Abstract
The presence of captan residues in apples shows high toxicity, which often causes eye and skin irritation, dermatitis, conjunctivitis, and vomiting in humans. In this context, an electrochemical biosensor based on acetylcholinesterase (AChE) immobilized on a ZnO nanorod interface has been proposed. In this work, Hill, dose-response, and first-, second-, and third-order polynomial regression models were successfully applied and the prediction ability of these models was tested with the use of current density obtained from the cyclic voltammograms of appropriate captan solutions. The Pt/ZnO/AChE bioelectrode showed a high sensitivity of 0.538 μA cm−2 μM−1 in the linear range from 0.05 to 25.0 μM with a limit of detection of 107 nM. The recovery results were observed between 98.4 and 102.4 % from the apple sample. This work provides a new promising tool for the detection of captan in apple samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blossom end rot, black rot, primary scab, and bull’s eye rot are the diseases of apple trees caused by fungi, namely Botrytis cinerea, Botryosphaeria obtusa, Venturia inaequalis, and Cryptosporiopsis curvispora, respectively [1]. Captan, a fungicide [2], is more active against diseases such as blossom end rot, black rot, primary scab, and bull’s eye rot [1]. Rawn et al. [3] observed captan levels in 142 apples ranging from 56.22 nM to 21.12 μM, which exceeded the acceptable maximum residue limit concentrations of captan in apples.
The presence of captan residues in apples can be a substantial path to human vulnerability. The symptoms of captan poisoning in humans include eye and skin irritation, dermatitis, conjunctivitis, and vomiting. The effects of captan poisoning in animals include hypothermia, listlessness, depression, diarrhea, weight loss, anorexia, abortion, increased thirst, and decreased immune function. Sheep, cattle, and fishes like brown trout, harlequin fish, coho salmon, brook trout, fathead minnow, and bluegill sunfish are susceptible to captan poisoning. Scientific reports showed that captan pesticide was rapidly metabolized in several animal species. Captan pesticide can also affect the human reproductive system at low levels of exposure. The most minimal dosage for humans that results in death is 1071 mg kg−1. As per the World Health Organization (WHO) limit, the daily intake for humans is 0.100 mg kg−1 day−1.
Singh et al. [2] developed an electrochemical biosensor for the detection of captan in contaminated water based on the inhibition of glutathione-S-transferase (GST) enzyme immobilized on the surface of (3-aminopropyl)triethoxysilane self-assembled monolayer modified gold electrode in which the proposed linear regression model was formulated using six different captan concentrations with a regression coefficient (R 2) value of 0.99. However, the relative standard deviation (RSD) of the calibrated current was very high (12 %), which was higher than the standard RSD value (RSD ≤ 5 %), indicating that the electrochemical analysis may lead to the inaccurate estimation of captan in contaminated water samples [2]. Moreover, the reported calibrated curve for estimating the unknown concentration of captan in contaminated water samples was linear. But the GST inhibition was nonlinear. Using nonlinear regression analysis, the RSD of the calibrated current can be minimized which in turn helps in the accurate estimation of unknown concentrations of captan in contaminated water samples. Hence, in this article, linear and nonlinear regression models were statistically analyzed to find out the appropriate model for calibration of the developed Pt/ZnO/AChE bioelectrode to determine unknown concentrations of captan in apples with high accuracy. In this work, the general toxicity of captan towards the inhibition of AChE enzyme activity is also reported.
