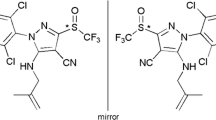
Abstract
A method for simultaneous enantioselective determination of fenarimol and nuarimol in apple, grape, cucumber, tomato, and soil was developed using liquid chromatography–tandem mass spectrometry. The enantioseparation results of the two fungicides through three different cellulose-based chiral columns are discussed. The influence of column temperature on the resolution of the enantiomers of the two fungicides was examined. Complete enantioseparation of the two fungicides’ enantiomers was obtained on a cellulose tris(4-methylbenzoate) column (Lux Cellulose-3) at 25 °C using methanol and 0.1 % formic acid solution (80:20, v/v) as mobile phase. The linearity, matrix effect, recovery, and precision were evaluated. Good linearity was obtained over the concentration range of 1–500 μg L−1 for each enantiomer in the standard solution and sample matrix calibration solution. There was no significant matrix effect in apple, grape, cucumber, or tomato samples, but signal suppression was typically observed with the soil extracts. The mean recoveries, repeatability, and reproducibility were 76.5–103 %, 2.1–9.0 %, and 4.2–11.8 %, respectively. The limit of quantification for enantiomers of the two fungicides in fruits, vegetables and soil was 5 μg kg−1. Moreover, the absolute configuration of the enantiomers of fenarimol and nuarimol was determined from a combination of experimentally determined and predicted electronic circular dichroism spectra.

Predicted ECD spectra of nuarimol enantiomers (a) and fenarimol enantiomers (b). Experimentally measured ECD spectra of nuarimol enantiomers (c) and fenarimol enantiomers (d) in acetonitrile (20 mg L−1)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fenarimol and nuarimol (Fig. 1) are two important pyrimidine fungicides. They are widely used to effectively control scab and powdery mildew in fruits and vegetables [1–3]. Their primary mode of action is based on the inhibition of sterol biosynthesis. Each of these compounds has an asymmetrically substituted carbon atom and is thus chiral with two enantiomers. As a result of enantioselectivity, one enantiomer of chiral pesticides may become enriched relative to the other during metabolism in plants or soil [4–6]. However, most chiral pesticides, including fenarimol and nuarimol, are presently administered and marketed as racemates. Therefore, effective enantioselective analysis of chiral pesticides has become increasingly important. Chiral separation of stereoisomers of pesticides is usually accomplished by gas chromatography (GC), capillary electrophoresis (CE), supercritical fluid chromatography (SFC), or high-performance liquid chromatography (HPLC) [7]. Baseline separation of the nuarimol enantiomers and partial separation of the fenarimol enantiomers were obtained with GC, using a 2,3-di-O-acetyl-6-O- thexyldimethyl-silyl-γ-cyclodextrin (ACTHDMS-γ-CD) column [8]. Separation of nuarimol enantiomers was also obtained by an affinity electrokinetic chromatography–partial filling technique using human serum albumin (HSA) as the chiral selector [9]. But as far as we know, no previous work has been reported on chiral determination of fenarimol and nuarimol by HPLC. Now, with the rapid development of chiral stationary phases (CSP), HPLC has been increasingly used for chiral separations because of its simplicity and ease of operation. Moreover, HPLC can today be efficiently coupled with mass spectrometry (MS), increasing selectivity and allowing simplified sample pretreatment. Recently, enantioselective HPLC–MS/MS methods for the determination of triazole fungicides in fruits, vegetables, soil, and water were published [10–13]. To date, achiral methods for the determination of fenarimol and nuarimol in various matrixes have been published using HPLC–MS/MS [14–16], but to our knowledge, no enantioselective method is currently available.
Because of their widespread use, fenarimol and nuarimol have the potential to cause adverse effects in humans, soil, and plants. Moreover, fenarimol is highly persistent in soil and known to have estrogenic and antiandrogenic activity, as well as moderate acute and long-term toxicity to fish and aquatic invertebrates [17–20]. Therefore, the development and validation of an enantioselective method for the quantitative determination of chiral fenarimol and nuarimol is urgently required.
The determination of the absolute configuration (AC) of chiral molecules is also an important aspect of chiral analysis. The rapid development of computerized ab initio time-dependent density functional theory (TDDFT) has allowed the widespread application of chiroptical methods, such as vibrational circular dichroism (VCD) and electronic circular dichroism (ECD), for the determination of the ACs of chiral molecules [21].
