Abstract
Ultraperformance convergence chromatography/tandem triple quadrupole mass spectrometry (UPC2-MS/MS) is a novel tool in separation science that combines the advantages of supercritical fluid chromatography with ultraperformance liquid chromatography/MS/MS technology. The use of nontoxic CO2 fluid and a postcolumn additive to complement MS/MS allows better control of analyte retention for chiral separation and high-sensitivity determination with different chiral stationary phases. This paper reports the stereoselective separation and determination of the chiral neonicotinoid sulfoxaflor in vegetables and soil by UPC2-MS/MS. Baseline resolution (Rs ≥ 1.56) of and high selectivity (LOQ ≤ 1.83 μg/kg) for the four stereoisomers were achieved by postcolumn addition of 1 % formic acid–methanol to a Chiralpak IA-3 using CO2/isopropanol/acetonitrile as the mobile phase at 40 °C, 2,500 psi, and for 6.5 min in electrospray ionization positive mode. Rearranged Van’t Hoff equations afforded the thermodynamic parameters ΔH ο and ΔS ο, which were analyzed to promote understanding of the enthalpy-driven separation of sulfoxaflor stereoisomers. The interday mean recovery, intraday repeatability, and interday reproducibility varied from 72.9 to 103.7 %, from 1.8 to 9.2 %, and from 3.1 to 9.4 %, respectively. The proposed method was used to study the pharmacokinetic dissipation of sulfoxaflor stereoisomers in soil under greenhouse conditions. The estimated half-life ranged from 5.59 to 6.03 d, and statistically nonsignificant enantioselective degradation was observed. This study not only demonstrates that the UPC2-MS/MS system is an efficient and sensitive method for sulfoxaflor stereoseparation, but also provides the first experimental evidence of the pharmacokinetic dissipation of sulfoxaflor stereoisomers in the environment.

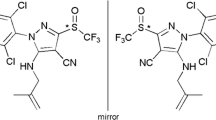
Chemical structure and UPC2-MS/MS separation chromatogram of sulfoxaflor. (* stereogenic center)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfoxaflor (N-[methyloxido[1-[6-(trifluoromethyl)-3-pyridinyl]ethyl]-λ4-sulfanylidene]cyanamide), is the first member of a novel insecticide class, the sulfoximines, which are being developed to control sap-feeding insects such as aphids, whiteflies, hoppers, and Lygus [1]. It exhibits high levels of insecticidal potency both in the laboratory and in the field because of its unique chemical moiety, a sulfoximine. The sulfoximines are mainly used as commercial agrochemicals, and present a unique set of structure–activity relationships (SAR) compared with other insecticides [2]. No cross-resistance to sulfoxaflor has been found in pest insect strains that exhibit high levels of resistance to the first three generations of neonicotinoids and other nAChR-acting insecticides [3, 4]. Sulfoxaflor is considered to be a fourth-generation neonicotinoid by the International Union of Pure and Applied Chemistry (IUPAC) (http://sitem.herts.ac.uk/aeru/iupac/1669.htm). Stereoisomeric mixtures of this compound are known to have a high potential to bioaccumulate and are highly toxic to honeybees. As such, sulfoxaflor has attracted a great deal of research attention (see http://sitem.herts.ac.uk/aeru/iupac/1669.htm).
Sulfoxaflor has two tetrahedral stereogenic atoms (the S and C atoms attached to position 3 of the pyridine ring), and presents two diastereomers, each of which gives rise to two enantiomers. Stereoisomers of chiral compounds, despite their similar structures and appearances, can exhibit very different bioactivities, toxicities, and metabolic and excretion characteristics when introduced into asymmetric biological or chemical systems [5, 6]. In most cases, only one of the pesticide isomers is active; the other isomer may have less or no activity, or it may exert toxic effects against nontarget organisms [7]. Thus, it is very important to develop stereoselective analytical methods to evaluate the risk to food safety and environmental risk from sulfoxaflor in its pure isomeric forms and to address issues related to its bioaccumulation. These methods may also help to shed light on the high toxicity of sulfoxaflor racemate to honeybees.
In this study, we report the efficient and sensitive chiral separation and determination of sulfoxaflor stereoisomers in vegetables and soil by ultraperformance convergence chromatography/tandem triple quadrupole mass spectrometry (UPC2-MS/MS). This work expands the limits on research into sulfoxaflor, which has traditionally focused on biological characterization [1, 3, 8], insecticidal activity [9, 10], mode of action [4, 11], and metabolism [2, 12] of the mixture of isomers. UPC2 is an excellent complement to MS spectrometry because it combines the advantages of supercritical fluid chromatography (SFC) and ultraperformance liquid chromatography (UPLC) technology [13]. A systematic discussion on how to improve the stereoselectivity of sulfoxaflor stereoisomers by varying the chiral stationary phases (CSPs), co-solvents, pressure of the automated backpressure regulator (ABPR), and temperatures—among other factors—is provided. Results are explained based on the perspectives of chiral recognition, retention, resolution, and Van’t Hoff plots. The proposed method was applied to investigate the enantioselective degradation of sulfoxaflor stereoisomers in soil under greenhouse conditions in order to estimate the half-lives of the stereoisomers as well as their preferentially degraded enantiomers. To the best of our knowledge, the present report provides the first experimental evidence of the pharmacokinetic degradation of sulfoxaflor stereoisomers in soil.
