studies, HLB column phase presented appropriate chemical properties to pre-concentrate and extract a wide variety of emerging contaminants [27, 45–50]. Here, particular preference was devoted to develop a method with a commercially available column, so that the method can be easily reproduced in any laboratory.
In order to warranty effective extraction, retention factor (k) of each compound was evaluated in the HLB SPE column using an isocratic mobile phase varying from 2 to 50 % ACN (B). Uracil was selected as void volume marker. According to Fig. 2, 5 % of B provided enough retention to all fluoroquinolones. For sulfonamides, a maximum 2 % of ACN was necessary to achieve enough retention of sulfacetamide; the same was observed to caffeine during loading procedure. Thus, sample loading used simple LC pump with an isocratic phase of ultra-pure water (pump A) to prevent the elution of analytes before the valve switching.
Analysis of lab-made sewage
Lab-made sewage was used to evaluate sample preparation performance. This solution is absent of antibiotics and caffeine, and also mimics sewage composition which allows to optimize and validate the proposed method in a complex sample.
Furthermore, this lab-made sewage has been used in many studies in lab-scale anaerobic reactors and batch reactors as organic substrate for studies related to new technologies for wastewater treatment [51, 52].
Samples of lab-made sewage were injected into the SPE column, about 1.5 min were necessary to eliminate the main matrix components, as shown Fig. 3.
Elution mode
A gradient mode using pump B was used for the elution of analytes as described above. Backflush and foreflush were compared based on asymmetry and number of plates using a lab-made sewage spiked sample. SAC was used to calculate these parameters since it requires 98 % of water to be retained in the SPE column.
In forwardflush mode, As and N were 2.5 and 256, respectively. As and N in backflush mode were 1.2 and 2304. Based on As and N data, a better peak focusing was obtained in the backflush mode, and this condition was adopted to elute all analytes during the method validation and sample analysis.
Chromatographic separation
The gradient and isocratic modes were tested using acetonitrile and water, both containing 0.1 % of formic acid in different mixtures. Most of the analytes co-eluted because of their structural similarity, however using the gradient mode provided a rapid determination. Besides the co-elution, the compounds were analyzed without any interference and cross-talk using the selected SRM transitions and MS/MS conditions.
The injection volume was evaluated from 5 to 100 μL (maximum autosampler capacity); 100 μL was used to obtain the lowest LOQs without compromising peak shape and efficiency.
A carryover study was necessary to avoid lack of precision and accuracy. The autosampler was programmed to clean internal and external needle parts with a mixture of acetonitrile, isopropanol, methanol, and water containing 0.1 % of acid formic (25 % (v/v) of each) pumped by a metering pump. In addition, the valve was switched to injection position to prevent adsorption of analytes. A minimal carryover of 0.5 % was observed between the calibrator at the highest concentration level and subsequent blank injection.
Method validation
The sample pre-treatment was adjusting the pH to 3.0 and filtration through a 0.22-μm membrane. In pH = 3.0, sulfonamides are mainly in neutral form and fluoroquinolones are in cationic form. HLB SPE phase showed enough selectivity to pre-concentrate and extract all of these polar compounds found in the lab-made sewage.
Figure 4 presents a typical chromatogram of spiked lab-made sewage at intermediate concentration levels.
Extracted ion chromatogram (XIC) from lab-made sewage sample spiked with 0.015 μg L−1 of SMZ, 0.149 μg L−1 of SDMX, 0.322 μg L−1 of SMTX, 0.645 μg L−1 of TMP, 0.660 μg L−1 of SMER, 0.594 μg L−1 of CIP, 1.45 μg L−1 of SDIZ, 1.58 μg L−1 of ENRO, 3.92 μg L−1 of NOR, 3.92 μg L−1 of PEF, 5.40 μg L−1 of OFLO, 6.93 μg L−1 of SAC, and 14.9 μg L−1 of CAF
Linearity
Linearity was evaluated spiking the analytes in the lab-made sewage in seven calibration points (3 orders of magnitude) and calculated by using least-squares linear regression analysis. Isotopically labeled IS were used in order to hinder the matrix effects. The calibration curves were tested using external standard method and IS. Both showed linearity without lack of fit. However, external standard was adopted since it contains the pure data obtained, also IS were not available for all analytes used in this proposed method, mainly for the less retained compounds, CAF and SAC.
