Abstract
A simple and quick online solid-phase extraction (SPE) coupled to liquid chromatography (LC)/tandem mass spectrometry (MS/MS) for the determination of the five antibiotics (florfenicol, FF; lincomycin, LCM; oxytetracyclin, OTC; tylosin, TS; valnemulin, VLM) in swine wastewater has been developed. After filtration, aliquots (100 μl) of wastewater samples were directly injected to a column-switching LC system. Some matrix interference was removed by washing up SPE column with 0.2% formic acid solution and acetonitrile. Antibiotics eluted from SPE column were separated on analytical column by converting switching valve and were detected by MS/MS. Calibration curves using the method of standard addition had very good correlation coefficients (r > 0.99) in the range of 0.1 to 2 ng/ml. The intra-day precision of the method was less than 12% and the inter-day precision was between 6 to 17%. The detection limits were 0.01–0.1 ng/ml. When this method was applied to wastewater samples in swine facilities, four compounds (LCM, OTC, TS, and VLM) were detected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Veterinary antibiotics are extensively used in livestock for therapeutic and prophylactic purposes. However, the large amounts of administrated antibiotics are excreted from animals in urine, feces, and manure. Animal agriculture plants usually use aerobic or anaerobic lagoon system for waste disposal. In the wastewater treatment system, the behavior of antibiotics has not been confirmed. If many antibiotics are accumulated in wastewater and soil, a portion of bacteria may develop strong resistance to antibiotics. There are a few case of investigation for antibiotic residues in water and soil at livestock farms [1–5]. For example, Schlüsener et al. reported that the maximum concentrations of antibiotics such as tiamulin (43 μg/kg) and salinomycin (11 μg/kg) was found in liquid manure [4]. The wastewater from livestock farms could directly drain out to rivers and lakes. Recent studies have also shown that the river and groundwater are contaminated with veterinary antibiotics [6–12]. Therefore, it is very important to know the concentrations of residual antibiotics in livestock wastewater.
Several methods using a liquid chromatography (LC)/tandem mass spectrometry (MS/MS) and offline solid-phase extraction (SPE) cartridges have been developed to detect antibiotics in aquatic environments [7–13]. Although offline SPE methods are sensitive and selective, they lack in simplicity, rapidity, and safety. The simple and quick methods to detect antibiotics in wastewater sample are expected. Recently, online SPE methods have been used to successfully determine antibiotics in animal tissues [14], ground and surface water [15, 16], and urban wastewater [17, 18]. On online SPE methods, sample has been directly loaded on HPLC system without complicated preparation like offline SPE. We also reported the more simple online SPE-LC/MS/MS system to determine simultaneously veterinary drugs (63 compounds) in animal tissues [19]. We have used conventional LC system consisting of an autosampler designed to typically inject 0.1–100 μl, a binary pump and a six-port valve. However, there were no effective and simple online SPE-LC/MS/MS methods available for measuring the antibiotics in livestock wastewater sample. Choi et al. [20] analyzed several sulfonamides and tetracyclines in agricultural wastewaters using online SPE-LC/MS. The more reliable and sensitive online SPE-LC/MS/MS analysis of drugs in the dirty matrices such as livestock wastewater has not been developed yet.
In this paper, we describe online SPE-LC/MS/MS procedure to simultaneously determine five antibiotics actually used in two piggeries; florfenicol (FF), lincomycin (LCM), oxytetracyclin (OTC), tylosin (TS), and valnemulin (VLM) in swine wastewater. These five antibiotics are frequently used for swine facilities in Japan.
Experimental
Chemicals
FF and TS were provided by Sigma-Aldrich (St. Louis, MO). LCM and VLM were purchased from Dr. Ehrenstorfer (Aufsburg, Germany). OTC, methanol, acetonitrile, and formic acid were obtained from Kanto Chemicals (Tokyo, Japan). All solvents used for standard preparation and mobile LC phase were of LC grade. Distilled water was purified using a Millipore system Milli Q (Molsheim, France).
Swine wastewater samples
Swine wastewater samples were obtained from a sewage treatment field exhausted from two different swine production facilities. At least five antibiotics were administered to the domestic animals in the facilities. A schematic diagram of the sewage treatment constructed at seven lagoons and a return channel in Miyagi (Japan) is shown in Fig. 1.
