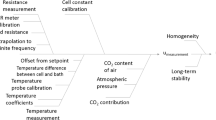
Abstract
This work presents the results from a series of stability studies for some batches of different aqueous pH reference materials, packed in high-density polyethylene (HDPE) bottles, taking primary pH measurements over long periods (time lengths from 18.4 to 21.0 months). The results obtained over time for acid and neutral buffer solutions (nominal pH values of 1.68, 4.00, and 6.86 at 25 °C), considering their uncertainties, were statistically similar, demonstrating the high stability of these materials. On the other hand, for the alkaline buffer solutions (nominal pH values of 9.18 and 10.01 at 25 °C), there was a clear decrease in the results over time, with pH variation rates around −8.5 × 10−4 per month. The results showed that reference materials of the acid and neutral buffer solutions can be easily provided with small uncertainty values and long shelf lives in simple HDPE bottles closed under air atmosphere, whereas reference materials of the alkaline buffer solutions must have more limited shelf lives and higher uncertainty values (taking into account the pH decrease over time) or must be provided in special packaging (such as ampoules) to prevent carbon dioxide interference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Practical pH measurements of real samples are mostly performed by potentiometry using a combined glass electrode, calibrated by reference pH buffer solutions [1, 2]. Confidence in the reliability of the measurement results requires the establishment of a metrological calibration hierarchy linking the quantity in the sample to a unit in the International System of Units or, where this is not possible, to another valid international reference. For pH, a traceability chain can be established by calibration using certified reference materials of pH buffer solutions, for which the pH values are obtained utilizing an internationally recognized measurement procedure, called primary method of pH measurement [3–5]. The primary pH measurement is the most precise known procedure for determining pH, with expanded uncertainties (U, coverage factor—k—equals to 2) typically from 0.002 to 0.004 [4].
According to ISO Guide 34 and ISO Guide 35 [6, 7], the certification process of a reference material must include a long-term stability study with, for instance, measurements of randomly selected units from the batch of reference material taken over time. Stability testing aims to determine the remaining degree of instability of the material after preparation or to confirm the stability of the material. In the case of the long-term stability of a reference material, it is associated with the behavior of the material on the shelves of the producer (storage conditions) [7]. However, a given stability study may not be long enough or it may not use analytical methods with enough precision for the analyst to detect small trends in the measurement data over time. In addition, no existing literature has reported results of stability studies for pH reference materials covering an extended period (such as 18 months or more) and using primary pH measurements.
Therefore, this paper presents the results obtained for a series of stability studies, for different aqueous pH reference materials (nominal pH values ranging from 1.68 to 10.01 at 25 °C), carried out over long periods (at least 18 months) and using primary pH measurements. The results were analyzed to check for possible trends over time in the pH values.
Materials and methods
Samples
Five batches of pH aqueous buffer solutions (nominal pH values of 1.68, 4.00, 6.86, 9.18, and 10.01 at 25 °C), with compositions as recommended by IUPAC [3], were prepared and analyzed over time using primary pH measurements. They were named as tetraoxalate (TO, pH 1.68) batch, phthalate (PT, pH 4.00) batch, phosphate (PP, pH 6.86) equimolal batch, tetraborate (TB, pH 9.18) batch, and carbonate (CN, pH 10.01) batch. Each batch was prepared to have an approximate total volume of 40 L of buffer solution. Deionized water was used directly from a water purifier, without any pretreatment (boiling or deaeration with argon), and no stabilizer was added to the solutions. After preparation, the batches were transferred to 250-mL HDPE bottles properly labeled and numbered. The bottles were closed manually immediately after filling and two more times in the next 2 days using a grip plier. Finally, the bottle caps were sealed using a paraffin tape.
Experimental procedure
A typical primary pH measurement system was used in this work. For each measurement, first, the six Harned cells were filled with a hydrochloric acid solution (about 0.01 mol kg−1 molality) whose molality was precisely and previously determined by coulometric titration [8]. Then, measurements of difference of potential were taken continuously for each cell, every minute, until they became stable. Then, the results were used to determine the standard potential of each of the Ag/AgCl electrodes. Next, the same cells (with the same electrodes) were filled with aliquots of the pH buffer solution with different chloride molalities added (concentration range from about 0.005 to 0.020 mol kg−1). For the TO, PT, PP, and TB buffer solutions, measurements of difference of potential were taken continuously until they became stable. For the CN buffer solution, measurements of difference of potential were continuously taken for 180 min, and the last 30 measurements were used to determine the difference of potential at zero time. Then, the results were used to determine the acidity function at zero chloride molality and, finally, the pH of the buffer solution, with the appropriate estimation of the measurement uncertainty according to guidelines [4, 9, 10]. Detailed descriptions and explanations about the measurement system and the calculation involved in the measurement (including the uncertainty) were extensively given previously [3–5]. All measurements were carried out at 25 °C.