Three SRM transitions were monitored, except for NOR and OFLO which presented two intense and stable transitions. Two transitions were used for confirmation and one for quantification. The quantification SRM transition was chosen according to the lack of fit, and the lowest values achieved to obtain the calibration curves were selected.
Weight calibration of 1/x 2 were applied for SMTX, SDMX, TMP, and ENRO; 1/y 2 for SDIZ, SAC, and OFLO; 1/y for PEF and NOR; and 1/x for CAF. The weighting factors were selected based on lower relative error and the sum of relative residual errors lower than 15 % in the LOQ level. Non-weighted calibrations were enough for CIP and SMZ. For the majority of analytes, regression data (R 2) was higher than 0.97, except for ENRO and SAC. These analytes presented a better R 2 value without weighted calibration, although with lower adjustment mainly for LOQ concentration level. Besides a lower R 2 value, better accuracy was observed using weighted calibration.
Figures of merit
The MDL and MQL were obtained based on a S/N ratio of 3 and 10, respectively. The MDL varied from 0.800 to 600 ng L−1, and MQL from 1.200 to 1200 ng L−1. These values are in the same range as previous studies applying offline and online SPE in the sample preparation step [4, 21, 29, 40, 46, 50, 53].
Table 3 shows in details the MDL, MQL, intra- and inter-day precision, and RR values. The intra-day and inter-day precision results are compatible with IUPAC and Anvisa guidelines [37, 38]. The intra- and inter-day precision values were lower than 16 and 21 %, respectively, which is acceptable for complex samples [4, 25, 29].
RR was evaluated by spiking three different concentration levels (MQL; medium and high) in ultra-pure water, and comparing those levels collected in the lab-made sewage. RR values varied from 7 to 72 %, the lowest RR values were observed for CAF and SAC, the most polar compounds analyzed (log P −0.07 and −1.0). RR average varied from 23 to 48 % for fluoroquinolones and 8.5 to 56 % for sulfonamides. Similar RR using online SPE were obtained by Feitosa-Felizzola et al. [54] and Khan et al. [55]. In both studies, the respective authors related the low RR to matrix effect caused by ionization suppression. In fact, a RR study cannot discern between the effect of matrix on the extraction efficiency and on the ionization performance at detector. In order to determine extraction efficiency (by means of absolute recovery), the use of post-extraction spiking is recommended. However, for online SPE methods, it is a laborious, lengthy, and uncertain procedure. On the other hand, in order to infer about the matrix effect on electrospray ionization, the next section presents a qualitative matrix effect study by means of standard T-infusion test associated with matrix processing by the online extraction procedure. The stability of spiked samples in a lab-made sewage was evaluated in three different situations. Both samples were stable within 48 h as well as for long-term stability over 3 weeks (504 h). The highest stability was achieved when stored at 4 °C, analyte areas differ less than 20 % for t 0 injection; the samples were then kept at 4 °C.
Matrix effect
A T-infusion was used for matrix effect evaluation; ultra-pure water sample was used as reference and compared with the lab-made sewage. Figure 5 shows an intense signal suppression in the range of 4.7 and 5.0 min. This chromatographic region corresponds to the retention time of most compounds in this analysis. The matrix effect was expected since lab-made sewage is a complex sample containing inorganic and organic compounds which may lead to an intense interference in the ionization of analytes.
Application to real samples
Real sewage samples and anaerobic pilot-scale reactor effluent samples (n = 15) were analyzed. Six antibiotics and caffeine were detected and quantified in both matrices as shown in Fig. 6. A simple and rapid standard addition calibration was applied to overcome the observed matrix effect and improve the quality of the antibiotic concentration measured.
The concentration level in the anaerobic reactor effluent for SMZ, TMP, and OFLO in some samples was higher than in the sewage. This fact has been observed by hydrolysis of conjugated metabolites of these antibiotics, typically found in the sewage [12, 25, 56, 57]. This phenomenon could be enhanced by the complex microbial community within the anaerobic reactor.