Instrumentation
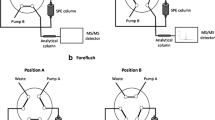
For an online SPE-LC/MS/MS analytical system, the LC system consisted of an Agilent 1100 (Agilent Technologies, Atlanta, GA) with a binary pump, an autosampler, a six-port switching valve and a thermostated column compartment. The SPE column was a 20 mm × 2.1 mm i.d., 25 μm Oasis HLB (Waters, Milford, MA), and a analytical column was a 100 mm × 2.1 mm i.d., 1.8 μm Zorbax SB-Aq (Agilent Technologies, Atlanta, GA). The mobile phase was consisted of 0.2% formic acid solution and acetonitrile with a linear gradient. The column temperature was 40 °C and the injection volume of the sample solution was 100 μl. The switching valve was set between SPE column and analytical column [19]. All chromatographic programs for the valve-switching procedure and the flow path of each step are summarized in Table 1. At waste valve position (Steps 1–4, 6–8), the solvent was eluted through only SPE column and then disposed. In case of MS/MS valve position (Step 4–6), elution solvent passed through SPE and analytical column to MS/MS detector.
The antibiotics in the eluate were quantitated with API3000 tandem mass spectrometer (triple quadrupole mass spectrometer) with electro spray ionization (Applied Biosystems, Foster, CA). The ions were detected using multiple reactions monitoring (MRM) with either positive (ionspray voltage, 3,500 V; entrance potential, 10 V) or negative (ionspray voltage, −3,500 V; entrance potential, −10 V) ion mode. The temperature of nebulizer gas was 500 °C, and nebulizer gas, curtain gas, and collision gas were fixed at 13, 9, and 5, respectively. The conditions of MS/MS of each antibiotic are shown in Table 2.
Sample preparation
Individual stock solutions of five antibiotics (100 μg/ml) were prepared in methanol, and each stock solution was mixed. The calibration standards solutions were prepared in distilled water at the following concentrations: 20, 10, 5, 1, and 0 ng/ml. To prepare the spiked sample, aliquots (100 μl) of appropriate standard solution were added in each swine wastewater sample (900 μl). The final concentrations of these calibration-spiked samples were 2, 1, 0.5, 0.1, and 0 ng/ml, and they were filtered by 0.20-μm disposable filter. If particles were suspended in the sample, it was centrifuged at 10,000 rpm for 10 min before filtration. A 100 μl volume of the calibration-spiked sample solution was injected into the online SPE-LC/MS/MS system. If the concentration of sample spiked at 0 ng/ml (diluted blank sample) was above 1 ng/ml, the sample was diluted from ten to thousand times with distilled water.
Calibration and quantification
The linearity curves of five antibiotics were assessed by preparing calibration-spiked samples (2, 1, 0.5, 0.1, and 0 ng/ml) using each wastewater sample or diluted one. The calibration curve was constructed by plotting the peak area of quantification ion against the concentration of each compound. Quantification of all analytes was performed by the standard addition method (SA). The diluted blank sample concentrations were calculated from the slope of the calibration curve and the peak area of the diluted blank sample. Because the isomers/epimers of OTC and TS exit, a peak within 3% of retention time from a standard peak was identified by the confirmation ion. The real sample concentration was estimated from the following formula.
Results and discussion
MS/MS parameters
To determine the optimal condition for each compound, the individual standard solution in 0.2% formic acid solution–acetonitrile (50/50, v/v) was introduced directly into the MS/MS by the infusion mode using syringe infusion pump. In ESI-MS, the drugs show intense [M+H]+ ion except for FF ([M–H]− ion) on their full-scan spectra. These ions were selected as precursor ions to obtain product ions in MRM mode. The MRM condition for the analysis is listed in Table 2.
Optimizing online SPE condition
Online SPE optimization was carried out on an OASIS HLB column. The offline HLB cartridges has been chosen to avoid the irreversible binding of tetracyclines to silanol groups in wastewater samples [6]. The online HLB cartridge was also selected in our previous paper which described the analytical method of veterinary drugs in animal muscle [19]. Although complete separation is not necessary for the selective MS/MS detection, it generally improves detectability and reduces ion enhancement/suppression effect. The gradient conditions using 0.2% formic acid solution and acetonitrile were examined with reference to our previous paper [19]. The five antibiotics were eluted according to the condition described in Table 1 using LC system with only online SPE column. The retention time of LCM, OTC, TS, FF, and VLM were 7.58, 9.20, 10.87, 11.26 and 11.66 min, respectively. The online SPE column fully separated five antibiotics, but the chromatographic peaks were broad. Thus, it is necessary to combine analytical column with SPE column. Moreover, the valve position was kept at the waste position until 6 min to remove the polar matrix as much as possible.