Materials and methods
Chemicals and reagents
Standard sulfoxaflor (stereoisomer ratio = 2:3:2:3; purity = 99.9 %) was obtained from J&K Scientific Ltd. (Beijing, China). High-purity CO2 (≥99.999 %) and N2 (≥99.999 %) were acquired from Haike Yuanchang Gas (Beijing, China). LC/MS-grade formic acid (FA) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). LC-grade acetonitrile (ACN), 2-propanol (IPA), methanol (MeOH), ethanol, n-hexane, and 1-butanol were purchased from Honeywell International Inc. (Morristown, NJ, USA). Ultrapure water was prepared from a Milli-Q system (Millipore, Bedford, MA, USA). Analytical grade sodium chloride (NaCl) and anhydrous magnesium sulfate (MgSO4) were purchased from Beihua Fine Chemicals Co. (Beijing, China). Cleanert PSA (primary/secondary amines, 40–60 μm), GCB (graphitized carbon black, 120–400 mesh), C18 (40–60 μm), and Florisil (120–400 mesh) were purchased from Bonna-Agela Technologies (Tianjin, China). Multi-walled carbon nanotubes (MWCNTs) with average external diameters of <8 nm, 10–20 nm, 20–30 nm were purchased from Boyu Technologies Inc. (Beijing, China).
Field trials
Field experiments were conducted in Langfang (116.4°E, 39.3°N), Hebei Province, China in 2012 according to the Guidelines for Pesticide Residue Field Trials (NY/T 788-2004) issued by the Ministry of Agriculture, People’s Republic of China [14]. The plots had no history of the application of neonicotinoids, and the application of compounds with structures similar to that of sulfoxaflor was forbidden during the trial period. The physicochemical characteristics of the soil in the field were as follows: organic matter, 1.2 %; pH (suspension of soil in 0.01 M CaCl2, 1:2.5, w/w), 7.82; sand, 15.1 %; silt, 45.1 %; and clay 39.8 %. Four trial plots, each with an area of 30 m2, were chosen; three plots were designated as dissipation plots to avoid random errors, and the fourth plot was used as a control (without sulfoxaflor). Each plot was separated from the next plot by a buffer area of 1 m. The temperature of the area was maintained in the range 22 ± 10°C throughout the experiment. Soil samples were taken from a composite of 8–12 subsamples collected from a depth of 0–15 cm at increasing time intervals on day 0 (2 h after spraying) and at 1, 3, 5, 7, 10, 14, 21, 28, and 35 days after treatment with 50 % sulfoxaflor water-dispersible granules at a dosage of 525 g a.i. ha−1 (grams of active ingredient per hectare). Treated samples were stored in the dark at −20 °C until analysis.
Sample preparation
The blank vegetable samples (cucumber and tomato) were obtained from trial plots of the Institute of Plant Protection conducted in Langfang, Hebei Province, China, and homogenized using a blender (Philips, Shanghai, China). Soil samples were air-dried at room temperature, homogenized, and then passed through a 2-mm sieve. A 10 ± 0.10 g representative portion of the prepared samples (cucumber, tomato, and soil) were weighed into a 50 mL PTFE centrifuge tube after thawing to room temperature. Tubes containing spiked samples with suitable concentrations of sulfoxaflor standard solutions were mixed well and equilibrated for 2 h at room temperature to allow the pesticide to distribute evenly through the tube and interact with the sample matrix. Five milliliters of ultrapure water and 10 mL of ACN were sequentially added to the soil samples. The tubes were capped and vigorously shaken at an oscillation frequency of 1,350 min−1 using a CK-2000 high-throughput grinder (TH Morgan, Beijing, China) for 10 min. Four grams of anhydrous MgSO4 and 1 g of NaCl were added to the mixture, which was subsequently vortexed for 1 min using an XW-80A vortex (Kirin Medical Instruments, Hangzhou, China). The tube was centrifuged for 5 min at 2,588×g relative centrifugal force (RCF) using a SIGMA 3–15 centrifuge (SIGMA Laborzentrifugen GmbH, Osterode am Harz, Germany). Afterwards, 1.5 mL of the ACN layer (upper) were transferred to a single-use centrifuge tube and 150 mg of anhydrous MgSO4 and 5 mg of MWCNTs (<8 nm in size) were added to it. The samples were again vortexed for 1 min and centrifuged at 2,188×g RCF for 5 min. The resulting supernatant (ACN) was finally filtered using a 0.22-μm nylon syringe filter for UPC2-MS/MS injection.
UPC2-MS/MS analysis
UPC2-MS/MS analysis was performed on a Waters ACQUITY UPC 2TM system (Milford, MA, USA) equipped with an ACQUITY UPC 2TM column manager, an ACQUITY UPC 2TM convergence manager, an ACQUITY UPC 2TM sample manager-FL, an ACQUITY UPC 2TM binary solvent manager, and a Waters 515 compensation pump. Four chiral columns purchased from Daicel Chemical Industries (Tokyo, Japan), including Chiralpak® IA-3 [amylose tris(3,5-dimethylphenylcarbamate), 3 μm], Chiralpak® IA-5 [amylose tris(3,5-dimethylphenylcarbamate), 5 μm], Chiralpak® IB-3 [cellulose tris(3,5-dimethylphenylcarbamate), 3 μm], and Chiralpak® IC-3 [cellulose tris(3,5-dichlophenylcarbamate), 3 μm], were employed. The columns had dimensions of 4.6 mm diam. × 150 mm i.d. and different enantioselective phases were immobilized onto 3- or 5-μm silica gel beads. A Chiralpak® IA-3 was used to discriminate the stereoisomers of sulfoxaflor. The flow rate of the CO2-based mobile phase containing IPA/ACN (3/2, v/v) as mixed co-solvents, the temperatures of the column and sample vial holder, and the ABPR were set to 2.2 mL/min, 40 °C, 4 °C, and 2,500 psi, respectively. In each run, 1 μL of the sample was injected and the following gradient conditions for the mixed co-solvents were employed: initial, 8 %; 0.2 min, 12 %; 5.5 min, 12 %; 5.8 min, 8 %; 6.5 min, 8 %. 1.0 % FA-MeOH (v/v), which was used as a postcolumn additive, was applied at a flow rate of 0.1 mL/min.
