Abstract
A poly(NMA-ST-co-TAIC-EDMA) monolithic column was prepared by redox initiation method using N-methylolacrylamide (NMA) and styrene (ST) as co-monomers. The composite monolith was characterized by scanning electron microscopy (SEM) and nitrogen adsorption–desorption isotherm and was used as a solid phase extraction (SPE) absorbent for enrichment of β-sitosterol by high performance liquid chromatography (HPLC). Under the optimum conditions for extraction and determination, the calibration equation was y = 1.02247x + 0.1766; the linear range was 0.015–0.75 mg mL−1 and the linear regression coefficient was 0.998. The limit of detection (LOD) and the limit of quantification (LOQ) were 0.006 mg mL−1 and 0.02 mg mL−1, respectively. The spiked recoveries of β-sitosterol in edible oil samples were 83.60–103.88%; precisions for intra-day and inter-day assays presented as relative standard deviations were less than 6.0% and 6.2%, respectively. The enrichment factor of β-sitosterol was 60 and the results showed that the monolithic column had high selectivity and good permeability as an on-line SPE absorbent for the enrichment and determination of β-sitosterol from edible oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytosterols, having a hydrocarbon chain at the C-24 position, as a kind of functional foods [1], have increasingly become a research focus for the prominent ability to reduce LDL cholesterol and thereby lower cardiovascular disorders [2]. Phytosterols are families of more than 200 different compounds [3] and β-sitosterol is one of the most common phytosterols [4]. The health beneficial properties of β-sitosterol have been widely reported, β-sitosterol has the capacities of reducing low-density lipoprotein (LDL) cholesterol levels [5], anti-inflammatory, anti-bacterial, anti-fungal, antiulcerative, and antitumor [6, 7]. β-Sitosterol is naturally occurring compound in plants [8], such as vegetable oil, products made from oil, nuts and seeds, which are all good sources of β-sitosterol [9, 10]. Despite these, there are still many difficulties in the efficient enrichment and recovery of β-sitosterol, such as difficult sample handling and purification, et al.
Society and the scientific community have payed much more attention to green analytical chemistry in recent decades that affect both the land and the aquatic environment. It is a necessity that newly developed methods meet the requirements of green chemistry and green, simple, fast analytical methods are now needed to determine the target compounds in complex matrices [11, 12]. Vegetable oil is a pretty complex sample with a variety of ingredients, which including energy, fat, protein, cholesterol, carbohydrates, vitamin E, et al. Therefore, sample petreatment is a critical part of the analytical process for β-sitosterol extracted from vegetable oils. At present, the common methods for extraction and separation of β-sitosterol are mainly including liquid–liquid extraction [13], solvent crystallization [14] and thin-layer chromatography (TLC) [15]. SPE has been favored by researchers and is recognized as beneficial alternative for sample pretreatment, because it overcomes many drawbacks distinct from other techniques [16]. SPE has its own unique advantages, it provides low solvent consumption, low intrinsic costs and reduction of processing time [16,17,18]. Zhu et al. developed a new approach applied for the extraction and determination of β-sitosterol from Salicornia herbacea L. using ionic liquids-based molecular imprinting polymer [19]. Alghamdi et al. provided an SPE purification method to harvest the physiologically-active compounds including β-sitosterol from the vegetable oils [20]. In recent years, online SPE is becoming much more widely used than off-line SPE because of its many superiorties such as simpler steps, larger volume injection and the higher detection sensitivity [21] in sample preparation. As an important separation materials used in HPLC, monolithic columns, with high permeability and selectivity, good mechanical stability and reproducibility [22], has proven triumphantly to be stationary phases regarded as absorbents for SPE due to their advantageous hydrodynamic features and their easy flexible preparation and versatility [23].
The aims of our study were to prepare a poly(NMA-co-ST-co-TAIC-co-EDMA) monolithic column and develop an online SPE-HPLC approach for simultaneous enrichment and determination of β-sitosterol. In this research, the preparation of the monolith was simple and its applicability for the enrichment of β-sitosterol had been validated by five different edible oil samples. The combination of HPLC and online SPE has provided a rapid and simple method that could be applied by routine laboratories for the determination and enrichment of β-sitosterol in plant oils.