In this article, HPLC–MS/MS was applied to the enantioseparation of fenarimol and nuarimol on three chiral columns. The mobile phase systems for HPLC–MS/MS analysis were optimized, and the influence of column temperature on the resolution was examined. To determine the ACs of the enantiomers, ECD spectra were predicted using the TDDFT methodology, and a comparison of experimental and predicted ECD spectra was performed. The validated method was applied to the analysis of enantioselective residues in fruit, vegetable, and soil samples (apple, grape, cucumber, tomato, and soil).
Experimental
Reagents and materials
HPLC-grade methanol (CH3OH) and acetonitrile (CH3CN) were purchased from Merck (Darmstadt, Germany). Purified water was prepared by using a Milli-Q water purification system (Millipore Corporation, Billerica, USA). Formic acid (HCOOH, ≥96 % purity) was purchased from TEDIA (Fairfield, USA). Acetic acid (≥99.7 % purity) and uracil (≥99.0 % purity) were obtained from Sigma-Aldrich (St Louis, USA). Silica-based sorbents including C18 (40 μm particle size) and primary secondary amine (PSA) (40 μm particle size) were obtained from Agilent (Wilmington, USA). All other chemicals were of analytical grade and purchased from commercial sources.
The analytical columns, cellulose tris(3,5-dimethylphenylcarbamate) (Lux Cellulose-1), cellulose tris(3-chloro-4-methylphenylcarbamate) (Lux Cellulose-2), and cellulose tris(4-methylbenzoate) (Lux Cellulose-3) were purchased from Phenomenex (Torrance, USA), and all the columns were sized 150 mm × 2.0 mm i.d., and packed with 3-μm particles.
Analytical standards of (±)-fenarimol (97.5 % purity) and (±)-nuarimol (96.0 % purity) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Stock standards (approximately 100 mg L−1) of the individual fungicides were prepared by dissolving the reference compounds in acetonitrile. Additional individual or mixed standards at lower concentrations were prepared by serial dilution of the individual stock standards. All solutions were stored in the dark at 4 °C.
Enantiomers of fenarimol and nuarimol with purity ≥98.0 % were obtained from Daicel (Shanghai, China). Stock standards (approximately 100 mg L−1) of each individual fungicide enantiomer were prepared in acetonitrile. Standard solutions of each enantiomer (20 mg L−1) were used to obtain ECD spectra.
HPLC–MS/MS analysis
HPLC–MS/MS analysis was performed on a TSQ Discovery quantum mass spectrometer and a Surveyor liquid chromatograph (Thermo Fisher Scientific, Waltham, USA). The Thermo Fisher Xcalibur software package (version 2.0.7) was used to control the system, and collect and analyze data.
Acetonitrile or methanol with 0.1 % formic acid was used as the mobile phase. The injection volume was 10 μL, and the flow rate was 0.2 mL min−1. The ESI–MS interface was operated in the positive ion mode with selected reaction monitoring (SRM). The ESI source conditions were as follows: ion-spray voltage 4,000 V, spray needle temperature 350 °C, sheath gas (N2) 35 U (arbitrary units), auxiliary gas (N2) 15 U, collision gas (Ar) 1.5 mTorr. For fenarimol, the transitions m/z 331 → 268 and m/z 331 → 259 were used for quantification and confirmation, respectively, and the collision energies were both 20 eV. For nuarimol, the transitions m/z 315 → 252 and m/z 315 → 243 were used for quantification and confirmation, respectively, and the collision energies were 19 eV and 22 eV, respectively. Uracil (100 mg L−1) was injected as a dead time marker under the same established chromatographic conditions, using 113 ([M + H]+) as the selected ion.
The capacity factor [k′ = (t R − t 0)/t 0] and the separation factor (α = k 2′ / k 1′ ) were calculated, where t R and t 0 are the retention time of the analyte and the column void time, respectively, and k 1′ and k 2′ are the capacity factors for the first- and second-eluted stereoisomer, respectively.
Circular dichroism spectroscopy
ECD spectroscopy was carried out using a Jasco J815 (Tokyo, Japan) circular dichroism spectropolarimeter at room temperature. Acetonitrile was used as a solvent. Spectra were collected over a wavelength range of 200–400 nm, with a 50 nm/min scan speed. A quartz cell with a path length of 0.1 cm was used for the scanning and the average of three scans was reported.