In all samples, the retention time deviation was less than ±0.3 %. Moreover, the relative abundance of two different SRM monitored for each analyte varied less than ±20 %. Thus, at least four identification points (IP) were accomplished in a minimum of three IPs as recommended by the EU criteria [58]. Of the seven compounds, five were confirmed as positive using these criteria. Only SMZ and CIP showed a SRM relative abundance transition with variation higher than ±20 % in all samples. This could be due to the matrix effect. Moreover, both of these compounds are in low nanogram-per-liter range, and the samples undergo much interference in their ion ratio.
Signal suppression was predominant in sewage and effluent samples for most of the analytes. However, signal enhancement was observed for SMZ and CIP which could be a key explanation for the SRM ion ratio deviation.
For the sewage, matrix suppression varied from 10 to 83 %, as observed in other studies [59, 60]. Previous studies using SPE offline related to analysis of hospital wastewater and urban influent wastewater demonstrated a ion suppression that varied from 30 to 90 % [57]. Even using online SPE, Ciofi et al. [61] observed ionization suppression for steroids analysis varying from 71.3 to 94.6 %. In the anaerobic reactor effluent, most of the samples showed suppression ranging from 12 to 91 %; however, some samples showed signal enhancement for SMZ, CIP, TMP, SMTX, NOR, and OFLO. Gros et al. [57] reported occurrence of signal enhancement for fluoroquinolones, and Zhou et al. [62] demonstrated the same behavior for SMZ and SMTX. This is attributed to the presence of dissolved organic matter which varied in the samples studied from 700 to 800 mg L−1 of chemical oxygen demand (COD). Moreover, a slight fluctuation on the anaerobic effluent composition also explains this fact. This shift is caused by typical day-to-day oscillations in the sewage composition, which directly influenced the effluent analysis, mainly, for LC-MS/MS causing ion suppression or enhancement.
Table 4 shows the concentration levels found in the sewage and reactor effluent, a wide range varying from 0.018 μg L−1 to SMZ until 1097 μg L−1 of CAF was observed in accordance with previous publications [2, 63–65]. The sewage was collected from a densely populated neighborhood, close to small farm yards explaining the high concentrations of CAF and NOR.
Conclusions
An online SPE method was successfully developed and applied for routine analysis of several antibiotics and caffeine found in the sewage and anaerobic reactor effluent. This setup used a simple, conventional HPLC system (autosampler and six-port valves) with a column-switching technique. Sample pre-treatment was limited to filtration, pH adjustment and IS spiking. An online SPE HLB column showed suitable selectivity to pre-concentrate the antibiotics from two different classes and caffeine; both had different physico-chemical properties. More than 200 analyses were performed without signal loss or even without SPE and analytical column clogging. The method was completely validated in backflush mode, presenting linearity, selectivity, precision, and detectability in sub-nanogram-per-liter levels in complex samples similar to sewage material (lab-made sewage). Standard addition was applied to monitor six of the most common antibiotics and caffeine in the environmental samples without a time-consuming and labor-intensive sample preparation step. The method presented provides a good basis for further studies on the removal and adsorption kinetics of antibiotics using lab-scale anaerobic reactors and batch studies.