Experimental Section
Materials and Instruments
N-Methylolacrylamide (NMA), N-hexanol and β-Sitosterol (≥ 98%) were purchased from Shanghai Aladdin Co. (Shanghai, China). Triallyl isocyanurate (TAIC) was purchased from Shanghai Energy Chemical Co., Ltd. (Shanghai, China). Styrene (ST) was purchased from Damao Chemical Reagent Factory (Tianjin, China). Ethylene glycol dimethacrylate (EDMA) was purchased from Macklin Co. Ltd. (Shanghai, China). Benzoyl peroxide (BPO) and ethanol were obtained from Tianjin Guangfu Fine Chemical Research Institute. N,N-dimethylaniline (DMA) and HPLC-grade methanol were products of Kermel Chemcal Reagent Co. Ltd. (Tianjin, China). Phytosterol corn oil, sesame oil, peanut oil, walnut oil and corn oil were bought from supermarket (Baoding, China). Aromatic compounds were purchased from Beijing Chemical Plant (Beijing, China). Ultrapure water was prepared with a UP Water Purification System (Ulupure, Chengdu, China). All solutions were filtered through a 0.22 µm membrane. The stainless-steel columns (50 mm × 4.6 mm i.d) were obtained from Beijing Xinyu Instrument Co. Ltd. (Beijing, China).
All analyses were performed on Thermo Unitmate 3000 system (Thermo scientific, USA). Ultrasonic cleaning machine was purchased from Kun Shan Ultrasonic Instruments Co. Ltd (Jiangsu, China). The electronic balance was acquired from Startorius Group (Startorius, Germany). The morphology was examined by SEM (Phenom, Eindhoven, The Netherlands). The surface area and porosity were obtained by nitrogen adsorption/desorption measurment on a TriStar II 3020 instrument (Micromeritics, the United State).
Preparation of Poly(NMA-ST-co-TAIC-co-EDMA) Monolithic Column
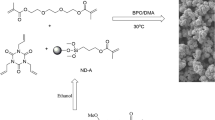
The preparation of the monolith was carried out as follows: NMA, ST, TAIC and EDMA were firstly dissolved in n-hexanol and then BPO was added. The prepolymerization solution was sonicated for 30 min at 30 °C, after 30 µL DMA being added and sonicated, the mixture was poured in a stainless steel column (50 mm × 4.6 mm i.d). After polymerization at 30 °C for 3.5 h, the column was connected with the HPLC pump and washed with methanol for 90 min to remove porogenic solvents and other soluble compounds present in the polymeric column.
Characterization of the Column
The monolithic material is formed by the interact reaction about the unsaturated bonds between monomer and crosslinker. The optimized monolith was further characterized by SEM. The structure of the column material was observed on a JEOL SEM 6700 microscopy. The column was washed by methanol for 2 h and dried in an oven for 48 h. The surface area of the column was performed by nitrogen adsorption–desorption on a Micromeritics Tristar II 3020.
Sample Preparation
Preparation of Aromatic Compounds
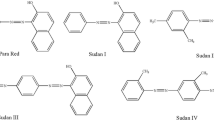
1 mg of small molecule (benzene, nitrochlorobenzene, biphenyl and phenanthrene) was separately dissolved in 4 mL methanol and then kept in refrigerator at 4 °C.
Preparation of β-Sitosterol Standard Solutions
1 mg of β-sitosterol standard was dissolved in 1 mL methanol. After filtered through a 0.22 µm membrane, the sample solution was stored in refrigerator at 4 °C.
Preparation of Edible Oil Sample
Firstly, 2.500 g edible oil was weighed in a 50.0 mL plastic centrifuge tube and 15.0 mL ethanol and 5.0 mL 50% KOH solution were added. Then the mixture was settled in a thermostatic shaker for 50 °C for 1 h. The product was cooled to room temperature and adjusted pH to 6–8 using acetic acid glacial. Then ethanol was added to 50.0 mL. After being settled in the refrigerator freezer for 2 h, the mixture was centrifugated at 10,000 r/min for 10 min. The supernatant was transferred to a 50.0 mL centrifuge tube and ethanol was added to 50.0 mL. The sample solutions were filtered through a 0.22 µm membrane and then stored in refrigerator at 4 °C.
Procedures of Online SPE
The SPE procedure was shown in Fig. 1. Firstly, β-sitosterol standard solutions were continuously injected into the monolithic column for different times using ethanol/H2O (4/96, v/v) as the mobile phase to enrich β-sitosterol. Then the column was connected with C18 column (150 mm × 4.6 mm i.d.) and 100% methanol was used as the mobile phase to elute β-sitosterol from the monolith.