Materials and methods
Materials and apparatus
Acetylthiocholine chloride (ATChCl) (≥99 % purity) with a molecular weight of 197.73 g M−1, acetylcholinesterase (AChE) from the electricus eel with specific activity (E.C. 3.1.1.7, activity 200–1000 U mg−1), captan, cerium acetate, zinc acetate, and 2-pyridine aldoxime methiodide were purchased from Sigma-Aldrich, USA (https://www.sigmaaldrich.com). Sodium hydroxide, potassium hydroxide, glucose, lactic acid, 0.5 wt% chitosan in 1 % acetic acid (degree of deacetylation of 82.5 %, molecular weight 140,000 g mol−1), ascorbic acid, monobasic sodium phosphate monohydrate, and dibasic sodium phosphate dehydrate were procured from Merck India Ltd., India (https://www.merk.com). Cadmium acetate dehydrate was purchased from Loba Chemie Pvt. Ltd., India (www.lobachemie.com). Urea, nickel chloride, and cupric acetate were purchased from Thermo Fisher Scientific Pvt. Ltd., India (www.thermofisher.com). Platinum wire counter electrode (CHI115, 0.5 mm diameter), KCl-saturated Ag/AgCl reference electrode (CHI111, 0.5 mm diameter), and platinum (Pt) electrode (CHI102, 2 mm diameter) were purchased from CH Instruments, Inc (www.chinstruments.com). All solutions and reagents were prepared using deionized water (AQUA Purifications Systems, India, www.aquapurification.com). Synthesis, immobilization procedures, and characterization results of ZnO and CeO2 nanoparticle-modified Pt electrode are given in the Electronic Supplementary Material (ESM, Fig. S1). Electrochemical characterization was performed using an electrochemical workstation (CHI600C, CH Instruments, USA, www.chinstruments.com) with the Pt/ZnO/AChE/chitosan as working electrode, Ag/AgCl saturated with 0.1 M KCl as a reference electrode, and Pt wire as a counter electrode. All electrochemical measurements were carried out at room temperature in pH 8.0 phosphate-buffered saline (PBS).
Preparation of real samples
Fresh apples were purchased from a local market, and 20 mg of skin of apple samples was homogenized with 30 mL of pH 8.0 PBS. This mixture was centrifuged at 8000 rpm, and the supernatant solution was transferred and made up to 20 mL with deionized water. Finally, under the optimal conditions, a recovery study was performed by adding different concentrations of captan to pH 8.0 PBS.
Data analysis
A biplot of principal component analysis (PCA) was carried out to study the effects and variances of various electrochemical parameters on the added captan. The Hill, dose-response, and first-order, second-order, and third-order polynomial regression analyses were carried out for the increasing captan concentrations (see ESM). Furthermore, ANOVA was carried out at the 0.05 level to analyze the significant difference between the regression coefficients. Finally, percentage recovery, root mean square error of cross validation (RMSECV), and relative prediction error (RPES) were utilized to assess the proposed regression model for the estimation of captan in apple samples (see ESM). All the statistical data analyses were carried out employing MATLAB 6.5 software.
Results and discussion
Electrochemical ATChCl sensing mechanism
The Pt/AChE bioelectrode hydrolyzed ATChCl to thiocholine (red) at 140 mV (vs. Ag/AgCl) (see ESM Table S1 and Fig. S2 (a)). After subjecting the Pt/AChE bioelectrode to 250 mV (vs. Ag/AgCl), thiocholine (red) oxidized to form dithio-bis-choline (thiocholine (ox)) [4]. This leads to an effective electron transfer in the Pt/AChE bioelectrode [4]. Similar electrochemical reactions were also noticed in CeO2- and ZnO-modified Pt electrodes. It can be seen that the values of the reduction and oxidation peak potentials were in good agreement with the previously reported AChE biosensor based on porous gold nanoparticle-CaCO3 hybrid material (cathodic peak potential E pc = 192 mV and anodic peak potential E pa = 275 mV), which were corresponding to the reduction and oxidation of the thiocholine (red)/thiocholine (ox) couple [4]. The electrochemical reaction that occurred at the surface of various modified Pt bioelectrodes was given as follows:
Among the two metal oxide nanostructures, namely CeO2 and ZnO, the electrochemical biosensor based on ZnO nanorods has displayed satisfactory results for ATChCl analysis because of its good conductivity, biocompatibility, high percentage relative signal (ESM Fig. S2 (b)), and rapid electron transfer between AChE and the Pt electrode. Therefore, Pt/ZnO/AChE was used as the working electrode for captan detection in all further studies.
Optimization of experimental parameters
The effect of pH (ESM Fig. S3), amount of immobilized AChE enzyme (ESM Fig. S4 and S5), scan rate (ESM Fig. S6, S7 and S8), ATChCl (ESM Fig. S9 (a) and (b)), and incubation time (ESM Fig. S10) on the Pt/ZnO/AChE bioelectrode response is given in the ESM. The optimum pH, amount of immobilized AChE enzyme, scan rate, ATChCl, and incubation time were pH 8.0, 0.25 U mL−1, 0.02–0.1 V s−1, 1.0 mM, and 15 min, respectively.