A triple quadrupole Xevo®-TQD mass spectrometer (Waters Inc.) equipped with an electrospray ionization source (ESI) was used to quantify the sulfoxaflor stereoisomers. ESI+ was selected for subsequent experiments because this mode yields higher signal to noise ratios (S/N) than ESI−. Analyses were performed with source and desolvation temperatures of 150 and 500 °C, respectively. The nebulizer gas was 99.999 % N2 and the collision gas was 99.999 % Ar (pressure, 2 × 10−3 mbar) in the T-wave cell. Cone and desolvation N2 flows of 50 and 900 L/h were applied. MS detection was performed in multiple reaction monitoring (MRM) mode. Masslynx NT v.4.1 SCN 882 was used to collect and analyze the data obtained. Details of the MRM parameters used to determine sulfoxaflor stereoisomers are summarized in Table 1.
Thermodynamic and pharmacokinetic calculation
The retention factor (k), selectivity factor (α), and resolution (Rs) of the sulfoxaflor stereoisomers may be obtained and calculated from the following equations:
where t 0 is the void time (t 0 = 0.85 min, determined using 1,3,5-tri-tert-butylbenzene) under the given chromatographic conditions, t 1 and t 2 are the retention times of less-retained and more-retained peaks, respectively, and ω 1 and ω 2 are the widths of less-retained and more-retained peaks at half height, respectively. The thermodynamic parameters for enantiomeric resolution can be calculated using the following Van’t Hoff equations [15, 16]:
where ΔH ο and ΔS ο are the respective standard transfer enthalpy and entropy of the analyte from the mobile phase to the CSP, R is the gas constant, T is the absolute temperature, ϕ is the phase ratio, and ΔΔH ο and ΔΔS ο are the differences in enthalpy (ΔH ο 2 − ΔH ο 1) and entropy (ΔS ο 2 − ΔS ο 1), respectively. Provided that \( \ln \phi \) is independent of temperature, a plot of ln k versus 1/T will be linear with a slope of −ΔH ο/R and an intercept of [ΔS ο/R +\( \ln \phi \)]. For the linear plot of lnα versus 1/T, the slope and intercept are −ΔΔH ο/R and ΔΔS ο/R, respectively. The degradation rate constants (K) of the sulfoxaflor stereoisomers in soil samples were estimated using first-order kinetics [17, 18] and calculated according to
where C 0 and C are the concentrations of the test chemical at time zero and time t, respectively. Regressive functions were all obtained on the basis of the mean value of three replicates. The half-life (T 1/2, in days) was estimated from
The enantiomeric fraction (EF) is used to measure the enantioselectivity of the dissipation of sulfoxaflor enantiomers in the soil. The following equations describe the EF of a pair of enantiomers.
where (+)A, (−)A, (+)B, and (−)B are the peak areas of the specified (+) and (−) diastereomers A and B of sulfoxaflor eluted from the Chiralpak IA-3 column. EF values range from 0 to 1, with EF = 0.50 representing a racemic mixture. All of the functions were obtained on the basis of the mean value of three replicates.
Results and discussion
Optimization of the UPC2-MS/MS conditions
Effect of polysaccharide CSPs on chiral recognition, retention, and resolution of sulfoxaflor stereoisomers
Four immobilization chiral columns (Chiralpak IA-3, Chiralpak IA-5, Chiralpak IB-3, and Chiralpak IC-3) were evaluated for their ability to discriminate sulfoxaflor stereoisomers in the present study. As shown in Fig. 1, optimal stereoselectivity was achieved on Chiralpak IA-3, as it yielded the best resolution (1.56, 2.33 and 5.07) and a relatively short retention time (4.21, 4.42, 4.72, and 5.41 min). Chiralpak IA-5, IB-3, and IC-3 showed insufficient discrimination ability for sulfoxaflor stereoisomers. Electron-withdrawing chlorines in the phenyl ring of the carbamate derivative (IC-3) decrease the k of the CSPs, whereas electron-donating methyl groups (e.g., those in IA-3, IA-5, IB-3) enlarge k. Chiralpak IA-3, in particular, presents the smallest k values (3.95, 4.20, 4.55, and 5.36). Electronegative atoms (N, S and O) contribute to the binding of the stereoisomers to the carbamate groups, thereby forming transient diastereoisomers through hydrogen bonding via S=O and S=N groups and dipole–dipole interactions [19, 20]. Results of previous studies on three polysaccharide CSPs [21–23] revealed that amylose tris(3,5-dimethylphenylcarbamate) produces excellent success rates for chiral resolution.
Chiralpak IA-3, which features 3-μm particles, showed better chiral recognition ability for sulfoxaflor stereoisomers than Chiralpak IA-5, which features 5-μm particles (Fig. 1a–b). This suggests that there are chiral cavities with specific configurations on the particles of different sizes associated with different CSPs [24, 25]. The 3-μm particles provide the most suitable sites for stereoisomers of sulfoxaflor, thus leading to a greater chance of the stereoisomers interacting with the CSP through different hydrogen bonds, π–π interactions, or dipole–dipole-induced interactions. Therefore, the chiral cavities with different polysaccharide selectors or particle sizes must have different stereorecognition levels.