ECD calculations
First, the geometry of the fenarimol and nuarimol enantiomers was optimized by Gaussian 09, using DFT at a level of B3LYP/6-31+G* in acetonitrile, with a CPCM solvent model. After the initial geometry optimization, the ECD spectra of the fenarimol and nuarimol enantiomers were predicted using the TDDFT method, at a level of B3LYP/6-31+G* in acetonitrile, with a CPCM solvent model following reported protocols [22, 23].
Sample preparation
Surface soil samples (depth 0–10 cm) were collected from the experimental field of the Zhejiang Academy of Agricultural Sciences (Hangzhou, China). No fenarimol or nuarimol was detected in these samples. Soil samples were air-dried at room temperature, homogenized, and sieved to yield a maximum particle size of less than 2 mm. Fruit and vegetable samples were finely chopped and homogenized using a blender (Philips, China). All samples were stored at −20 °C until required. Analyses were performed according to the following protocol.
Soil sample
Five grams of soil was weighed into a 50-mL polypropylene centrifuge tube. Then, 10 mL of water and 20 mL of 1 % acetic acid in acetonitrile were added, and the mixture was vortexed for 1 min, followed by sonication for 15 min. Subsequently, 6 g of anhydrous magnesium sulfate (MgSO4) and 1.5 g of anhydrous sodium acetate were added, and the tube was shaken vigorously by hand for 5 min, followed by centrifugation at 6,000 rpm for 2 min. An aliquot of the supernatant (10 mL) was evaporated to near dryness in a rotary evaporator, over a water bath at 40 °C, and dried under a gentle stream of nitrogen. The residue was dissolved in 5 mL of methanol/0.1 % formic acid solution (80:20, v/v), and the resulting solution was filtered through a 0.22-μm Teflon filter prior to HPLC–MS/MS analysis.
Fruit and vegetable samples
Fifteen grams of sample (apple, grape, cucumber, or tomato) was weighed into a 50-mL polypropylene centrifuge tube, and 15 mL acetonitrile containing 1 % of acetic acid was added. The mixture was vortexed for 1 min and sonicated for 15 min. Subsequently, 6 g of anhydrous MgSO4 and 1.5 g of anhydrous sodium acetate were added, and the tube was shaken vigorously by hand for 5 min and centrifuged at 6,000 rpm for 2 min. A 1-mL aliquot of the acetonitrile extracts (supernatant) was transferred into a 2-mL centrifuge tube containing 50 mg PSA sorbent, 50 mg C18 sorbent, and 150 mg anhydrous MgSO4 for cleanup. The tube was shaken and centrifuged, after which a 0.5-mL aliquot of the supernatant was transferred into a 2-mL centrifuge tube containing 0.5 mL water. The resulting solution was filtered through a 0.22-μm Teflon filter for HPLC–MS/MS analysis.
Method validation
A series of mixed standard solutions of fenarimol and nuarimol were prepared at concentrations of 1, 2.5, 10, 25, 50, 125, and 500 μg L−1 for each enantiomer. According to the procedure described above, blank matrix was prepared and a series of matrix-matched calibration standards with the same concentrations were also prepared. The calibration curves were obtained by plotting analyte concentrations against peak areas of quantification ion transition, and applying regression analysis. The linearity was expressed as a correlation coefficient. The limits of detection (LODs) were calculated as three times the signal-to-noise (peak to peak) ratio of the quantitative ion transition by analyzing samples spiked with fenarimol and nuarimol at low concentration levels with five replicate extractions. The limit of quantification (LOQ) was defined as the lowest spiking level of each enantiomer with acceptable recovery [24].
For the analysis of spiked samples, blank samples were fortified with mixed standard solutions at three concentration levels (5, 50, and 250 μg kg−1). Five replicates were prepared for each level. Fortified samples were left to equilibrate for 1 h prior to analysis, and the recoveries were calculated. The precision of the method was determined by the repeatability and reproducibility. To determine the repeatability, the five replicates of each fortified sample at three concentration levels were analyzed on the same day, with the same instrument and by the same operator, and the intraday RSDs were calculated. To determine the reproducibility, each fortified sample was analyzed on three different days, with the same instrument but by different operators, and the interday RSDs were calculated.
Results and discussion
Determination of the absolute configurations of the enantiomers
For the analysis of chiral molecules, it is critical to determine their absolute configurations. In this paper, we applied the TDDFT methodology to predict the ECD spectra of nuarimol and fenarimol, and determined the absolute configuration of their enantiomers through the combination of experimental and predicted ECD spectra.