Validation study of online SPE-LC/MS/MS
An analytical column was equipped in LC system and the mixed standard solutions were measured at conditions shown in Table 1. Under the optimized conditions, the calibration curves of standard solutions (2, 1, 0.5, 0.1, and 0 ng/ml) diluted with water were linear at the range of 0.1–2 ng/ml. The correlation coefficients were obtained 0.9954–0.9998. The other validation data was shown in Table 3. The precision of the method was determined by analyzing six replicates of 0.5 ng/ml standard solutions. A relative standard deviation (%RSD) of the retention time was from 0.070 to 0.10% and that of the peak area was 4.9 to 9.9%. The accuracy for five antibiotics (spiked at 1 ng/ml) in typical wastewater samples (sites 4 and 6 of Fig. 1) was calculated by the absolute calibration curve method (AC) and SA. The recoveries by AC were 15–120%, and that by SA were 87–100%. Thus, SA was selected for accurate quantification. Method precision was evaluated by SA on the same day (intra-day) and three different days (inter-day) using a typical sample spiked at the 1 ng/ml level. Intra-day precision was between 2.1 and 12%RSD, and inter-day precision ranged from 6.0 to 17%RSD. Recoveries (normally between 70 and 120%) and precision (%RSD < 20%) by SA were satisfied.
Quantitative analysis of antibiotics in swine wastewater
The online SPE-LC/MS/MS method established in this study was applied for the determination of the residual antibiotics in swine wastewater samples. The samples were collected at eleven sites of a drainage canal exhausted from two piggeries (Fig. 1). The typical chromatograms of standard solution, blank sample and blank sample spiked standard are shown in Fig. 2. The wastewater matrix was sure to cause signal suppression or enhancement. SA is required for accurate determination of antibiotics concentration because of matrix suppression or enhancement.
MRM chromatograms of: a standard solution (1 ng/ml); b typical blank swine wastewater sample; c blank sample spiked at 1 ng/ml. Blank sample (not dilution) of tylosin and valnemulin are the site 4, and the others (not dilution) are the site 6. FF florfenicol, LCM lincomycin, OTC oxytetracyclin, TS tylosin, VLM valnemulin
The standard addition calibration curve and concentration of five antibiotics in swine wastewater samples are shown in Table 4. FF was not detected in samples at all sites. VLM was only detected in wastewater in facility B. LCM, OTC and TS were found at both facilities A and B. LCM and TS found at site 7 in river A could trace back to facility A and B, respectively. OTC was diluted or decomposed in river A and was not detected at site 7 in upstream of river A, while LCM and TS were detected in downstream of river A (site 10 and 11). Loftin et al. reported that LCM and TS were higher stable than OTC in water of pH 5–9 [21]. We presume that LCM and TS might be difficult to degrade in this sewage treatment system.
SA using the offline SPE method needs some purification handling. It has to use several SPE cartridges per a sample. On the other hand, the online SPE method has a complicated system consisting of two pumps, a large volumetric injection needle and a ten-port valve. We have used conventional LC system consisting of an autosampler designed to typically inject 0.1–100 μl, a binary pump and a six-port valve. Our method has more simple system than other online SPE system and higher sampling throughput than offline SPE methods.
Identification of OTC and TS isomers/epimers
The OTC and TS in sample had peaks at the retention time different from the standard. It is well known that OTC has an epimer (4-epi-OTC) [21–25]. TS also has multiple isomers and its epimer [26]. It is necessary to identify the isomers/epimers of antibiotics existing in each sample, because the isomers/epimers may affect drug resistance of bacteria by different mechanism. However, it was difficult to determine the structure of the isomers/epimers in this study. The epimers of OTC and TC had the same precursor ion and product ion (OTC: 461 > 426 m/z, TS: 917 > 174 m/z), but they did not always have same intensities [22–25]. We identified isomers/epimers OTC and TS by retention time (within 3% of standard compound) and confirmation ion (OTC: 461 > 201 m/z, TS: 917 > 101 m/z). Retention time and a ratio of MS/MS intensity for OTC and TS isomers/epimers are shown in Table 5. The retention time and the ratio of quantification ion intensity and confirmation ion intensity (QI/CI) tentatively judge the isomer/epimer same or not. We guessed that OTC in site 2, 3, and 5 samples was the same epimer, but OTC in site 4 was not. In the same way, we presumed that TS in site 2, 3, 5, and 6 were different isomer/epimer each other, but TS in site 7 to 11 were identical. We supposed that OTC epimerization was occurred at aerobic lagoon (Lagoon A) as between site 3 and 4. On the other hand, it was conjectured that TS was isomerized and/or epimerized at between sites 2 and 3, sites 5 and 6 (Lagoon B), and sites 6 and 7 (Lagoon C), respectively. The air was bubbled through Lagoon A to C for the whole day. OTC and TS could be oxidized to yield the isomer/epimer in aerobic lagoons. Total concentrations of OTC or TS in samples were often uncertain because of different intensity of isomers/epimers. However, the results of measurement are approximately accurate, because the correlation coefficients of OTC and TS were greater than 0.994 (Table 4). We think the rapid detection was rather necessary than accurate quantification of antibiotics in swine wastewater.