Effect of CO2-based mobile-phase composition and flow rate on retention and separation factors
The mobile phase is an essential component that is substantially involved in the chiral selector–selectand (CS-SA) association mechanism at multiple levels, because it defines the properties of the interaction environment [26, 27]. Compressed liquid CO2 was used as the primary mobile phase throughout the UPC2-MS/MS procedure. Six co-solvents (ACN, IPA, MeOH, ethanol, n-hexane, and 1-butanol) were evaluated individually in terms of their ability to achieve optimum separation of the sulfoxaflor stereoisomers on Chiralpak IA-3. Considering that single modifiers were incapable of achieving satisfactory resolution of sulfoxaflor, mixed modifiers were introduced into the system. The mixture of IPA and ACN facilitated the best stereoisomeric resolution of the four stereoisomers (Fig. 2a).
k appeared to decrease as the content of co-solvents increased, likely because the solvating power of the CO2-based mobile phase increases upon the addition of IPA and ACN. As the ACN content increased from 0 to 50 % (v/v), k decreased from 5.49, 5.85, 5.96, and 6.53 to 3.74, 3.94, 4.33, and 5.12, respectively, offering greater efficiency and shorter retention times for the sulfoxaflor stereoisomers. However, decreases in stereoselectivity and resolution were noted. In a plot of the variation in Rs as a function of co-solvent ratio, the Rs values (1.56, 2.33, and 5.07) actually peaked at an IPA/ACN ratio of 60/40 (v/v). The α values showed a similar curve and were 1.06, 1.08, and 1.18 at this point. The flow rate of the mobile phase was also evaluated for the stereoseparation of sulfoxaflor stereoisomers (Fig. 2b). As the flow rate increased from 1.0 to 3.0 mL/min, α increased from 1.03, 1.06, and 1.13 to 1.09, 1.13, and 1.26, respectively. By contrast, Rs decreased significantly from 1.30, 2.80, and 5.22 to 1.38, 1.93, and 4.43. Considering a flow rate of 2.2 mL/min, the best resolution (1.56, 2.33, and 5.07) was achieved in 6.5 min. Thus, mixed co-solvents comprising IPA/ACN = 60/40 (v/v) in the mobile phase and a flow rate of 2.2 mL/min were selected for subsequent experiments.
The stereoselectivity of sulfoxaflor stereoisomers varies greatly according to the nature and proportions of the co-solvents used. The supramolecular structure of the polysaccharide backbone and the binding sites available are altered by IPA and ACN, and IPA/ACN = 60/40 (v/v) may facilitate the access of the stereoisomers from the mobile phase to the CS. Likewise, a leading interaction based on hydrogen bonding has been predicted to determine the retention of the stereoisomers, and at least one other type of interaction (e.g., π–π and steric hindrance) that is independent of solvent polarity is responsible for the enantioselectivity [28, 29], which depends upon the stereoenvironment [26]. According to M. Lammerhofer, the mechanism has only been partially established so far [29], and the identity of the strongest CS–SA interaction, which determines the stereoselectivity profiles, needs to be explored further in a follow-up study.
Effect of ABPR on the stereoselectivity of sulfoxaflor stereoisomers
The pressure of the supercritical system is believed to influence the density of CO2 [13, 30]. ABPR obviously influences the eluotropic strength of the fluid in UPC2-MS/MS. The effects of ABPR on the retention and separation of sulfoxaflor stereoisomers using CO2/IPA/ACN as a ternary mobile phase were thoroughly studied under pressures ranging from 1,500 to 4,000 psi, increased in 500-psi increments (Fig. 2c). The retention times and k values of the four stereoisomers decreased as the pressure decreased from 1,500 to 4,000 psi. The retention time decreased by approximately 1.4 min and k decreased from 4.65, 4.85, 5.18, and 6.13 to 3.02, 3.32, 3.66, and 4.51, respectively. These results indicated that the mobile phase density increases inside the column, with greater solvating power and higher elution efficiency occurring at higher ABPRs. However, stereoselectivity remained fairly constant, with only slight variations (≤0.3) for both pairs of enantiomers. These results are consistent with those observed during SFC by V Abrahamsson [31] and Wang [30]. Despite its minimal effects on enantiomeric resolution, the backpressure greatly affects solute retention. Also, the mobile and stationary phase interactions control the rate of change of retention with pressure. As discussed above, the best resolution (≥1.56) was obtained at 2,500 psi with relatively short retention times (4.21, 4.42, 4.72, and 5.41 min).
Column temperature optimization using Van’t Hoff plots
Direct stereoisomeric separation with CSPs can be achieved by measuring thermodynamic parameters (ΔH ο and ΔS ο) over a certain temperature range from Van’t Hoff plots. During separation of the sulfoxaflor stereoisomers, the column temperature was increased from 25 to 40 °C in 5 °C increments. The natural logarithm of k (lnk), lnα, and Rs were analyzed to obtain the information necessary to understand stereoisomeric retention, selectivity, and the relevant mechanism on the Chiralpak IA-3 column. As shown in Fig. 3, Van’t Hoff plots of lnk versus 1/T (R 2 ≥ 0.9879) and lnα versus 1/T (R 2 ≥ 0.9858) for the sulfoxaflor stereoisomers were mainly linear, with transitions occurring between 25 and 40 °C. The retention and selection processes governing separation are unchanged over the temperature range studied [32]. The fact that the ΔH ο values (7.09, 6.75, 6.24, and 3.68) of the stereoisomers calculated from the slopes of the plots were positive indicates that the transfer of stereoisomers from the mobile phase to the stationary phase is entropically favorable according to Peter et al. [15] and O’Brien et al. [16]. However, considering that the resolutions achieved and the values of ΔΔH ο (−0.34, −0.51, and −2.57) and ΔΔS ο (−0.07, −0.11, and −0.82) were negative, enthalpy-driven separation of the sulfoxaflor stereoisomers appears to take place. In conclusion, increasing the temperature from 25 to 40 °C is beneficial when attempting to resolve the sulfoxaflor stereoisomers. Since the suggested upper temperature limit of Chiralpak IA-3 is 40 °C, and a reduction in analysis time may be achieved below this temperature, the final column temperature was set to 40 °C.