Quality Assurance/Quality Control (QA/QC)
QA/QC of the analysis was performed with five spiked edible oil samples containing three different concentration levels of β-sitosterol. Spiked samples were prepared by adding different amounts of solution (analytes). The solutions were homogenised by sonication and analysed by using the procedure described in the section Procedures of online SPE. Extraction and analysis of spiked samples were carried out by several times in one day and in different days.
Results and Discussions
Optimization and Characterization of the Monolithic Columns
In order to obtain a monolithic column with uniform structure, the effect of the concentration of the monomer, the crosslinker and the porogen on permeability and mechanical stability were investigated, which was shown in Table 1. The SEM images of the monoliths were shown in Fig. 2.
When the amounts of n-hexanol increased from 1.2 mL to 1.0 mL (column 1, 3, 4), the pressure of the column increased from 12 bar to 17 bar and the permeability correspondingly reduced and the column became harder. From Fig. 2a (column 1) and Fig. 2b (column 4), it could be seen that, the pore size distribution got slightly narrower and the mobile phase was hard to pass through with the decrease of n-hexanol. When the amount of EDMA decreased from 0.25 mL (column 1) to 0.20 mL (column 6), column 6 possessed lower pressure and better permeability. From Fig. 2a, c, it could be seen that column 6 had smaller size globules and more uniform structure. Moreover, the size of small microglobules decreased and the pore size distribution got larger with the addition of ST and TAIC, which was shown in Fig. 2d (column 9), and the material tends to be with skeletal stucture.
In summary, column 9 possessed better permeability and mechanical stability than other columns. So column 9 was employed in the following experiments.
The nitrogen adsorption–desorption isotherm of the column 9 was shown in Fig. S1a (see Electronic Supplementary Material) and exhibited the third type adsorption isotherm which demonstrated that the column had macroporous structure. The surface area of column 9 was 10.14 m2 g−1.
Permeability of the Polymer-Based Monolithic Column
As shown in Fig. S1b (see Electronic Supplementary Material), a good linear (correlation coefficient r > 0.99) relationship between the flow rate and back pressure was obtained, which indicated that the monolith possessed good mechanical and chemical stability. This was corresponding to the high permeability (5.58 × 10−14 m2 in Table 1) of the monolith.
Retention Properties of the Monolithic Column
In order to investigate the retention properties of the monolithic column, three small molecule compounds were separated by the monolith. The elution order of three aromatic compounds was benzene, biphenyl and phenanthrene, which indicated that the column was a reversed phase separation mechanism. To further validate this conclusion, retention factors which were calculated by the following formula: k = (tR − t0)/t0 (k stands for retention factor, tR stands for the retention time of small molecules, t0 stands for the retention time of thiourea) [24] of the three aromatic compounds were recorded under various content of methanol and the results were shown in Fig. S1c (see Electronic Supplementary Material). With an increase of methanol content from 55 to 80%, the retention factors decreased as expected, which indicated that the separation mechanism of the column was based on the reversed phase retention.
Separation Performance
In order to investigate the separation ability, the columns were firstly used to separate aromatic compounds including neutral (Fig. 3a) and basic analytes (Fig. 3b). In Fig. 3c, six small molecules of different kinds could achieve baseline separation. The results illustrated that the column had the ability to separate different kinds of small molecular compounds.
Separation of different small molecules with the obtained polymer monolith a was the neutral small molecules: (1) benzene, (2) nitrochlorobenzene, (3) biphenyl, (4) phenanthrene; b was basic compounds: (1) p-nitroaniline, (2) diphenylamine, (3) triphenylamine; c was different kinds of compounds: (1) resorcinol, (2) benzene, (3) nitrochlorobenzene, (4) naphthalene, (5) biphenyl, (6) phenanthrene. mobile phase: a MeOH/H2O (80/20, v/v); b MeOH/H2O (80/20, v/v); c MeOH/H2O (65/35, v/v). Flow rate: 1.0 mL min−1; Detection wavelength: 254 nm; Injection volume, 5 µL; Temperature: 30 °C
Optimization of the Online SPE–HPLC Conditions
Effect of Organic Solvent in Mobile Phase on Enrichment
In general, methanol, acetonitrile and ethanol are commonly used as mobile phase for RP-HPLC. In order to obtain a suitable solvent for enrichment, an optimization experiment about the loss rate of β-sitosterol on the polymer monolith was investigated and the result was shown in Fig. 4a. It could be seen that lower loss rate of β-sitosterol was obtained with the decrease of the content of organic solvent. It also could be seen that more than 99% β-sitosterol was kept on the column successfully when no more than 4% ethanol in the mobile phase. In consideration of the cost and toxicity, 4% ethanol was chosen as the optimal mobile phase for enrichment.
