Abstract
This paper describes the synthesis of novel molecularly imprinted polymers (MIPs), prepared by a noncovalent imprinting approach, for cleanup and preconcentration of curcumin (CUR) and bisdemethoxycurcumin (BDMC) from medicinal herbal extracts and further analysis by high-performance liquid chromatography with fluorescence detection (HPLC-FLD). Two molecular mimics, a mixture of reduced BDMCs and 4-(4-hydroxyphenyl)-2-butanone (HPB), have been synthesized and applied as templates for MIP synthesis. The polymers were prepared using N-(2-aminoethyl) methacrylamide (EAMA) as functional monomer, ethylene glycol dimethacrylate (EDMA) as the cross-linker (in a 1:5 molar ratio), and a mixture of acetonitrile/dimethylsulfoxide (90 %, v/v) as porogen. MIPs prepared using a mixture of reduced BDMCs as template showed higher selectivity for CUR and BDMC than those obtained with HPB, with imprinting factors of 3.5 and 2.7 for CUR and BDMC, respectively, using H2O/acetonitrile (65:35, v/v) as mobile phase. The adsorption isotherms for CUR in the MIP and the nonimprinted polymer (NIP) were fitted to the Freundlich isotherm model, and the calculated average binding affinities for CUR were (17 ± 2) and (8 ± 1) mM−1 for the MIP and the NIP, respectively. The polymers were packed into solid-phase extraction (SPE) cartridges, and the optimized molecularly imprinted solid-phase extraction (MISPE-HPLC) with fluorescence detection (FLD) method allowed the extraction of both curcuminoids from aqueous samples (50 mM NH4Ac, pH 8.8) followed by a selective washing with acetonitrile/NH4Ac, 50 mM at pH 8.8 (30:70 %, v/v), and elution with 3 × 1 mL of MeOH. Good recoveries and precision ranging between 87 and 92 %, with relative standard deviation (RSD) of <5.3 % (n = 3), were obtained after the preconcentration of 10-mL solutions containing both CUR and BDMC at concentrations in the range of 0–500 μg L−1. The optimized method has been applied to the analysis of both curcuminoids in medicinal herbal extracts.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food additives, such as antioxidants, can be applied to extend the shelf life of foods and maintain their safety, nutritional quality, and functionality [1]. In the last decade, there has been an increasing interest in the consumption of diets rich in polyphenols, and other food phenolics, due to their potential benefits on human health. These compounds confer stability against oxidation, and several studies have shown their essential role in the prevention of cancer, cardiovascular diseases, diabetes, osteoporosis, or neurodegenerative diseases [2]. Polyphenolic compounds are secondary metabolites of plants widely distributed in vegetables, fruits, cereals, and other food commodities. The interest of food manufacturers and consumers in natural additives, consumed for generations in contrast to their synthetic counterparts, have stimulated the use of natural antioxidants such as curcuminoids in the food industry [3].
Curcumin is a hydrophobic polyphenol derived from the rhizome (turmeric) of the herbal Curcuma longa with antioxidant and anti-inflammatory properties that has been proposed as a cancer chemopreventive agent and is often employed as a natural dye in foodstuffs [4–6]. This yellow pigment is authorized as a food additive in the EU [7], as well as in other countries. In 2003, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) established an acceptable daily intake of 0–3 mg/kg bw/day [8]. Commercial curcumin contains 77 % 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (CUR), 17 % demethoxycurcumin (DMC), and 3 % bisdemethoxycurcumin (BDMC), and these compounds exhibit significantly different antioxidant (CUR > DMC > BDMC), antitumor, and anti-inflammatory activities [9–11]. Therefore, efficient analytical methods for the isolation and quantification of curcuminoids are essential for their application in the control of pharmaceutical preparations as well as in food analysis.
Several chromatographic methods based on high-performance thin-layer chromatography [12], high-performance liquid chromatography [13, 14], or micellar electrokinetic capillary chromatography [15] have been reported so far for the determination of curcuminoids in raw materials, biological samples, foods, and pharmaceutical products. Most of them require laborious sample extraction procedures followed by subsequent cleanup and preconcentration by solid-phase extraction (SPE) [16].