Effect of postcolumn additives and their flow rates on ESI-MS/MS
Post-column addition was adopted in the proposed UPC2-MS/MS procedure to prevent distinct resolution of interferences by UPC2 and maximize the ionization efficiency of ESI-MS/MS. Acid additives are particularly favored during ESI-MS/MS because several studies indicate a 3- to 10-fold increase in the S/N ratio when FA is applied instead of acetic acid [33], trifluoroacetic acid (TFA) [34], or ammonium acetate [35]. In this study, the effects of different FA concentrations (0.2 %, 1.0 %, and 2.0 %) in MeOH on the S/N ratios of sulfoxaflor stereoisomers were investigated. The highest S/N ratios for the four stereoisomers were obtained when the concentration of FA was 1.0 %. The effects of different flow rates (0.1, 0.2, 0.3, and 0.45 mL/min) of 1.0 % FA-MeOH were also determined. The lowest flow rate, 0.1 mL/min, was selected for further analysis because there was hardly any change in the signal responses of the sulfoxaflor stereoisomers with flow rate. Even more importantly, the problem of lower signal suppression but poorer resolution of the pre-column additive FA used in LC-MS/MS for chiral separation, as reported by Garcia [35] and Corradini et al. [36], was successfully avoided by using the postcolumn additive method in this study.
Elution order of sulfoxaflor stereoisomers
The elution order of the sulfoxaflor stereoisomers was determined by measuring the optical rotations (ORs) of the individual optically pure stereoisomers. Based on our previous study of stereoisomer separation using the ChromegaChiral CCA column, the following stereoisomers were identified: (−) sulfoxaflor A (diastereomer A of sulfoxaflor), (+) sulfoxaflor B (diastereomer B of sulfoxaflor), (−) sulfoxaflor B, and (+) sulfoxaflor A [37]. The elution order of the four stereoisomers of sulfoxaflor on Chiralpak IA-3 was determined using UPC2-MS/MS by injecting an aliquot of each individual optically pure stereoisomer. Micropreparation of the individual stereoisomers was obtained using an Agilent 1100 series HPLC system (Agilent Technology, Waldbronn, Germany) equipped with a ChromegaChiral CCA Chiral column [amylose tris(3,5-dimethylphenylcarbamate), 4.6 mm diam. × 250 mm i.d., 5 μm, ES Industries, West Berlin, NJ, USA] using n-hexane/ethanol/methanol (90:2:8, v/v/v) as the mobile phase at 20 °C. The sulfoxaflor stereoisomers eluted from the Chiralpak IA-3 in the following order: (+) sulfoxaflor B, (−) sulfoxaflor A, (−) sulfoxaflor B, and (+) sulfoxaflor A (Fig. 4). The reversal of the elution order of sulfoxaflor stereoisomers on the amylose CSP associated with steric environment of chiral cavities. Because it was responsible for the bonding of the different magnitude between CS-SA by different mobile phases [38].
Sample preparation development using a modified dSPE cleanup process
To obtain better recoveries and improve the purification efficiency, the application of modified dispersive solid-phase extraction (dSPE) was explored and validated by comparing the MWCNTs with conventional sorbents such as Cleanert PSA, GCB, C18, and Florisil. Ten sorbents were investigated, including 50 mg of PSA, 50 mg of C18, 50 mg of Florisil, 10 mg of GCB, 50 mg of PSA + 10 mg of GCB, 50 mg of C18 + 10 mg of GCB, 50 mg of Florisil + 10 mg of GCB, and three sizes of MWCNTs (<8 nm, 10–20 nm, 20–30 nm). The recoveries of the sulfoxaflor stereoisomers from cucumber, tomato, and soil matrices spiked at different levels were calculated, and the colors of the ACN sample extracts were evaluated. The <8 nm MWCNTs (5 mg) showed the best purification efficiency and recovery, which ranged from 72.9 to 103.7 % (Fig. 5), due to their extremely large surface areas and unique structure [39, 40].
Recoveries of sulfoxaflor stereoisomers obtained using MWCNTs and conventional dSPE sorbents from cucumber spiked with 0.1 mg/kg sulfoxaflor racemate, and photograph showing the colors of the resulting ACN sample extracts. Sorbents: (A) 50 mg PSA, (B) 50 mg C18, (C) 50 mg Florisil, (D) 50 mg PSA + 10 mg GCB, (E) 50 mg C18 + 10 mg GCB, (F) 50 mg Florisil + 10 mg GCB, (G) 10 mg GCB, and (H–J) 5 mg MWCNTs with internal diameters of < 8 nm (H), 10–20 nm (I), and 20–30 nm (J)
Assay validation
Selectivity, linearity, and LOQ
Blank analyses of vegetable and soil samples performed by monitoring the characteristics of selected ion chromatograms for each stereoisomer investigated demonstrated no interferences in the retention time interval expected for their elution (Fig. 6). As shown in Table 2, good linearity was observed among the four stereoisomers, and mean correlation coefficients (R 2) of higher than 0.9945 were obtained. The LOQs of the sulfoxaflor stereoisomers were determined at S/N = 10 and were estimated to be 1.28–1.68 μg/kg in cucumber, 1.34–1.83 μg/kg in tomato, and 1.21–1.58 μg/kg in soil on the basis of an acceptable RSD of 10 % [41]. This sensitivity is far better than the maximum residual levels of sulfoxaflor estimated by the Joint Meeting On Pesticide Residues (JMPR, 0.50 mg/kg for cucumber and 1.50 mg/kg for tomato) (http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report11/Sulfoxaflor.pdf).