The individual enantiomers of nuarimol and fenarimol were stereochemically determined by CD spectroscopy and almost mirror-imaged CD curves were provided. The resulting overall curves of the predicted ECD (Fig. 2a and b) and experimental ECD (Fig. 2c and d) were very similar. On the basis of the predicted ECD spectra, we were able to correctly determine the configurations of the nuarimol and fenarimol enantiomers that eluted from the column. Accordingly, peak 1 and peak 2 of the chromatograms shown in Fig. 5 were assigned to (R)-nuarimol and (S)-nuarimol, (R)-fenarimol and (S)-fenarimol, respectively.
MS/MS optimization and fragmentation pathway
To identify the optimal MS/MS conditions, HPLC–MS/MS with ESI was preliminarily operated in the positive and negative modes. To obtain a constant signal, a syringe pump was used to infuse the analyte (5 mg L−1, in the range of 5 μL min−1) into the LC eluent via a T connection. It was found, for full spectrum scans, that the two fungicides had high sensitivity in the positive mode, but in the negative mode the signal strength was very low. Thus, the positive mode was selected for the analyses. The base peak was observed at [M + H]+. Mass fragments were produced from the [M + H]+ ion by collision-induced dissociation (CID) using argon at 1.5 mTorr. As a result, fragment ions at m/z 268, 259, and 81, produced from m/z 331, were detected for fenarimol, whereas fragment ions m/z 252, 243, and 81, produced from m/z 315, were detected for nuarimol. In addition, accurate ion masses (331.0409, 268.0531, 259.0097, 81.0458, 315.0699, 252.0831, and 243.0380) were measured by HPLC/quadrupole-time-of-flight MS (Bruker micrOTOF-QII Q-TOF MS, USA). The proposed fragmentation pathways of the molecular ions of fenarimol and nuarimol, along with tentative structures, are shown in Fig. 3a and b. The mass measurement error of the TOF–MS was lower than 2.1 mDa. For fenarimol, transitions m/z 331 → 268 and 331 → 259 were selected for quantification and confirmation, respectively, whereas for nuarimol, transitions m/z 315 → 252 and 315 → 243 were selected for quantification and confirmation, respectively. These transitions were selected on the basis of their high response and low background interference.
Effect of CSP and mobile phase composition
It is common to use either acetonitrile or methanol as organic modifiers under reversed-phase conditions. In this experiment, the initial conditions were set at 80 % methanol or 70 % acetonitrile, a flow rate of 0.2 mL min−1, and column temperature of 25 °C. The two fungicides were separated on three cellulose-based columns (Lux Cellulose-1, Lux Cellulose-2, and Lux Cellulose-3). If enantioseparation was unsatisfactory, optimization steps such as changing the mobile phase compositions were considered. Generally, chiral resolution improved with the increase of the aqueous solution content in the mobile phase.
In this study, the best resolution of fenarimol and nuarimol was achieved with Lux Cellulose-3 and methanol. The structures of Lux Cellulose-1 and Lux Cellulose-2 contain carbamate moieties, whereas that of Lux Cellulose-3 contains benzoate moieties. It has been reported that the use of columns with carbamate groups result in better chiral separation of triazole fungicides [25, 26]. However, for fenarimol and nuarimol, Lux Cellulose-3 gave better results than Lux Cellulose-1 or Lux Cellulose-2. This suggests that the retention mechanism of the individual enantiomers is different in every CSP. Although there are many reports on the chiral separation of compounds, the chiral recognition mechanism of the cellulose-based CSPs is still unclear, so chiral recognition is still a “trial and error” process.
Moreover, methanol and acetonitrile may induce different effects on the separation of the same compound on the same CSP column. Figure 4 shows the differences in the enantioseparation of fenarimol and nuarimol when methanol and acetonitrile were used on the same column (Lux Cellulose-3).
In summary, the best simultaneous resolution of the two fungicides was achieved on Lux Cellulose-3, with a solvent system consisting of methanol/0.1 % formic acid (80:20, v/v). Figure 5 shows the separation and typical SRM chromatograms of the two fungicides. Under the optimized conditions, a baseline separation of the enantiomers of fenarimol and nuarimol was achieved in less than 8 min. However, it took approximately 1 h to obtain baseline separation of the nuarimol enantiomers and partial separation of the fenarimol enantiomers with GC [8]. With regards to CE, partial separation of the nuarimol enantiomers was obtained within nearly 17 min [9].