Conclusion
The screening methods using online SPE-LC/MS/MS was developed for the determination of five antibiotics in swine wastewater. It was possible to measure five antibiotic compounds without pretreatment by offline SPE. The analysis was more simple, rapid and safe than offline SPE methods and other online SPE system. This method was applied for measuring five antibiotics actually used in swine facilities (Miyagi, Japan), and detected four compounds (lincomycin, oxytetracyclin, tylosin, valnemulin) in the wastewater. The detection limit of this system was 0.01–0.1 ng/ml. Most veterinary antibiotics in swine wastewater sample purified by offline C18 SPE could be measured by our online SPE-LC/MS/MS method. The isomers/epimers of OTC and TS were also separated by our online SPE-LC/MS/MS method. The retention time and the ratio of quantification ion intensity and confirmation ion intensity tentatively judged the isomer/epimer same or not. We are sure that our method can be useful for output investigation of antibiotics in most livestock wastewater.
References
Hamscher G, Sczesny S, Höper H, Nau H (2002) Anal Chem 74:1509–1518
Mueller SR, Singer H, Stoob K, Burkhardt M, Hartmann N, Goetz C, Stamm C, Waul C (2003) Mitt Lebensmittelunters Hyg 94:574–578
Christian T, Schneider RJ, Faerber HA, Skutlarek D, Goldbach HE, Meyer MT (2003) Acta Hydrochim Hydrobiol 31:36–44
Schlüsener MP, Bester K, Spiteller M (2003) Anal Bioanal Chem 375:942–947
Martínez-Carballo E, González-Barreiro C, Scharf S, Gans O (2007) Environ Pollut 148:570–579
Lindsey ME, Meyer M, Thurman EM (2001) Anal Chem 73:4640–4646
Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R (2003) Environ Sci Technol 37:1241–1248
Seino A, Furusho S, Masunaga S (2004) J Jpn Soc Water Environ 27:685–691 (in Japanese)
Hao C, Lissemore L, Nguyen B, Kleywegt S, Yang P, Solomon K (2006) Anal Bioanal Chem 384:505–513
Kim S-C, Carlson K (2007) Anal Bioanal Chem 387:1301–1315
Hirsch R, Ternes TA, Haberer K, Mehlich A, Ballwanz F, Kratz K-L (1998) J Chromatogr A 815:213–223
Sacher F, Lange FT, Brauch H-J, Blankenhorn I (2001) J Chromatogr A 938:199–210
Petrović M, Hernando MD, Diaz-Cruz MS, Barcelò D (2005) J Chromatogr A 1067:1–14
Tang HP, Ho C, Lai SS (2006) Rapid Commun Mass Spectrom 20:2565–2572
Stoob K, Singer HP, Goetz CW, Ruff, M, Mueller SR (2005) J Chromatogr A 1097:138–147
Pozo OJ, Guerrero C, Sancho JV, Ibáñez M, Pitarch E, Hogendoorn E, Hernández F (2006) J Chromatogr A 1103:83–93
Feitosa-Felizzola J, Temime B, Chiron S (2007) J Chromatogr A 1164:95–104
Segura PA, Gagnon C, Sauv, é S (2007) Anal Chim Acta 604:147–157
Tagiri-Endo M, Yanagita N (2007) Bunseki Kagaku 56:317–326 (in Japanese)
Choi K-J, Kim S-G, Kim C-W, Kim S-H (2007) Chemosphere 66:977–984
Loftin KA, Meyer MT, Adams CD, Surampalli R (2008) J Environ Qual 37:378–386
Halling-Sørensen B, Lykkeberg A, Ingerslev F, Blackwell P, Tjørnelund J (2003) Chemosphere 50:1331–1342
Khong S-P, Hammel Y-A, Guy PA (2005) Rapid Commun Mass Spectrom 19:493–502
Bogialli S, Coradazzi C, Di Corcia A, Lagana A, Sergi M (2007) J AOAC Int 90:864–871
Xu J-Z, Ding T, Wu B, Yang W-Q, Zhang X-Y, Liu Y, Shen C-Y, Jiang Y (2008) J Chromatgr B 868:42–48
Paesen J, Cypers W, Busson R, Roets E, Hoogmartens J (1995) J Chromatgr A 699:99–106
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tagiri-Endo, M., Suzuki, S., Nakamura, T. et al. Rapid determination of five antibiotic residues in swine wastewater by online solid-phase extraction–high performance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 393, 1367–1375 (2009). https://doi.org/10.1007/s00216-008-2543-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2543-5