Typical UPC2-MS/MS (MRM) chromatograms of the blanks and various spiked samples. a-1 Cucumber blank, a-2 cucumber spiked at 0.05 mg/kg, b-1 tomato blank, b-2 tomato spiked at 0.05 mg/kg, c-1 soil blank, c-2 soil spiked at 0.05 mg/kg; c-3, c-4, and c-5 show chromatograms of the sulfoxaflor stereoisomers in soil after 0, 7, and 28 days, respectively
Matrix effect
Matrix-matched calibration solutions were used to compensate for errors associated with signal suppression and enhancement (SSE) in the MS/MS (MRM mode) detector and modified dSPE method. The data in Table 2 indicate that significant signal enhancements (≥10 %, using ACN as the reference) ranging from +11 to +46 % were observed for the sulfoxaflor stereoisomers in three matrices. As such, external matrix-matched standards were selected for the accurate quantitation of different samples.
Precision and accuracy
Recovery assays were carried out to investigate the accuracy and precision of the proposed method by assaying samples spiked with sulfoxaflor racemate at three concentration levels (0.05, 0.10, and 0.50 mg/kg) in five replicates. The process was repeated for 3 days using the same instrument but handled by different operators. The results showed excellent average recoveries in the range 72.9–103.7 % at all spiking levels. Good repeatability, as indicated by relative standard deviations (RSD) of less than 9.4 %, was also obtained. In general, the intra-day (n = 5) and inter-day (n = 15) RSDs for the proposed method ranged from 1.8 to 9.2 % and from 3.1 to 9.4 %, respectively (Table 3). These results demonstrate that UPC2-MS/MS was able to achieve satisfactory precision and accuracy for the stereoisomeric analysis of sulfoxaflor in vegetables and soil matrices. Figure 6a1–c2 shows typical UPC2-MS/MS (MRM) chromatograms of the blanks and various spiked samples.
Stability
The stabilities of stock solutions of sulfoxaflor and spiked cucumber, tomato, and soil samples were tested monthly using the same UPC2-MS/MS instrument. Results were analyzed using Student’s paired t-test at the 95 % confidence limit, and no significant differences (P > 0.05) were observed. Quality control included one standard for each ten samples analyzed. Sulfoxaflor standards were injected repeatedly to determine the reproducibility of the EF measurements. EFA and EFB were calculated to be 0.50 ± 0.007 (n = 5) and 0.50 ± 0.009 (n = 5), respectively.
Practical application
Application to market samples
Ten cucumber samples and ten tomato samples were purchased from a market in Beijing, China, treated with the sample preparation procedures described above, and analyzed by UPC2-MS/MS under the same conditions specified in the “UPC2-MS/MS analysis” section. Results showed that the residual contents of the four stereoisomers in all the samples were lower than their corresponding LOQs.
Application in enantioselective degradation of sulfoxaflor stereoisomers in soil
The proposed method was applied to study the enantioselective degradation of two pairs of sulfoxaflor enantiomers in soil under greenhouse conditions. The concentrations of the sulfoxaflor stereoisomers in soil measured after application achieved maxima within 2 h and gradually decreased as time elapsed. As shown in Table 4, the degradation of the four sulfoxaflor stereoisomers in soil followed first-order kinetics (R 2 = 0.9018–0.9304). The estimated half-lives (T 1/2) of the four stereoisomers, which are important indicators of pesticide efficacy and pollution, were also determined [42]. The T 1/2 values of the sulfoxaflor stereoisomers in soil ranged from 5.59 to 6.03 days under greenhouse conditions—longer than those measured under field conditions [43]. Enantioselectivity during sulfoxaflor degradation was evaluated by monitoring the changes in the EF values of the different diastereomers (EFA and EFB) at predetermined intervals. EFA and EFB were nearly 0.5 two hours after application in soil; thereafter, EFA increased gradually from 0.49 to 0.53, and EFB decreased from 0.50 to 0.48. The difference between (−)-sulfoxaflor and (+)-sulfoxaflor was evaluated, and statistical nonsignificance between EFA and EFB (P > 0.05, Student’s paired t-test, 95 % confidence limit) was observed. Previous research reported that microbial decomposition plays an important role in the enantioselective degradation of many chiral chemicals in soils [44, 45], which may explain the stereoselectivity of the sulfoxaflor stereoisomers observed in the present study. Typical UPC2-MS/MS (MRM) chromatograms of the sulfoxaflor stereoisomers are shown in Fig. 6c3–c5.
Conclusions
UPC2-ESI-MS/MS was applied to the stereoisomeric separation and determination of sulfoxaflor stereoisomers in vegetables and soil. Baseline resolutions were achieved using a Chiralpak IA-3 column with IPA and ACN (2:3, v:v) as co-solvents at 40 °C and 6.5 min. High S/N ratios were obtained using postcolumn addition of 1.0 % FA-MeOH at a flow rate of 0.1 mL/min. Higher ABPRs were conducive to solute retention but prompted slight differences in the stereoisomeric resolution of sulfoxaflor. Analysis of the Van’t Hoff plots led to a better understanding of enthalpy-driven separation by UPC2-MS/MS. High purification efficiencies and recoveries (which varied from 72.9 to 103.7 %) were obtained using MWCNTs (<8 nm, 5 mg). The proposed method was applied to study the enantioselective degradation of sulfoxaflor isomers in soil. Results showed that the estimated half-lives of the stereoisomers range from 5.59 to 6.03 days. Statistical nonsignificance of the enantioselectivity of the two pairs of enantiomers was also observed. The results of this stereoselective separation and the practical application of it provide a solid foundation for future studies that attempt to trace the different bioactivities, toxicities, and metabolic and environmental behavior of sulfoxaflor stereoisomers in order to minimize risks posed by the insecticide to beneficial insects and the environment.