References
Kemper N (2008) Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic 8:1–13. doi:10.1016/j.ecolind.2007.06.002
Le-Minh N, Stuetz RM, Khan SJ (2012) Determination of six sulfonamide antibiotics, two metabolites and trimethoprim in wastewater by isotope dilution liquid chromatography/tandem mass spectrometry. Talanta 89:407–416. doi:10.1016/j.talanta.2011.12.053
Gil García MD, Belmonte Gallegos A, Valverde RS, Martínez Galera M (2012) Determination of (fluoro)quinolones in environmental water using online preconcentration with column switching linked to large sample volumes and fluorescence detection. J Sep Sci 35:823–831. doi:10.1002/jssc.201100911
Gros M, Rodríguez-Mozaz S, Barceló D (2012) Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem. J Chromatogr A 1248:104–121. doi:10.1016/j.chroma.2012.05.084
Seifrtová M, Aufartová J, Vytlacilová J, Pena A, Solich P, Nováková L (2010) Determination of fluoroquinolone antibiotics in wastewater using ultra high-performance liquid chromatography with mass spectrometry and fluorescence detection. J Sep Sci 33:2094–2108. doi:10.1002/jssc.201000215
Zhou L-J, Ying G-G, Liu S, Zhao J-L, Yang B, Chen Z-F, Lai H-J (2013) Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci Total Environ 452–453:365–376. doi:10.1016/j.scitotenv.2013.03.010
Zwiener C (2007) Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal Bioanal Chem 387:1159–1162. doi:10.1007/s00216-006-0818-2
Qi W, Singer H, Berg M, Müller B, Pernet-Coudrier B, Liu H, Qu J (2015) Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 119:1054–1061. doi:10.1016/j.chemosphere.2014.09.027
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res 44:417–426. doi:10.1016/j.watres.2009.07.010
Buerge IJ, Poiger T, Muller MD, Hans-Rudolf B (2003) Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ Sci Technol 37:691–700. doi:10.1021/es020125z
Hillebrand O, Nödler K, Licha T, Sauter M, Geyer T (2012) Caffeine as an indicator for the quantification of untreated wastewater in karst systems. Water Res 46:395–402. doi:10.1016/j.watres.2011.11.003
Brandt EMF, de Queiroz FB, Afonso RJCF, Aquino SF, Chernicharo CAL (2013) Behaviour of pharmaceuticals and endocrine disrupting chemicals in simplified sewage treatment systems. J Environ Manag 128:718–726. doi:10.1016/j.jenvman.2013.06.003
Cruz-Morató C, Ferrando-Climent L, Rodriguez-Mozaz S, Barceló D, Marco-Urrea E, Vicent T, Sarrà M (2013) Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res 47:5200–5210. doi:10.1016/j.watres.2013.06.007
Locatelli MAF, Sodré FF, Jardim WF (2011) Determination of antibiotics in Brazilian surface waters using liquid chromatography-electrospray tandem mass spectrometry. Arch Environ Contam Toxicol 60:385–393. doi:10.1007/s00244-010-9550-1
Abraham W-R, Macedo AJ, Gomes LH, Tavares FCA (2007) Occurrence and resistance of pathogenic Bacteria along the Tietê River downstream of São Paulo in Brazil. Clean – Soil, Air, Water 35:339–347. doi:10.1002/clen.200700011
Boxall ABA, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley JP, Verslycke T, Ankley GT, Beazley KF, Belanger SE, Berninger JP, Carriquiriborde P, Coors A, Deleo PC, Dyer SD, Ericson JF, Gagné F, Giesy JP, Gouin T, Hallstrom L, Karlsson MV, Larsson DGJ, Lazorchak JM, Mastrocco F, McLaughlin A, McMaster ME, Meyerhoff RD, Moore R, Parrott JL, Snape JR, Murray-Smith R, Servos MR, Sibley PK, Straub JO, Szabo ND, Topp E, Tetreault GR, Trudeau VL, Van Der Kraak G (2012) Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect 120:1221–1229. doi:10.1289/ehp.1104477
Huerta B, Marti E, Gros M, López P, Pompêo M, Armengol J, Barceló D, Balcázar JL, Rodríguez-Mozaz S, Marcé R (2013) Exploring the links between antibiotic occurrence, antibiotic resistance, and bacterial communities in water supply reservoirs. Sci Total Environ 456–457:161–170. doi:10.1016/j.scitotenv.2013.03.071