Optimization of online-SPE conditions. a Loss rate of β-sitosterol from the monolithic column using different concentration of organic solvent as the mobile phase. b Recoveries of β-sitosterol retained on the monolith at different flow rate. c Recoveries of β-sitosterol eluented from the monolith at different concentration of organic solvent. Conditions: monolithic column: 50 × 4.6 mm i.d.; analysis column: C18 Cosmosil column: 150 × 6 mm i.d.; Injection sample: 1.0 mL min−1β-sitosterol standard solution; Detection wavelength: λ = 217 nm. Injection volume: 20 µL; Mobile phase: a, c different content of methanol, acetonitrile or ethanol b ethanol/H2O (4/96, v/v)
Influence of Flow Rate on Enrichment
Figure 4b showed that different flow rate led to various recoveries for enrichment. It could be seen that the recoveries increased when the flow rate increased from 0.4 mL min−1 to 1.0 mL min−1. But when the flow rate was 1.2 mL min−1, the obtained recovery decreased. So the flow rate of 1.0 mL min−1 was chosen for enrichment in the following experiment.
Option of Eluting Condition
In order to select a suitable solvent to elute β-sitosterol, the effect of the content of organic solvent on the recovery of β-sitosterol was investigated. The results in Fig. 4c indicated that the recovery of β-sitosterol increased when the content of organic solvent increased, and 100% methanol was adopted as the optimum elution solvent.
Investigation of Maximum Adsorption Capacity
In order to test the adsorption performance of the monolithic column for β-sitosterol, β-sitosterol standard solution was continuously injected for different times. From Fig. 5, it could be seen that the adsorption amount of β-sitosterol increased correspondingly when the injection volume increased. The maximum adsorption capacity of β-sitosterol adsorbed on the monolithic column was 18.88 mg g−1 and the enrichment factor defined as the ratio of the elution volume of β-sitosterol in the extract to that in the original β-sitosterol sample was 60, which indicated that the obtained column had the ability to enrich β-sitosterol.
Adsorption of β-sitosterol by the monolithic column. Conditions: monolithic column: 50 × 4.6 mm i.d.; analysis column: C18 Cosmosil column: 150 × 4.6 mm i.d.; Injection sample: 1.0 mg mL−1β-sitosterol standard solution; Detection wavelength: 217 nm. Flow rate: 1.0 mL mL−1; Mobile phase: ethanol/H2O (4/96, v/v) for enrichment; 100% methanol for elution
In the process of online enrichment and elution, the maximum pressure of the system would be able to reach to 10 MPa. Good repeatability of the experiment indicated that the monolithic column had good mechanical stability.
Enrichment of β-Sitosterol from Five Different Plant Oil Samples
The proposed online SPE-HPLC procedure was applied in the analysis of β-sitosterol in the edible oil samples. Figure 6 represented the enrichment results of β-sitosterol in five different plant oils and each sample was continuously injected for different times. The results indicated that the amount of β-sitosterol from oil samples increased correspondingly when the injection times increased.
Chromatograms of five different oil samples after enrichment. a, b, c, d, e represented sesame oil sample, peanut oil sample, coin oil sample, walnut oil sample and phytosterol corn oil, respectively; 1, 2 and 3 represented the injection volume of 20, 200 and 400 µL, respectively. Conditions: monolithic column: 50 × 4.6 mm i.d.; analysis column: C18 Cosmosil column: 150 × 4.6 mm i.d.; flow rate: 1.0 mL min−1; temperature: 30 ± 0.1 °C; detector wave length: λ = 217 nm
Quality Parameters
Instrumental quality parameters (linearity, limit of quantification, limit of detection, precision, accuracy and repeatability) for the analysed β-sitosterol were tested as below.
Linearity was assessed by β-sitosterol standard calibration curves which produced by a series of standard solutions of β-sitosterol. The calibration equation was y = 1.02247x + 0.1766. The correlation coefficient was 0.998, confirming that there was a good linear relationship between peak areas (y) and concentration (x) in a range of 0.015–0.75 mg mL−1. The limit of detection (LOD, S/N = 3) and limit of quantification (LOQ, S/N = 10) were 0.006 mg mL−1 and 0.02 mg mL−1, respectively.