Abbreviations
- ABPR:
-
Pressure of automated backpressure regulator
- CSP:
-
Chiral stationary phase
- CS-SA:
-
Selector–selectand
- dSPE:
-
Dispersive solid-phase extraction
- EF:
-
Enantiomer fraction
- ESI:
-
Electrospray ionization
- MRL:
-
Maximum residue limit
- MRM:
-
Multiple reaction monitoring
- MWCNTs:
-
Multi-walled carbon nanotubes
- nAChR:
-
Nicotinic acetylcholine receptor
- Rs:
-
Resolution
- RSD:
-
Relative standard deviation
- SSE:
-
Signal suppression and enhancement
References
Zhu Y, Loso MR, Watson GB, Sparks TC, Rogers RB, Huang JX, Gerwick BC, Babcock JM, Kelley D, Hegde VB, Nugent BM, Renga JM, Denholm I, Gorman K, DeBoer GJ, Hasler J, Meade T, Thomas JD (2011) Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J Agric Food Chem 59(7):2950–2957
Sparks TC, Watson GB, Loso MR, Geng CX, Babcock JM, Thomas JD (2013) Sulfoxaflor and the sulfoximine insecticides: chemistry, mode of action and basis for efficacy on resistant insects. Pestic Biochem Physiol 107(1):1–7
Longhurst C, Babcock JM, Denholm I, Gorman K, Thomas JD, Sparks TC (2013) Cross-resistance relationships of the sulfoximine insecticide sulfoxaflor with neonicotinoids and other insecticides in the whiteflies Bemisia tabaci and Trialeurodes vaporariorum. Pest Manag Sci 69(7):809–813
Cutler P, Slater R, Edmunds AJ, Maienfisch P, Hall RG, Earley FG, Pitterna T, Pal S, Paul VL, Goodchild J, Blacker M, Hagmann L, Crossthwaite AJ (2013) Investigating the mode of action of sulfoxaflor: a fourth-generation neonicotinoid. Pest Manag Sci 69(5):607–619
Garrison AW (2006) Probing the enantioselectivity of chiral pesticides. Environ Sci Technol 40(1):16–23
Zhang H, Wang X, Zhuang S, Qian M, Jiang K, Wang X, Xu H, Qi P, Wang Q (2012) Enantioselective separation and simultaneous determination of fenarimol and nuarimol in fruits, vegetables, and soil by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 404(6–7):1983–1991
Tonon MA, Jabor VA, Bonato PS (2013) Enantioselective analysis of zopiclone in rat brain by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 405(1):267–273
Babcock JM, Gerwick CB, Huang JX, Loso MR, Nakamura G, Nolting SP, Rogers RB, Sparks TC, Thomas J, Watson GB, Zhu Y (2011) Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag Sci 67(3):328–334
Lysandrou M, Ahmad M, Longhurst C (2010) Comparative efficacy of sulfoxaflor against cotton leafhopper, Amrasca devastans (Distant)(Cicadellidae: Homoptera) under field conditions of punjab and sindh. J Agric Res 48(4):517–524
Siebert M, Thomas J, Nolting S, Leonard B, Gore J, Catchot A, Lorenz G, Stewart S, Cook D, Walton L (2012) Field evaluations of sulfoxaflor, a novel insecticide, against tarnished plant bug (Hemiptera: Miridae) in cotton. J Cotton Sci 16:129–143
Watson GB, Loso MR, Babcock JM, Hasler JM, Letherer TJ, Young CD, Zhu Y, Casida JE, Sparks TC (2011) Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem Mol Biol 41(7):432–439
Sparks TC, DeBoer GJ, Wang NX, Hasler JM, Loso MR, Watson GB (2012) Differential metabolism of sulfoximine and neonicotinoid insecticides by Drosophila melanogaster monooxygenase CYP6G1. Pestic Biochem Physiol 103(3):159–165
Zhou Q, Gao B, Zhang X, Xu Y, Shi H, Yu LL (2014) Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra-performance convergence chromatography combined with a quadrupole time-of-flight mass spectrometry. Food Chem 143:199–204
Ministry of Agriculture (2004) NY/T788: Guidelines for pesticide residue field trials. Ministry of Agriculture, People's Republic of China, Beijing
Péter A, Vékes E, Armstrong DW (2002) Effects of temperature on retention of chiral compounds on a ristocetin A chiral stationary phase. J Chromatogr A 958(1):89–107
O’Brien T, Crocker L, Thompson R, Thompson K, Toma P, Conlon D, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Mechanistic aspects of chiral discrimination on modified cellulose. Anal Chem 69(11):1999–2007
Diao J, Xu P, Wang P, Lu Y, Lu D, Zhou Z (2010) Environmental behavior of the chiral aryloxyphenoxypropionate herbicide diclofop-methyl and diclofop: enantiomerization and enantioselective degradation in soil. Environ Sci Technol 44(6):2042–2047
Li Z, Zhang Y, Li Q, Wang W, Li J (2011) Enantioselective degradation, abiotic racemization, and chiral transformation of triadimefon in soils. Environ Sci Technol 45(7):2797–2803
Kažoka H, Koliškina O, Veinberg G, Vorona M (2013) Separation of piracetam derivatives on polysaccharide-based chiral stationary phases. J Chromatogr A 1281:160–165
Khan M, Viswanathan B, Rao DS, Reddy R (2007) Chiral separation of Frovatriptan isomers by HPLC using amylose based chiral stationary phase. J Chromatogr B 846(1):119–123
Ward TJ, Ward KD (2011) Chiral separations: a review of current topics and trends. Anal Chem 84(2):626–635
Zhang T, Nguyen D, Franco P (2008) Enantiomer resolution screening strategy using multiple immobilised polysaccharide-based chiral stationary phases. J Chromatogr A 1191(1):214–222
Ali I, Naim L, Ghanem A, Aboul-Enein HY (2006) Chiral separations of piperidine-2,6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta 69(4):1013–1017