Ingerslev F, Halling-Sørensen B (2000) Biodegradability properties of sulfonamides in activated sludge. Environ Toxicol Chem 19:2467–2473. doi:10.1002/etc.5620191011
Picó Y, Andreu V (2007) Fluoroquinolones in soil—risks and challenges. Anal Bioanal Chem 387:1287–1299. doi:10.1007/s00216-006-0843-1
García-Galán MJ, Silvia Díaz-Cruz M, Barceló D (2010) Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trends Anal Chem 27:1008–1022. doi:10.1016/j.trac.2008.10.001
García-Galán MJ, Villagrasa M, Díaz-Cruz MS, Barceló D (2010) LC-QqLIT MS analysis of nine sulfonamides and one of their acetylated metabolites in the Llobregat River basin. Quantitative determination and qualitative evaluation by IDA experiments. Anal Bioanal Chem 397:1325–1334. doi:10.1007/s00216-010-3630-y
Jelic A, Fatone F, Di Fabio S, Petrovic M, Cecchi F, Barcelo D (2012) Tracing pharmaceuticals in a municipal plant for integrated wastewater and organic solid waste treatment. Sci Total Environ 433:352–361. doi:10.1016/j.scitotenv.2012.06.059
Seifrtová M, Nováková L, Lino C, Pena A, Solich P (2009) An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal Chim Acta 649:158–179. doi:10.1016/j.aca.2009.07.031
Pan J, Zhang C, Zhang Z, Li G (2014) Review of online coupling of sample preparation techniques with liquid chromatography. Anal Chim Acta 815:1–15. doi:10.1016/j.aca.2014.01.017
García-Galán MJ, Díaz-Cruz MS, Barceló D (2010) Determination of 19 sulfonamides in environmental water samples by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS). Talanta 81:355–366. doi:10.1016/j.talanta.2009.12.009
Rodríguez E, Navarro-Villoslada F, Benito-Peña E, Marazuela MD, Moreno-Bondi MC (2011) Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography. Anal Chem 83:2046–2055. doi:10.1021/ac102839n
Stoob K, Singer HP, Goetz CW, Ruff M, Mueller SR (2005) Fully automated online solid phase extraction coupled directly to liquid chromatography-tandem mass spectrometry. Quantification of sulfonamide antibiotics, neutral and acidic pesticides at low concentrations in surface waters. J Chromatogr A 1097:138–147. doi:10.1016/j.chroma.2005.08.030
Huntscha S, Singer HP, McArdell CS, Frank CE, Hollender J (2012) Multiresidue analysis of 88 polar organic micropollutants in ground, surface and wastewater using online mixed-bed multilayer solid-phase extraction coupled to high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1268:74–83. doi:10.1016/j.chroma.2012.10.032
López-Serna R, Pérez S, Ginebreda A, Petrović M, Barceló D (2010) Fully automated determination of 74 pharmaceuticals in environmental and waste waters by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 83:410–424. doi:10.1016/j.talanta.2010.09.046
Wode F, Reilich C, van Baar P, Dünnbier U, Jekel M, Reemtsma T (2012) Multiresidue analytical method for the simultaneous determination of 72 micropollutants in aqueous samples with ultra high performance liquid chromatography-high resolution mass spectrometry. J Chromatogr A 1270:118–126. doi:10.1016/j.chroma.2012.10.054
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229. doi:10.1016/j.envint.2011.07.012
Dolar D, Gros M, Rodriguez-Mozaz S, Moreno J, Comas J, Rodriguez-Roda I, Barceló D (2012) Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO. J Hazard Mater 239–240:64–69. doi:10.1016/j.jhazmat.2012.03.029
Kosutic K, Dolar D, Asperger D, Kunst B (2007) Removal of antibiotics from a model wastewater by RO/NF membranes. Sep Purif Technol 53:244–249. doi:10.1016/j.seppur.2006.07.015
Wang X-H, Lin AY-C (2012) Phototransformation of cephalosporin antibiotics in an aqueous environment results in higher toxicity. Environ Sci Technol 46:12417–12426. doi:10.1021/es301929e
Fan X, Hao H, Shen X, Chen F, Zhang J (2011) Removal and degradation pathway study of sulfasalazine with Fenton-like reaction. J Hazard Mater 190:493–500. doi:10.1016/j.jhazmat.2011.03.069