Captan determination
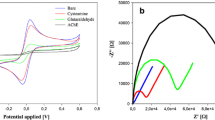
Figure 1(a) shows the cyclic voltammograms of Pt/ZnO/AChE in pH 8.0 PBS solution containing 1.0 mM ATChCl after inhibition with captan (0.05, 1.5, 3.5, 6.0, 10, 15, 20, and 25 μM) for 15 min. It was observed that the redox current reduced slowly with the increase in the concentration of captan owing to the inhibition of AChE enzyme. It also showed the applicability of the Pt/ZnO/AChE bioelectrode to monitor captan at different micromolar concentrations. Inhibition curves of the Pt/ZnO/AChE biosensor for captan determination after 15 min incubation are shown in Fig. 1(b). The inhibition curves from cathodic and anodic responses were inclined towards maximum values at high captan concentrations, suggesting that binding with active sites of AChE attained saturation as the captan concentrations increased from 0.05 to 25 μM. The inhibition curves from cathodic and anodic responses were related to the Michaelis-Menten relationship. The degree of co-operativity (p), apparent Michaelis-Menten constant (K app M ), and maximum percentage inhibition (I max %) estimated from the Hill plot of cathodic and anodic degrees of inhibition (see ESM) were determined to be 1.05, 7.43 μM, and 96.82, and 1.00, 5.48 μM, and 59.27, respectively, indicating positive co-operativity. It also showed that the binding of captan to the redox mediator of AChE successively increased the affinity of further entering captan molecules. The AChE inhibition curve obtained from the cathodic response held the maximum I max % (96.82) value in comparison with the AChE inhibition I max % (59.27) value measured from the anodic response. Since the Pt/ZnO/AChE bioelectrode showed higher inhibition in the cathodic process than in the anodic process, the AChE inhibition curve from the cathodic response was chosen for further analysis considering the higher degree of inhibition reached. The obtained LOD (IC10) and IC50 were 107 nM and 7.43 μM, respectively.
a Cyclic voltammograms of Pt/ZnO/AChE in pH 8.0 PBS solution containing 1.0 mM ATChCl after inhibition with captan (0.05, 1.5, 3.5, 6.0, 10, 15, 20, and 25 μM) for 15 min, b inhibition curves of Pt/ZnO/AChE biosensor for captan determination after 15 min incubation, and c PCA biplot of the estimated electrochemical parameters
The percentage of immobilized AChE residual activity decreased with increasing captan concentration (ESM Fig. S11). This was because the higher the captan concentration, the interaction between the immobilized AChE enzyme and captan was more and specific that resulted in greater inhibition. In the PCA biplot (Fig. 1 (c)), 25 μM concentration of captan was placed in the upper left quadrant and characterized by high FWHM (full width at half maximum of the cathodic peak height) (ESM Table S2), which indicated that the number of electrons transferred in the cathodic process inclined towards the minimum value at a high captan concentration. In the left quadrant, 0.05, 1.5, and 3.5 μM concentrations of captan were characterized by a high cathodic peak current density (J pc), electron transfer rate constant (K S ), surface coverage (Γ), and amount of charge consumed (Q), indicating that captan showed a lower inhibition rate at low captan concentrations. In other words, the electrochemical response of ATChCl altered by captan (0.05, 1.5, and 3.5 μM) was low.