Precision was evaluated by the calculation of the relative standard deviation (RSD) values of the measured concentrations on the same day (intraday) and three consecutive days (interday). Accuracy expressed by the recovery, was the value of the measured concentration divided by the target concentration and multiplied 100%. Table 2 listed the values of precision (< 6.24%), recovery (83.60–103.88%) and confidence interval in detail; these values confirmed the feasibility of the method.
The established and validated method was adopted to determine the concentrations of β-sitosterol in five oil samples. The contents of β-sitosterols in five samples were between 866.14 and 4183.99 mg kg−1. The highest concentration of β-sitosterol was found in corn oil.
To investigate the repeatability of the method, five monolithic columns were prepared under the same conditions and then used as the SPE columns. RSD values for the retention times and peak areas were less than 4.15% (n = 5) and 5.24% (n = 5), respectively, which indicated that the repeatability of the method was good.
Comparison with Other Known Methods
Several known methods to determin β-sitosterol in various samples were shown in Table 3. GC-FID method was employed with long analysis time, complex procedures and high LODs [25]. LC-MS method was with high sensitivity and lower method detection limits, but its application was limited on account of pretty high cost and more advanced instrument requirements, especially for ordinary analysis laboratories [26]. UPLC-ELSD, HPLC-C18 and MIP-HPLC methods were available in routine analysis but they could not achieve the large amounts enrichment or high cost of β-sitosterol in different samples [19, 27, 28]. Nevertheless, SPE-HPLC method in this study developed a novel technique for simultaneous determination and enrichment of β-sitosterol in edible oil samples, which had the advantages of reduction in the amount and the toxicity of solvents and reagents, higher enrichment factor and recoveries, much simpler process steps and lower cost.
Conclusion
The study on β-sitosterol in plant foods has brought great concerns. Therefore, establishing analytical methods for determination and enrichment of β-sitosterol in edible oil samples is of great importance. In this work, an on-line SPE-HPLC method by using poly(NMA-ST-co-TAIC-co-EDMA) monolith as absorbent for simultaneous determination and enrichment of β-sitosterol in five different edible oil samples was successfully established. The advantages of this method included simplicity, rapidity, good selectivity and better detectability of targeted analytes. The good extraction efficiency, linearity, lower LOD, trueness and precision of the proposed method indicated that the method was reliable and sensitive. Therefore, the technique could be reliable and suitable for routine determination and enrichment of β-sitosterol in different edible oil samples.
References
Asım O, Cesarettin A, Birgül VK, Serap Y, Cihan O, Ayse K, Ebru P, Jerzy Z (2017) Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J Funct Foods 31:311–319
Gylling H, Plat J, Turley S, Ginsberg HN, Ellegard L, Jessup W, Jones PJ, Lutjohann D, Maerz W, Masana L (2014) Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 232:346–360
Moreau RA, Whitaker BD, Hicks KB (2002) Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41:457–500
Bacchetti T, Masciangelo S, Bicchiega V, Bertoli E, Ferretti G (2011) Phytosterols, phytostanols and their esters: from natural to functional foods. Mediter J Nutr Metab 4:165–172
Liu SY, ANaErGuLi M (2012) Determination of β-sitosterol with chemical course and material applications in Jatropha seed oil by high performance liquid chromatography. Adv Mater Res 577:69–72
Micallef MA, Garg ML (2009) Beyond blood lipids: phytosterols, statins and omega-3 polyunsaturated fatty acid therapy for hyperlipidemia. J Nutr Biochem 20:927–939
Franca M, Andrea P (2010) Phytosterols and cardiovascular health. Pharmacol Res 61:193–199
Naiyer S, Wajahatullah K, Shadab MD, Asgar A, Sundeep SS, Sadhana S, Faisal A, Al-Allaf ZA, Ibrahim AAI (2017) Phytosterols as a natural anticancer agent: current status and future perspective. Biomed Pharmacother 88:786–794
Vieno P, David GL, Tatu AM, Jari T, Anna-Maija L (2000) Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric 80:939–966