Molecularly imprinted polymers (MIPs) are organic materials with the ability to recognize and bind specific target molecules and separate them from other matrix components. These materials have proven to be very useful in analytical separations, especially as SPE sorbents (molecularly imprinted solid-phase extraction (MISPE)) [17]. There are only a few examples in the literature on the application of MIPs for curcuminoid analysis. Wang et al. [18] described the preparation of curcumin-imprinted poly(methacrylamide-co-methacrylic acid) membranes using a porous filter paper as support for the polymerization. Long incubation times (12 h) were required for the extraction of CUR, DMC, and BDMC from methanol solutions, and the composite membranes showed the highest selectivity for CUR. The use of methacrylic acid (MAA) as functional monomer was also reported by Aziz et al. [19] for the synthesis of CUR-selective bulk polymers using acetonitrile (MeCN), tetrahydrofurane (THF), or CHCl3 as porogens. The best imprinting factor (IF) for CUR (IF = 1.27) was reported for the polymer prepared with THF. Recently, Kitabatake et al. [20] described the preparation of CUR-imprinted polymers by precipitation polymerization and its application for the extraction of curcuminoids from C. longa L. The authors concluded that MAA was not a suitable monomer for the preparation of CUR-selective MIPs, choosing a mixture of 4-4-vynilpiridine and methacrylamide, as functional comonomer, and divinylbenzene as cross-linker, for MIP synthesis. The development of MIPs for the extraction of other bioactive phenolic compounds, such as kukoamine A, from vegetal samples has also been reported in the literature [21].
The use of close structural analogs of the analyte as templates (known as mimics, surrogate, or dummy templates) for MIP synthesis has proven to be very useful when the polymers are intended to be used for trace analysis [22]. Moreover, this approach is also necessary when the structure of the target molecule contains double bonds that can be involved in the polymerization process, as in the case of CUR. As a result, the template molecule may be covalently attached to the polymer backbone, and less cavities will be available for analyte rebinding decreasing efficiency of the generated material [23–25]. This would explain the relatively low imprinted factors reported previously with CUR-imprinted polymers [19, 20].
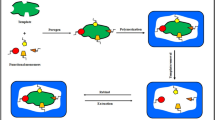
This paper describes the synthesis of curcuminoid-selective MIPs for their application as SPE sorbents for the selective extraction of these compounds in food samples. Two structural analogs of CUR, namely 4-(4-hydroxyphenyl)-2-butanone (HPB) (MIP-1) and a mixture of reduced BDMCs (MIP-2) (Fig. 1), have been synthesized and applied as templates for MIP synthesis using a noncovalent imprinting approach. The interaction of CUR with N-(2-aminoethyl) methacrylamide (EAMA) in acetonitrile (MeCN) solutions has been evaluated by UV-Vis spectrophotometry. The binding affinity and heterogeneity of the binding sites in the polymers have been assessed in equilibrium rebinding experiments, and their cross-selectivity for CUR, BDMC, and related compounds has been determined by high-performance liquid chromatography (HPLC). A MISPE method has been optimized for CUR and BDMC extraction from medicinal herbal samples.
Experimental
Reagents
Bisdemethoxycurcumin (>98 %) and 4-(4-hydroxyphenyl)-2-butanone (HPB, >99 %) were purchased from TCI Europe (Zwijndrecht Belgium), while N-(2-aminoethyl) methacrylamide hydrochloride (EAMA, 98 %) was purchased from Polysciences Inc. (Eppelheim, Germany), and prior to use, it was neutralized by adding a stoichiometric amount of 1.0 M tetrabutylammonium hydroxide (TBA) in MeOH (the solvent was eliminated in the rotavapor), ethylene glycol dimethacrylate (EDMA) (98 %, with monomethyl hydroquinone as inhibitor), and 2-(diethylamino)ethyl methacrylate (99 %, with 100 ppm of phenothiazine inhibitor). The monomers were purified by using an inhibitor removal column from Sigma-Aldrich (Milwaukee, WI). 2,2′-Azobis (2′4-dimethyl valeronitrile) (ABDV) was purchased from Wako (Neuss, Germany). Bisphenol A (BPA) was from Fluka (Buchs, Switzerland); resorcinol (RESOR), α-zearalenol (α-ZOL), 4-methoxyphenol (4-MetPOH), and ammonium acetate (NH4Ac) were from Sigma-Aldrich (Madrid, Spain); and water purified by a Millipore Milli-Q system (Millipore, Bedford, MA, USA) was used throughout. 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (98 %), ethanol absolute (EtOH, synthesis grade), acetonitrile (MeCN, HPLC grade), dichloromethane (synthesis grade), methanol (MeOH, HPLC grade), tetrahydrofuran (THF, synthesis grade), and extra dry DMSO (99.9 %) were purchased from Acros Organics (Geel, Belgium).