Batista APS and Nogueira RFP (2012) Parameters affecting sulfonamide photo-Fenton degradation – Iron complexation and substituent group. J Photochem Photobiol A Chem 232:8–13. doi:10.1016/j.jphotochem.2012.01.016.37. U.S. Food and Drug Administration, Bioanalytical Method Validation in: Guidance for Industry (2001), p. 1–22
Thompson M, Elisson SLR, Wood R (2002) Harmonized guidelines for single-laboratory validation of methods of analysis. Pure Appl Chem 74:835–855
Agência Nacional de Vigilância Sanitária (ANVISA), Guia para Validacão de Métodos Analíticos e Bioanalíticos, Resolucão RE no 899, de 29/05/2003, 2003. Available from http://www.anvisa.gov.br/legis/resol/2003/re/89903re.htm
Moura RB, Damianovic MHRZ, Foresti E (2012) Nitrogen and carbon removal from synthetic wastewater in a vertical structured-bed reactor under intermittent aeration. J Environ Manag 98:163–167. doi:10.1016/j.jenvman.2012.01.009
Pozo OJ, Guerrero C, Sancho JV, Ibáñez M, Pitarch E, Hogendoorn E, Hernández F (2006) Efficient approach for the reliable quantification and confirmation of antibiotics in water using on-line solid-phase extraction liquid chromatography/tandem mass spectrometry. J Chromatogr A 1103:83–93. doi:10.1016/j.chroma.2005.10.073
Díaz-Cruz MS, García-Galán MJ, Barceló D (2008) Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J Chromatogr A 1193:50–59. doi:10.1016/j.chroma.2008.03.029
Le Fur C, Legeret B, de Sainte Claire P, Wong-Wah-Chung P, Sarakha M (2013) Liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry for the analysis of sulfaquinoxaline byproducts formed in water upon solar light irradiation. Rapid Commun Mass Spectrom 27:722–730. doi:10.1002/rcm.6507
Pfeifer T, Tuerk J, Bester K, Spiteller M (2002) Determination of selected sulfonamide antibiotics and trimethoprim in manure by electrospray and atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 16:663–669. doi:10.1002/rcm.624
Brenner CGB, Mallmann CA, Arsand DR, Mayer FM, Martins AF (2011) Determination of sulfamethoxazole and trimethoprim and their metabolites in hospital effluent. Clean - Soil, Air, Water 39:28–34. doi:10.1002/clen.201000162
Carballa M, Omil F, Lema JM, Llompart M, García-Jares C, Rodríguez I, Gómez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38:2918–2926. doi:10.1016/j.watres.2004.03.029
García-Galán MJ, Díaz-Cruz MS, Barceló D (2011) Occurrence of sulfonamide residues along the Ebro River basin: removal in wastewater treatment plants and environmental impact assessment. Environ Int 37:462–473. doi:10.1016/j.envint.2010.11.011
Gómez MJ, Gómez-Ramos MM, Malato O, Mezcua M, Férnandez-Alba AR (2010) Rapid automated screening, identification and quantification of organic micro-contaminants and their main transformation products in wastewater and river waters using liquid chromatography-quadrupole-time-of-flight mass spectrometry with an accurate-mass. J Chromatogr A 1217:7038–7054. doi:10.1016/j.chroma.2010.08.070
Mutavdzić Pavlović D, Babić S, Dolar D, Asperger D, Kosutić K, Horvat AJM, Kastelan-Macan M (2010) Development and optimization of the SPE procedure for determination of pharmaceuticals in water samples by HPLC-diode array detection. J Sep Sci 33:258–267. doi:10.1002/jssc.200900571
Seifrtová M, Pena A, Lino CM, Solich P (2008) Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal Bioanal Chem 391:799–805. doi:10.1007/s00216-008-2020-1
Tagiri-Endo M, Suzuki S, Nakamura T, Hatakeyama T, Kawamukai K (2009) Rapid determination of five antibiotic residues in swine wastewater by online solid-phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 393:1367–1375. doi:10.1007/s00216-008-2543-5
Camiloti PR, Mockaitis G, Domingues Rodrigues JA, Rissato Zamariolli Damianovic MH, Foresti E, Zaiat M (2014) Innovative anaerobic bioreactor with fixed-structured bed (ABFSB) for simultaneous sulfate reduction and organic matter removal. J Chem Technol Biotechnol 89:1044–1050. doi:10.1002/jctb.4199