Klingberg S, Andersson H, Mulligan A, Bhaniani A, Welch A, Bingham S, Khaw KT, Andersson S, Ellegård L (2008) Food sources of plant sterols in the EPIC Norfolk population. Eur J Clin Nutr 62:695–703
Andrey S, Andrey B, Marcello L, Simone C, Vasil A (2017) Application of deep eutectic solvents in analytical chemistry: a review. Microchem J 135:33–38
Salgueiro-González N, Castiglioni S, Zuccato E, Turnes-Carou I, López-Mahía P, Muniategui-Lorenzo S (2018) Recent advances in analytical methods for the determination of 4-alkylphenols and bisphenol A in solid environmental matrices: a critical review. Anal Chim Acta 1024:39–51
Wajs-Bonikowska A, Stobiecka A, Bonikowski R, Krajewska A, Sikora M, Kula J (2017) A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from blackberry (Rubusfruticosus) growing in Poland. Soc Chem Ind 97:3576–3583
Hrabovski N, Sinadinović-Fišer S, Nikolovski B, Sovilj M, Borota O (2012) Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur J Lipid Sci Technol 114:1204–1211
Azadmard-Damirchi S, Dutta PC (2006) Novel solid-phase extraction extraction method to separate 4-desmethyl-4-monomethyl and 4,4ʹ-dimethylsterols in vegetable oils. J Chromatogr A 1108:183–187
Płotka-Wasylka J, Szczepańska N, Guardia MDL, Namieśnik J (2016) Modern trends in solid phase extraction: new sorbent media. Trends Anal Chem 77:23–43
Płotka-Wasylka J, Szczepańska N, Guardia M, Namieśnik J (2015) Miniaturized solid-phase extraction techniques. Trends Anal Chem 73:19–38
Yolanda P, Mónica F, MariaJose R, Guillermina F (2007) Current trends in solid-phase-based extraction techniques for the determination of pesticides in food and environment. J Biochem Biophys Methods 70:117–131
Zhu T, Row KH (2010) Extraction and determination of β-sitosterol from Salicornia herbacea L. using monolithic cartridge. Chromatographia 71:981–985
Alghamdi E, Piletsky S, Piletska E (2018) Application of the bespoke solid-phase extraction protocol for extraction of physiologically-active compounds from vegetable oils. Talanta 189(1):157–165
Natália FT, Milena GM, Caio RS, Susanne R (2016) On-line solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry as a powerful technique for the determination of sulfonamide residues in soils. J Chromatogr A 1452:89–97
Georges G (2007) Monolithic columns in high-performance liquid chromatography. J Chromatogr A 1168:101–168
Catalá-Icardo M, Torres-Cartas S, Meseguer-Lloret S, Gómez-Benito C, Carrasco-Correa E, Simó-Alfonso EF, Ramis-Ramos G, Herrero-Martínez JM (2017) Preparation of organic monolithic columns in polytetrafluoroethylene tubes for reversed-phase liquid chromatography. Anal Chim Acta 960:160–167
Li XJ, Jia M, Zhao YX, Liu ZS, Akber Aisa H (2016) Preparation of phenylboronate affinity rigid monolith with macromolecular porogen. J Chromatogr A 1438:171–178
Seçmeler Ö, Üstündağ ÖG (2017) A rapid in-house validated GC-FID method for simultaneous determination of lipophilic bioactives in olive oil: squalene, α-tocopherol, and β-sitosterol. Eur J Lipid Sci Technol 119(1):1–14
Martínez-Vidal JL, Garrido-Frenich A, Escobar-García MA, Romero-González R (2007) LC-MS determination of sterols in olive oil. Chromatographia 65(11–12):695–699
Ma SB, Zhang YR, Qu XL, Jin FY (2015) Determination of β-sitosterol content in ethnodrug guoshangye. Agric Sci Technol 16(11):2546–2548
Qiu FY, Ding L, Cao HY (2014) Determination of β-sitosterol in oils and fats by high performance liquid chromatography. Chin Oil Fat 39(7):91–94
Zhu T, Yoon C, Row K (2011) Solid-phase extraction of β-sitosterol from Salicornia herbacea L. using molecular imprinting polymer. Chin J Chem 29:1246–1250
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Nos. 21575033, 21505030), the Natural Science Foundation of Hebei Province (Nos. H2016201221, B2018201270), and the Hebei Province Science and Technology Research Project (grant number QN 2016128).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Published in the topical collection Recent Trends in Solid-Phase Extraction for Environmental, Food and Biological Sample Preparation with guest editors Anna Laura Capriotti, Giorgia La Barbera, and Susy Piovesana.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Guo, B., Pang, X. et al. Simultaneous Determination and Enrichment of β-Sitosterol From Edible Oil Samples Using Poly(NMA-ST-co-TAIC-co-EDMA) Monolith as Sorbent with On-line SPE-HPLC. Chromatographia 82, 1285–1293 (2019). https://doi.org/10.1007/s10337-018-3646-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3646-6