Stability evaluation
Randomly selected bottles of each batch of the buffer solutions were stored at room temperature (21 °C) for the stability studies. Then, primary pH measurements were taken over time (classical approach) as follows: five measurements over 21.0 months for the TO batch, six measurements over 19.5 months for the PT batch, five measurements over 18.4 months for the PP batch, six measurements over 20.5 months for the TB batch, and four measurements over 20.4 months for the CN batch (one primary pH measurement from one bottle for each time point). For each batch of the buffer solutions, the stability was assessed according to the similarity of the measurement results over time, using the statistics from the chi-square test and linear regression based on the least squares method.
Results and discussion
Figures 1 and 2 show all pH results obtained over time, with expanded uncertainties (k = 2). All numerical pH data are also given in Electronic Supplementary Material (ESM) Table S1. Table 1 shows statistical data from the chi-square test and linear regression for assessing the stability of the buffer solutions. As can be seen from Fig. 1, for each of the TO, PT, and PP batches, all pH results can be considered similar, taking into account their uncertainties, and with a random variation over time. This was confirmed by the chi-square test, where the calculated chi-square (χ 2 cal) values were lower than the tabled chi-square (χ 2 tab) values, as shown in Table 1. In addition, the differences between the highest and lowest results (ΔpH) and the standard deviations (SDs) between the results, considering each batch, ranged from 0.0022 to 0.0036 and from 0.0009 to 0.0017, respectively, which are similar to or lower than the typical expanded uncertainty (k = 2) values found for a single primary pH measurement. Moreover, and as can be seen in Table 1, the absolute slope from the linear regression of the data (|b|) was lower than the uncertainty value associated with the slope (u b) multiplied by the tabled Student’s t (t tab) value for each of these batches. This indicates that the TO, PT, and PP batches of buffer solution were stable over the time lengths studied in relation to their pH value.
As can be observed from Fig. 2, each of the TB and CN batches showed a clear trend, with decreasing pH values over time. In addition, as can be seen in Table 1, the χ 2 cal values were higher than the χ 2 tab values, showing that the pH results cannot be considered similar taking into account their uncertainties. Moreover, the ΔpH and SD values, considering each batch, ranged from 0.0176 to 0.0217 and from 0.0073 to 0.0077, respectively, which are significantly higher than the typical expanded uncertainty (k = 2) values found for a single primary pH measurement. Furthermore, and as shown in Table 1, the |b| value was higher than the u b value multiplied by the t tab value for each of these batches, showing that the TB and CN batches of buffer solution were not stable over the time lengths studied. The pH variation rates found for these batches (around −8.5 × 10−4 per month) cannot be neglected and indicate that similar pH reference materials of TB and CN (aqueous solutions in HDPE bottles) should include uncertainty contributions around 0.010 for a recommended shelf life of 1 year. For instance, for a new batch of TB or CN buffer solution with standard uncertainties of 0.0015, 0.0010, and 0.0015 (typical values) for the characterization, homogeneity, and short-term stability, respectively, the final standard uncertainty would be (0.00152 + 0.00102 + 0.00152 + 0.0102)1/2≈0.010, for 1 year of shelf life. That is, for new batches of TB and CN buffer solutions, the uncertainty contribution related to the long-term stability, for 1 year of shelf life, will be probably predominant over the uncertainties from the other studies. This variation over 1 year is similar to or lower than the typical expanded uncertainty (k = 2) values usually obtained in pH measurements with combined glass electrodes (0.01 to 0.03 at best conditions [4]), showing that these observed trends in the pH results over time would be hardly sensed by simple potentiometric measurements with glass electrodes. The detection of these trends in the pH results using differential potentiometry (secondary method of pH measurement [4, 11]) would be also difficult, since a reference sample with stable pH over time would be necessary. These trends in the results can probably be explained by neutralization reactions between the buffer solutions and carbon dioxide coming from the atmosphere, which must have occurred despite the care taken to close the bottles (see “Samples”). Such an outcome could explain why some national metrology institutes prefer to provide pH reference materials as pure salts or as solutions in ampoules, where the carbon dioxide interference can be considered negligible, allowing small final uncertainty values with long shelf lives. On the other hand, the results showed that pH reference materials of TO, PT, and PP can be directly provided as aqueous solutions (instead of pure salts, which require additional work by the customer to prepare the buffer solutions), with no special packing (such as ampoules), keeping small uncertainty values even for long shelf lives (recommended shelf lives of at least 18 months). Despite the carbon dioxide interference shown in this work for alkaline buffer solutions, resulting in decreasing pH values over time, surprisingly, another work has observed a slow pH increase over similar time periods for Tris buffer solutions in synthetic seawater (pH around 8.1) [12].