Qiu J, Dai S, Zheng C, Yang S, Chai T, Bie M (2011) Enantiomeric separation of triazole fungicides with 3-μm and 5-μml particle chiral columns by reverse-phase high-performance liquid chromatography. Chirality 23(6):479–486
Li J, Dong F, Cheng Y, Liu X, Xu J, Li Y, Chen X, Kong Z, Zheng Y (2012) Simultaneous enantioselective determination of triazole fungicide difenoconazole and its main chiral metabolite in vegetables and soil by normal-phase high-performance liquid chromatography. Anal Bioanal Chem 404(6–7):2017–2031
Sardella R, Ianni F, Lisanti A, Marinozzi M, Scorzoni S, Natalini B (2014) The effect of mobile phase composition in the enantioseparation of pharmaceutically relevant compounds with polysaccharide-based stationary phases. Biomed Chromatogr 28(1):159–167
West C, Bouet A, Routier S, Lesellier E (2012) Effects of mobile phase composition and temperature on the supercritical fluid chromatography enantioseparation of chiral fluoro-oxoindole-type compounds with chlorinated polysaccharide stationary phases. J Chromatogr A 1269:325–335
Berthod A (2006) Chiral recognition mechanisms. Anal Chem 78(7):2093–2099
Lämmerhofer M (2010) Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A 1217(6):814–856
Wang C, Zhang Y (2013) Effects of column back pressure on supercritical fluid chromatography separations of enantiomers using binary mobile phases on 10 chiral stationary phases. J Chromatogr A 1281:127–134
Abrahamsson V, Rodriguez-Meizoso I, Turner C (2012) Determination of carotenoids in microalgae using supercritical fluid extraction and chromatography. J Chromatogr A 1250:63–68
Wang F, O’Brien T, Dowling T, Bicker G, Wyvratt J (2002) Unusual effect of column temperature on chromatographic enantioseparation of dihydropyrimidinone acid and methyl ester on amylose chiral stationary phase. J Chromatogr A 958(1):69–77
Liu H, Berger SJ, Chakraborty AB, Plumb RS, Cohen SA (2002) Multidimensional chromatography coupled to electrospray ionization time-of-flight mass spectrometry as an alternative to two-dimensional gels for the identification and analysis of complex mixtures of intact proteins. J Chromatogr B 782(1):267–289
Marwah A, Marwah P, Lardy H (2002) Analysis of ergosteroids. VIII: Enhancement of signal response of neutral steroidal compounds in liquid chromatographic–electrospray ionization mass spectrometric analysis by mobile phase additives. J Chromatogr A 964(1–2):137–151
Garcia M (2005) The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography–electrospray mass spectrometry. J Chromatogr B 825(2):111–123
Corradini D, Huber CG, Timperio AM, Zolla L (2000) Resolution and identification of the protein components of the photosystem II antenna system of higher plants by reversed-phase liquid chromatography with electrospray–mass spectrometric detection. J Chromatogr A 886(1):111–121
Chen Z, Dong F, Xu J, Liu X, Chen Y, Liu N, Tao Y, Zheng Y (2014) Stereoselective determination of a novel chiral insecticide, sulfoxaflor, in brown rice, cucumber and apple by normal-phase high-performance liquid chromatography. Chirality 26(2):114–120
Wang T, Wenslow RM Jr (2003) Effects of alcohol mobile-phase modifiers on the structure and chiral selectivity of amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase. J Chromatogr A 1015(1):99–110
El-Sheikh AH, Sweileh JA, Al-Degs YS, Insisi AA, Al-Rabady N (2008) Critical evaluation and comparison of enrichment efficiency of multi-walled carbon nanotubes, C18 silica and activated carbon towards some pesticides from environmental waters. Talanta 74(5):1675–1680
Zhao P, Wang L, Zhou L, Zhang F, Kang S, Pan C (2012) Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J Chromatogr A 1225:17–25
Dong F, Li J, Chankvetadze B, Cheng Y, Xu J, Liu X, Li Y, Chen X, Bertucci C, Tedesco D (2013) The chiral triazole fungicide difenoconazole: absolute stereochemistry, stereoselective bioactivity, aquatic toxicity and environmental behavior in vegetables and soil. Environ Sci Technol 47(7):3386–3394
Zhang H, Wang X, Qian M, Wang X, Xu H, Xu M, Wang Q (2011) Residue analysis and degradation studies of fenbuconazole and myclobutanil in strawberry by chiral high-performance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 59(22):12012–12017
Xu J, Dong F, Liu X, Li J, Li Y, Shan W, Zheng Y (2012) Determination of sulfoxaflor residues in vegetables, fruits and soil using ultra-performance liquid chromatography/tandem mass spectrometry. Anal Methods 4(12):4019
Dong F, Cheng L, Liu X, Xu J, Li J, Li Y, Kong Z, Jian Q, Zheng Y (2012) Enantioselective analysis of triazole fungicide myclobutanil in cucumber and soil under different application modes by chiral liquid chromatography/tandem mass spectrometry. J Agric Food Chem 60(8):1929–1936
Wong CS (2006) Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal Bioanal Chem 386(3):544–558
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC, 31272071 and 31171879).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Z., Dong, F., Xu, J. et al. Stereoselective separation and pharmacokinetic dissipation of the chiral neonicotinoid sulfoxaflor in soil by ultraperformance convergence chromatography/tandem mass spectrometry. Anal Bioanal Chem 406, 6677–6690 (2014). https://doi.org/10.1007/s00216-014-8089-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8089-9