Model fitting and validation
The plots of the first-, second-, and third-order polynomial regression analyses of log (%inhibition) versus log (captan) and dose-response curve for varying concentrations of captan are shown in ESM Fig. S12 (a), (b) (c) & (d), respectively. With the measurements (ESM Table S3), individual equations were formulated and fitted with the measured percentage inhibition for the first- (Eq. (1)), second- (Eq. (2)), and third-order polynomial (Eq. (3)), nonlinear (Hill (Eq. (4)), and dose-response (Eq. (5))) regression analyses as follows:
First-order polynomial regression equation:
Second-order polynomial regression equation:
Third-order polynomial regression equation:
Hill equation:
Dose-response equation:
The ANOVA showed that the first-, second-, and third-order polynomial and nonlinear (Hill and dose-response) regression models were significant at 95 % confidence limit (p < 0.05). It was observed that as the order of polynomial regression increased from one to three, standard error decreased considerably, which indicated that only the third-order polynomial model was adequate to fit all concentrations of captan. Even though Hill and dose-response analyses depicted the AChE enzyme inhibition behavior with an R 2 of 0.99, the standard error values of individual parameters (p, K app M , I max %, log(I 0)and I max %) were comparatively higher than that of the case of the third-order polynomial regression analysis. On comparing the figure of merits (ESM Table S4 and S5), the third-order polynomial regression model was observed best among all the polynomial and nonlinear regression models as they depicted least values of RPEs (0.015), %recovery (99.982), and RMSECV (0.203). Based on the evaluation and substantiation results, the third-order polynomial regression model was suggested to be the most beneficial and precise model in sensing the captan in apple samples.
Determination of the type of inhibition and interference study
From the Lineweaver-Burk (ESM Fig. S13) and Dixon plots (ESM Fig. S14), it can be concluded that the inhibition was reversible and competitive. The value of inhibition constant (K i ) for the Pt/ZnO/AChE-modified bioelectrode was calculated to be 3.043 μM. The K i to K app M ratio (K i : K app M = 0.006 : 1) suggested that immobilized AChE enzyme has a greater affinity for captan than ATChCl.
The J pc for 0.1 mM concentration of ATChCl was compared with the J pc observed in the presence of various interferents after the Pt/ZnO/AChE bioelectrode was incubated in 1.5 μM captan for 15 min. No observable changes of J pc were noticed in the presence of 0.1 mM Ni2+ (I % = 0.28), 0.1 mM Cd2+ (I % = 0.83), 0.1 mM Zn2+ (I % = 0.43), 0.1 mM Cu2+ (I % = 0.93), 0.1 mM glucose (I % = 0.28), 0.1 mM urea (I % = 0.63), 0.1 mM ascorbic acid (I % = 0.62), and 0.1 mM lactic acid (I % = 0.92), respectively.
The maximal inhibition due to the potential interferents present in the apple samples might change the values of the inhibition Michaelis-Menten constant (K app M ) and the maximum degree of inhibition (I max %). Hence, a prediction band (ESM Fig. S14) with upper and lower limits was formulated for the measured current density at 95 % confidence interval so that the Pt/ZnO/AChE bioelectrode can selectively detect the presence of captan in PBS (0.1 M, pH 8.0) when the same experiment was repeated.
Among the various potential interferents, glucose showed the highest cross-reactivity (see ESM) of 2.1 % and it was followed by Ni2+ with a cross-reactivity of 1.1 % (ESM Table S6). They depicted minimal cross-reactivity values. These cross-reactivity studies suggested that the detection of captan would not be interfered by these interferents.
Precision, accuracy, stability, and reactivation
The intra-assay precision of the Pt/ZnO/AChE bioelectrode was assessed by examining the modified electrode for seven replicates, and the calculated RSD was lesser than 2.12 % at the ATChCl concentration of 0.1 mM in 1.5, 5, and 15 μM captan individually for 15 min. The lower RSD showed the good repeatability of measurements. The inter-assay precision was calculated by estimating the current density for 0.1 mM ATChCl at seven different electrodes, which were plunged in 1.5, 5, and 15 μM captan individually for 15 min. The RSD was estimated to be lesser than 3.17 %, showing satisfactory fabrication reproducibility. For 10, 15, and 20 μM of captan, the predicted captan concentrations ± relative error for four trials were found to be 10 ± 0.011, 15 ± 0.007, and 20 ± 0.005 μM, indicating acceptable accuracy of the Pt/ZnO/AChE bioelectrode in the determination of captan. As shown in ESM Fig. S15, the measured current density values were within the control limits for 18 days, indicating that ZnO nanorods offered a good microenvironment for the immobilized AChE enzyme to retain its catalytic activity. The Pt/ZnO/AChE bioelectrode showed a stable current density for a period of 18 days and retained approximately 91.7 % activity. The results of AChE enzyme reactivation depicted that the inhibited AChE enzyme can be reactivated more than 95.2 % of its original catalytic activity after immersing the Pt/ZnO/AChE bioelectrode in 4.0 mM 2-PAM for 15 min.