Conclusions
A series of stability studies were successfully carried out for different aqueous pH reference materials (nominal pH values ranging from 1.68 to 10.01 at 25 °C) packed in HDPE bottles by, for the first time, using only primary pH measurements taken over long time periods (at least 18 months). This approach was chosen to allow the detection of possible small trends in the measurement data over time, which could not be sensed with shorter stability studies or when using analytical methods with less precision.
The results showed that the pH values for the TO, PT, and PP buffer solutions were stable over time, whereas the pH values for the TB and CN buffer solutions clearly decreased during the study. This pH decrease can be attributed to neutralization reactions between the solutions and carbon dioxide, revealing a need to increase the final uncertainties for pH reference materials of alkaline buffer solutions (taking into account the pH decrease over time) or a need for special packaging (such as ampoules) to prevent carbon dioxide interference when the production of pH reference materials with small uncertainty values and long shelf lives is desired. The high stability of the other reference materials (TO, PT, and CN) showed that they can be easily provided with small uncertainty values and long shelf lives in simple HDPE bottles closed under air atmosphere.
References
Spitzer P, Meinrath G (2002) Anal Bioanal Chem 374:765–766
Naumann R, Alexander-Weber C, Eberhardt R, Giera J, Spitzer P (2002) Anal Bioanal Chem 374:778–786
Bates RG (1973) Determination of pH theory and practice. Wiley, New York
Buck RP, Rondinini S, Covington AK, Baucke FGK, Brett CMA, Camões MF, Milton MJT, Mussini T, Naumann R, Pratt KW, Spitzer P, Wilson GS (2002) Pure Appl Chem 74:2169–2200
Baucke FGK (2002) Anal Bioanal Chem 374:772–777
ISO (2009) ISO Guide 34. General requirements for the competence of reference material producers. International Organization for Standardization, Geneva
ISO (2006) ISO Guide 35. Reference materials—general and statistical principles for certification. International Organization for Standardization, Geneva
Pratt KW, Ortiz-Aparicio JL, Matehuala-Sanchez FJ, Pawlina M, Kozlowski W, Borges PP, Júnior WBS, Borinsky MB, Puelles AH, Hatamleh N, Acosta O, Nunes J, Lito MJG, Camões MF, Filipe E, Hwang E, Lim Y, Bing W, Qian W, Chao W, Hioki A, Asakai T, Máriássy M, Hanková Z, Nagibin S, Manska O, Gavrilkin V (2013) Metrologia 50:08001
ISO/IEC (2008) ISO/IEC Guide 98–3. Uncertainty of measurement—part 3: guide to the expression of uncertainty in measurement. International Organization for Standardization, Geneva
EURACHEM/CITAC (2012) EURACHEM/CITAC Guide CG 4. Quantifying uncertainty in analytical measurement. EURACHEM
Gonzaga FB, Dias JC, Jehnert D, Werner B, Schräpler K, Vyskočil L (2013) Electroanalysis 25:1955–1959
Nemzer BV, Dickson AG (2005) Mar Chem 96:237–242
Acknowledgments
The authors wish to thank CNPq (Process 310258/2012) and FAPERJ (Process 103.600/2012) for fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Reference Materials for Chemical Analysis with guest editors Hendrik Emons and Stephen A. Wise.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 6 kb)
Rights and permissions
About this article
Cite this article
Gonzaga, F.B., Dias, J.C. Long-term stability monitoring of pH reference materials using primary pH method. Anal Bioanal Chem 407, 3249–3252 (2015). https://doi.org/10.1007/s00216-014-8263-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8263-0