Mockaitis G, Pantoja JLR, Rodrigues JAD, Foresti E, Zaiat M (2014) Continuous anaerobic bioreactor with a fixed-structure bed (ABFSB) for wastewater treatment with low solids and low applied organic loading content. Bioprocess Biosyst Eng 37:1361–1368. doi:10.1007/s00449-013-1108-y
Garcia-Ac A, Segura PA, Viglino L, Fürtös A, Gagnon C, Prévost M, Sauvé S (2009) On-line solid-phase extraction of large-volume injections coupled to liquid chromatography-tandem mass spectrometry for the quantitation and confirmation of 14 selected trace organic contaminants in drinking and surface water. J Chromatogr A 1216:8518–8527. doi:10.1016/j.chroma.2009.10.015
Feitosa-Felizzola J, Temime B, Chiron S (2007) Evaluating on-line solid-phase extraction coupled to liquid chromatography-ion trap mass spectrometry for reliable quantification and confirmation of several classes of antibiotics in urban wastewaters. J Chromatogr A 1164:95–104. doi:10.1016/j.chroma.2007.06.071
Khan GA, Lindberg R, Grabic R, Fick J (2012) The development and application of a system for simultaneously determining anti-infectives and nasal decongestants using on-line solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 66:24–32. doi:10.1016/j.jpba.2012.02.011
Queiroz FB, Brandt EMF, Aquino SF, Chernicharo CAL, Afonso RJ (2012) Occurrence of pharmaceuticals and endocrine disruptors in raw sewage and their behavior in UASB reactors operated at different hydraulic retention times. Water Sci Technol 66:2562–2569. doi:10.2166/wst.2012.482
Gros M, Rodríguez-Mozaz S, Barceló D (2013) Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A 1292:173–188. doi:10.1016/j.chroma.2012.12.072
Commission Decision (2002/657/EC) of 12 August 2002, Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, Official Journal of the European Communities L221, Brussels, Belgium, p. 8
Barreiro JC, Vanzolini KL, Cass QB (2011) Direct injection of native aqueous matrices by achiral-chiral chromatography ion trap mass spectrometry for simultaneous quantification of pantoprazole and lansoprazole enantiomers fractions. J Chromatogr A 1218:2865–2870. doi:10.1016/j.chroma.2011.02.064
Renew JE, Huang C-H (2004) Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography–electrospray mass spectrometry. J Chromatogr A 1042:113–121. doi:10.1016/j.chroma.2004.05.056
Ciofi L, Fibbi D, Chiuminatto U, Coppini E, Checchini L, Del Bubba M (2013) Fully-automated on-line solid phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometric analysis at sub-ng/L levels of selected estrogens in surface water and wastewater. J Chromatogr A 1283:53–61. doi:10.1016/j.chroma.2013.01.084
Zhou LJ, Ying GG, Liu S, Zhao JL, Chen F, Zhang RQ, Peng FQ, Zhang QQ (2012) Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A 1244:123–138. doi:10.1016/j.chroma.2012.04.076
Batt AL, Snow DD, Aga DS (2006) Occurrence of sulfonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 64:1963–1971. doi:10.1016/j.chemosphere.2006.01.029
Li B, Zhang T, Xu Z, Fang HHP (2009) Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 645:64–72. doi:10.1016/j.aca.2009.04.042
Senta I, Terzić S, Ahel M (2008) Simultaneous determination of sulfonamides, fluoroquinolones, macrolides and trimethoprim in wastewater and river water by LC-tandem-MS. Chromatographia 68:747–758. doi:10.1365/s10337-008-0780-6
Acknowledgments
The authors are grateful to Fapesp (projects no. 12/07153-4, 10/19910-9, and 09/15984-0) and CNPq projects no. 475756/2013-4 for financial support.
Conflict of interest
The authors declare no conflict of interest relating to the material presented in this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 218 kb)
Rights and permissions
About this article
Cite this article
Lima Gomes, P.C.F., Tomita, I.N., Santos-Neto, Á.J. et al. Rapid determination of 12 antibiotics and caffeine in sewage and bioreactor effluent by online column-switching liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 407, 8787–8801 (2015). https://doi.org/10.1007/s00216-015-9038-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9038-y