Captan detection in apple samples
Recovery study
Table 1 presents the determination results of captan in apple samples (n = 3). Spiked recovery is an effective approach to study the accuracy of the proposed method. The captan concentration in the apple sample was calculated using the calibrated third-order polynomial equation. The observed recovery was in the range 98.4–102.4 %, which manifested a low matrix effect on the cyclic voltammetric response. In other words, the chitosan membrane offered low diffusional resistance to captan and high diffusional resistance to macromolecules found in the apple sample. Thus the passage of interferents to the immobilized AChE layer was hindered by the chitosan membrane. This recovery study also suggested that the potential interferents in the apple sample did not interfere with the quantification of captan.
Accuracy study
Comparison of captan values in three levels of determination was assessed by the Pt/ZnO/AChE bioelectrode (y) with the added captan values (x) showing a good correlation coefficient with r = 99 %, the regression equation being y = 1.020x − 0.035. Moreover, the analytical RPE and RMSECV of the added captan in the apple sample were 0.004–0.215 and 0.002–0.023, respectively, demonstrating the satisfactory accuracy of the third-order log (J pc) versus the log (captan) calibration equation. According to Student’s t test, as the t critical value (t critical = 2.306) was found to be higher than the calculated t exp value (t exp = 0.677) at the 95 % confidence interval, there was no significant differences between the mean captan concentrations. These results also showed the accuracy of the proposed Pt/ZnO/AChE bioelectrode.
Precision study
Within and between the estimated RSD values were 0.781 and 1.626 %, respectively. This high precision depicted the good repeatability and reproducibility of the Pt/ZnO/AChE bioelectrode. The F test was utilized at a 95 % confidence interval to assure any significant differences between the standard deviation of the mean concentration obtained using the reference method (added captan concentrations) in the spiked apple sample. The F critical value (F critical = 6.390) was found to be higher than the estimated F exp value (F exp = 1.042). Thus, the null hypothesis was accepted, and this confirmed that the third-order log (J pc) versus the log (captan) calibration equation was precise. Moreover, the maximum inhibition, recovery, RSD of captan recovery, and accuracy at the Pt/ZnO/AChE bioelectrode were found to be better than at the glutathione-S-transferase-modified gold electrode (ESM Table S7) [2].
Conclusion
An analytical method has been developed for the detection of captan, which was based on the inhibition of immobilized AChE enzyme activity. Investigation of five nonlinear models with observed current density values was the most effective calibration method for the accurate quantification of captan in apple samples. The immobilized AChE on the ZnO nanorod surface showed a high accuracy, a high degree of inhibition, and an acceptable recovery towards captan determination. All these results depicted that the Pt/ZnO/AChE bioelectrode could be utilized for the detection of captan in apple samples.
References
Smith FD, MacHardy WE (1984) Phytopathol 74:894–899
Singh RP, Kim YJ, Oh BK, Choi JW (2009) Electrochem Commun 11:181–185
Rawn DF, Quade SC, Shields JB, Conca G, Sun WF, Smith M, Fouquet A, Belanger A (2007) Food Addit Contam 24:149–155
Chauhan N, Narang J, Pundir CS (2011) Int J Biol Macromol 49:923–929
Acknowledgments
The authors are grateful to the Department of Science & Technology, New Delhi, for their financial support (DST/TSG/PT/2008/28, SR/FST/ETI-284/2011 (C) and Nano Mission Council (No. SR/NM/PG-16/2007)). We also thank SASTRA University for financial support (TRR fund).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1087 kb)
Rights and permissions
About this article
Cite this article
Nesakumar, N., Sethuraman, S., Krishnan, U.M. et al. Cyclic voltammetric acetylcholinesterase biosensor for the detection of captan in apple samples with the aid of chemometrics. Anal Bioanal Chem 407, 4863–4868 (2015). https://doi.org/10.1007/s00216-015-8687-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8687-1