Apparatus
Hydrogenation of BDMC was performed in a H-Cube™ hydrogenation reactor from ThalesNano (with 10 % Pd/C CatCart as the catalyst cartridge). MS spectrometry was performed in a Bruker Esquire-LC equipment in electrospray ionization (ESI)-negative mode, and proton nuclear magnetic resonance (1H-NMR) spectra were recorded in a Bruker Avance DPX 300 MHz (UCM Central Instrumentation Facilities). The chromatographic system consisted of a HP-1100 series high-performance liquid chromatography from Agilent Technologies (Palo Alto, CA) equipped with a quaternary pump, an online degasser, an autosampler, an automatic injector, a column thermostat, and a diode-array (DAD), or a fluorescence (FLD), detector. A peristaltic pump miniplus 3 (Gilson) was used for sample preconcentration in the cartridges. Chromatographic separation of the curcuminoids was performed on an AQUA C18 (2) (250 mm × 4.6 mm i.d.) HPLC column from Phenomenex (Torrance, CA). The mobile phase consisted of a mixture of MeCN/water 49:51 (v/v) at a flow rate of 1.0 mL min−1. The column was kept at room temperature. The injection volume was 8 μL, and all the compounds eluted within 10 min. The FLD detector excitation and emission wavelengths were set at λ exc = 428 nm and λ em = 535 nm, respectively. All solutions prepared for HPLC were passed through a 0.45-μm nylon filter before use. Quantification was performed using external calibration and peak area measurements.
Synthesis of BDMC
A solution of 41.8 mg of BDMC (0.135 mmol) in 45 mL of a mixture of absolute ethanol and dichloromethane (2:1, v/v) was circulated through the H-Cube™ hydrogenator (1 mL min−1 flow rate, at 20 °C, full hydrogen mode) through a 10 % Pd/C catalyst; 32.1 mg (ca. 76 % yield) of a mixture of partially reduced (4H-BDMC) and totally reduced (8H-BDCM) BDMC was obtained as shown by MS-ESI spectrometry and 1H-NMR spectroscopy.
UV-Vis titration
UV-Vis absorption spectra were measured using a Varian Cary 3-Bio (Palo Alto, CA, USA) spectrophotometer. The affinity constants and stoichiometry of the CUR complex with EAMA were determined by monitoring the intensity changes in the absorption band (ΔA) of a solution of CUR in MeCN (c = 24 μM) at 418 nm when adding increasing amounts of the functional monomer. The molar ratio CUR/EAMA was increased from 1:0 to 1:50. Total volume for each analysis was 1 mL, and all the spectra were recorded between 300 and 600 nm. The association constants for the 1:1 and 1:2 complexes, K 11 and K 12, were calculated using the following equations (Eqs. 1 and 2) [26]:
where β 11 = K 11 and β 12 = K 11 K 12; L t is the total concentration of functional monomer (mol L−1); [L] is the concentration of free functional monomer (mol L−1); S 0 = 2.4 × 10−5 (mol L−1), Δε is the molar absorption coefficient (mol−1 L cm−1), and b is the optical path length (1 cm).
Polymer synthesis
MIP-2 was synthesized by dissolving in a glass vial 27.5 mg (0.088 mmol) of the reduced BDMC template molecule, 148 mg (0.352 mmol) of EAMA, HCl-TBA (the commercial hydrochloride salt of EAMA was previously neutralized by adding a stoichiometric amount of TBA), and 331.9 μL (1.76 mmol) of EDMA as cross-linker (molar ratio 1:5), in a mixture of 64 μL of DMSO and 573 μL of MeCN. The mixture was left to stand for 15 min and purged with Ar for 5 min. After addition of 24.0 mg of ABDV, the vials were purged with Ar for 10 min, sealed, and kept at 60 °C for 24 h for polymerization. The resulting bulk polymers were grounded in a mortar and sieved with 25–100-mm sieves. The same procedure was followed for the synthesis of MIP-1 using HPB (29.5 mg, 0.179 mmol) as the template molecule. A nonimprinted polymer (NIP) was prepared in the same way, in the absence of the template molecule. The resulting MIP and NIP particles were washed with methanol/HCl (10 %, v/v) in a Soxhlet (24 h) for the extraction of the template molecules. Prior to use, the solid was settled in MeOH/water (80:20, v/v) to remove fine particles.
Chromatographic evaluation of the imprinted polymers
MIP-1, MIP-2, or NIP polymers were slurry-packed in methanol into stainless steel HPLC columns (50 mm × 2.1 mm), using an HPLC pump (M45, Waters) at a flow rate of 1 mL min−1. The temperature was kept at 25 °C, and the analytical excitation and emission wavelengths were set at λ exc = 428 nm and λ em = 535 nm, respectively. Methanol was used as void volume marker. The columns were equilibrated with the mobile phase (using mobile phases based on binary mixtures of H2O and MeCN ranging from 100 % H2O to 100 % MeCN), and for each run, 8 μL of solutions of CUR, BDMC, HPB, and other related/nonrelated compounds (50 mg L−1) was injected separately into the column. The capacity factor (k) for each analyte was calculated as k = (t − t 0)/t 0, where t and t 0 are the retention times of the analyte and the void marker, respectively. The IF was calculated as k MIP/k NIP, where k MIP and k NIP are the retention factors of a solute on the MIP and the NIP columns, respectively.