Effect of column temperature
Temperature is considered to be an important parameter in enantiomer separations. To investigate the effect of temperature on the resolution of the two fungicides, the column (Lux Cellulose-3) temperature was increased from 10 to 40 °C, with 5 °C increments. Methanol/0.1 % formic acid (80:20, v/v) was used as a mobile phase. The thermodynamic parameters were calculated using the following van’t Hoff equations [27, 28]:
where ΔH and ΔS are the standard transfer enthalpy and entropy of the analyte from the mobile phase to the stationary phase, R is the gas constant, T is the absolute temperature, ф is the phase ratio, ΔΔH 0 and ΔΔS 0 are the differences (ΔH 2 − ΔH 1) and (ΔS 2 − ΔS 1), respectively. Provided that ln ф is independent of temperature, a plot of ln k′ vs. 1/T will be linear with a slope of −(ΔH /R) and an intercept of ΔS*. For the linear plot of ln α vs. 1/T, the slope and intercept are −(ΔΔH 0/R) and ΔΔS 0/R, respectively.
Figure 6 shows the linear van’t Hoff plots of fenarimol and nuarimol, for temperatures from 0 to 40 °C, using methanol/0.1 % HCOOH (80:20, v/v) as the mobile phase. Plots of ln k′ and ln α versus 1/T for fenarimol and nuarimol were linear (R 2 > 0.98). The calculated ΔH, ΔS*, ΔΔH 0, and ΔΔS 0 values for every van’t Hoff plot are given in Table 1. The fact that the ΔH values were negative suggests that the transfer of the stereoisomers from the mobile to the stationary phase was enthalpically favored, and the conclusions that the values of ΔΔH 0 and ΔΔS 0 were negative indicate an enthalpy-driven separation [27, 28].
Method validation
Fungicide extraction based on the buffered QuEChERS method [29, 30] was performed, omitting the ultrasound-assisted extraction mentioned in the article. Validation of the proposed method included linearity, recovery, and repeatability. Good linear calibration curves for each enantiomer were obtained over the range of 1–500 μg/L. Table 2 lists the linear equations for the enantiomers in standard solutions and matrix-matched solutions. The matrix effect was calculated by comparing the slope of matrix-matched standard curve with the slope of the standard calibration curve [31], and Table 2 shows the slope ratios of matrix-matched to solvent-based calibration. In general, there was no significant matrix effect for the four enantiomers in apple, grape, cucumber, and tomato, as the slope ratios of matrix-matched to solvent-based calibration were in the range of 0.943–1.030. However, signal suppressions for the four compounds were observed in the soil matrix extracts, as the slope ratios for (R)-nuarimol, (S)-nuarimol, (R)-fenarimol, and (S)-fenarimol were 0.829, 0.841, 0.845, and 0.862. As a result, matrix-matched calibration standards were used to remove the matrix effect error and to obtain more accurate results for the soil samples. The recoveries were calculated by comparing the concentrations of the blanks to the concentrations of the fortified samples. Repeatability was expressed as relative standard deviation (RSD). Recoveries in low, intermediate, and high spiked levels were in the ranges of 76.5–87.7 %, 83.8–103 %, and 85.4–98.6 %, respectively. RSDs of the recoveries were in the range of 2.1–11.8 %. The recoveries and repeatability obtained prove that the method was efficient and reliable (Table 3). In this article, the LODs of fenarimol and nuarimol enantiomer in fruit and vegetable samples were estimated to be 0.8 and 0.5 μg kg−1, respectively, whereas those of fenarimol and nuarimol enantiomers in soil samples were estimated to be 1.2 and 0.75 μg kg−1, respectively. The LOQs were estimated to be 5 μg kg−1 for each enantiomer, based on the lowest fortified level in apple, grape, cucumber, tomato, and soil.
Conclusion
A reliable quantitative HPLC–MS/MS method for the analysis of fenarimol and nuarimol enantiomers in fruit, vegetable, and soil samples was developed. Complete enantioseparation was achieved on cellulose tris(4-methylbenzoate) by optimizing the experimental conditions. In the proposed method, the LOQs of the enantiomers of both fungicides were 5 μg kg−1. The recoveries at spiked levels of 5–250 μg kg−1 in five matrixes were in the range of 76.5–103 %, with associated RSDs of 11.8 % or lower. In addition, the absolute configurations of fenarimol and nuarimol enantiomers were determined by the combination of experimental and predicted ECD spectra. The proposed method was useful for determining trace fenarimol and nuarimol and their enantiomers in fruit, vegetable, and soil samples and could be used for enantioselective degradation studies in plant and environmental samples.
References
Paolini M, Mesirca R, Pozzetti L, Sapone A, Cantelli-Forti G (1996) Cancer Lett 101:171–178
Poli P, de Mello MA, Buschini A, de Castro VLSS, Restivo FM, Rossi C, Zucchi TMAD (2003) Mutat Res 540:57–66
Demirci F, Denizhan E (2010) Phytoparasitica 38:125–132
Maier NM, Franco P, Lindner W (2001) J Chromatogr A 906:3–33
Nillos MG, Gan J, Schlenk D (2010) J Chromatogr B 878:1277–1284
Garrison AW, Avants JK, Miller RD (2011) Int J Environ Res Publ Health 8:3453–3467
Hühnerfuss H, Shah MR (2009) J Chromatogr A 1216:481–502
Bicchi C, Cravotto G, D'Amato A, Rubiolo P, Galli A, Galli M (1999) J Microcolumn Sep 11:487–500
Martinez-Gomez MA, Escuder-Gilabert L, Villanueva-Camanas RM, Sagrado S, Medina-Hernandez MJ (2008) J Sep Sci 31:3265–3271
Li Y, Dong F, Liu X, Xu J, Li J, Kong Z, Chen X, Song W, Wang Y, Zheng Y (2011) J Chromatogr A 1218:6667–6674
Zhang H, Wang XQ, Qian MR, Wang XY, Xu H, Xu MF, Wang Q (2011) J Agric Food Chem 59:12012–12017
Li Y, Dong F, Liu X, Xu J, Li J, Kong Z, Chen X, Liang X, Zheng Y (2012) J Chromatogr A 1224:51–60
Liang HW, Qiu J, Li L, Li W, Zhou ZQ, Liu FM, Qiu LH (2012) J Sep Sci 35:166–173
Sannino A (2004) J AOAC Int 87:991–996
Wang SL, Xu YJ, Pan CP, Jiang SR, Liu FM (2007) Anal Bioanal Chem 387:673–685
Hengel MJ (2011) J Am Soc Brew Chem 69:121–126
Ari F, Dere E (2010) Acta Vet (Beogr) 60:31–38
Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jorgensen EC (2002) Toxicol Appl Pharmacol 179:1–12
Bonefeld-Jorgensen EC, Grunfeld HT (2004) Toxicol Lett 151:467–480
Thibaut R, Porte C (2004) J Steroid Biochem 92:485–494
Allenmark S, Gawronski J (2008) Chirality 20:606–608
Ding S, Jia L, Durandin A, Crean C, Kolbanovskiy A, Shafirovich V, Broyde S, Geacintov NE (2009) Chem Res Toxicol 22:1189–1193
Xu YX, Zhang H, Zhuang SL, Yu M, Xiao H, Qian MR (2012) J Agric Food Chem 60:4173–4178
European Union (2009) Method validation and quality control procedures for pesticide residues analysis in food and feed (document no. SANCO/10684/2009). European Commission, Brussels
Aboul-Enein HY, Ali I, Gubitz G, Simons C, Nicholls PJ (2000) Chirality 12:727–733
Zhang H, Qian MR, Wang XQ, Wang XY, Xu H, Wang Q, Wang MH (2012) J Sep Sci 35:773–781
Peter A, Vekes E, Armstrong DW (2002) J Chromatogr A 958:89–107
O'Brien T, Crocker L, Thompson R, Thompson K, Toma PH, Conlon DA, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Anal Chem 69:1999–2007
Lehotay SJ, Mastovska K, Lightfield AR (2005) J AOAC Int 88:615–629
Wang J, Chow W, Leung D (2010) Anal Bioanal Chem 396:1513–1538
Gosetti F, Mazzucco E, Zampieri D, Gennaro MC (2010) J Chromatogr A 1217:3929–3937
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (no. 21107094), the National High Technology R&D Program of China (The 863 Program, no. 2011AA100806), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Wang, X., Zhuang, S. et al. Enantioselective separation and simultaneous determination of fenarimol and nuarimol in fruits, vegetables, and soil by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 404, 1983–1991 (2012). https://doi.org/10.1007/s00216-012-6325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6325-8