Determination of the binding capacity
The binding capacity of the polymers has been evaluated in equilibrium rebinding experiments. To that aim, 10 mg of the MIP and the NIP materials was transferred into 1.5-mL glass vials and mixed with 1 mL of H2O/MeCN (70:30, v/v) solutions containing increasing concentrations of CUR (10–4,000 μM) and left shacking at 70 rpm, for 24 h, at room temperature. The vials were centrifuged (5 min, 2,000 rpm), and the concentration of ENRO in the supernatant was monitored by HPLC-FLD. The amount of CUR bound to the polymer (B) was calculated by subtracting the nonbounded amount (F) from the initial CUR concentration. The experimental data were fitted to the Freundlich adsorption isotherm (FI) (Eq. 3) using the nonlinear fitting module of SigmaPlot 11.0 software based on the Marquardt-Levenberg algorithm.
where a is the binding capacity and m is the heterogeneity index that ranges between 0 and 1, increasing with decreasing heterogeneity of the material.
The affinity distribution (AD) was calculated with Eq. 4 and the experimentally derived FI fitting parameters (a and m) [27]:
This equation is valid for a certain range of binding affinities with K min and K max set by the free concentrations (F max and F min) in the binding experiments (Eq. 5).
The apparent number of binding sites (\( {\overline{N}}_{{}_{K1- K2}} \)) and the apparent weighted average affinity (\( {\overline{K}}_{{}_{K1- K2}} \)) were calculated with Eqs. 6 and 7, as described in the literature [27].
Molecularly imprinted solid-phase extraction
Solid-phase extraction cartridges (Varian, Spain), 1 mL in volume, capped with fritted polyethylene disks at the top and at the bottom, were packed with 25 mg of the corresponding MIP or the NIP. The cartridges were equilibrated with 5 mL of buffer (NH4Ac, 50 mM, pH 8.8), and the sample containing the curcuminoids (NH4Ac, 50 mM, pH 8.8) was percolated at a constant flow rate of 0.50 mL min−1 with the aid of a peristaltic pump. The washing step was carried out with 1 mL of MeCN/NH4Ac, 50 mM, pH 8.8 (30:70 %, v/v). Curcuminoids were eluted with 3 × 1 mL of MeOH. The cartridges were re-equilibrated with 10 mL of buffer (NH4Ac, 50 mM, pH 8.8), before a new application. Quantification was performed using external calibration peak area measurements. Linear calibration graphs were obtained in the 0–5,000-μg L−1 concentration range for CUR and BDMC (R 2 > 0.9993). All the experiments were carried out in triplicate.
Determination of CUR and BDMC in medicinal herbal extracts
Medicinal herbal extracts (25 g), containing Curcumae domesticae rhizome and tamarind pulp extract (http://www.sidomuncul.com), purchased from a local supermarket in Bandung (West Java, Indonesia), were dissolved in 500 mL of NH4Ac (pH 8.8) and stored in the fridge at 4 °C, protected from light, until use. Aliquots of 15 mL were spiked with standard solutions of both curcuminoids to achieve final concentrations in the range of 0–500 μg L−1 for CUR and 0–150 μg L−1 for BDMC. The samples were centrifuged for 10 min at 3,500 rpm, and thereafter, 10 mL of the supernatant was analyzed using the optimized MISPE-HPLC-FLD protocol described above (“Experimental” section, “Apparatus”). The procedure was carried out in triplicate.
For quantification purposes, in the recovery experiments, 10 mL of standard solutions of CUR and BDMC (100, 200, 300, 400, and 500 μg L−1) was prepared in NH4Ac, 50 mM, pH 8.8, and preconcentrated in the MIP-2 columns described above (“Experimental” section, “Molecularly imprinted solid-phase extraction”). Calibration curves were obtained plotting the CUR and BDMC peak areas against the curcuminoid concentration in the standard solutions. Linear calibration graphs were obtained in the studied concentration range for both compounds (R 2 > 0.995).
Results and discussion
Synthesis of BDMC
The vinyl groups in the BDMC molecule were hydrogenated in order to prevent copolymerization of the template during the MIP synthesis. The reduction yielded a mixture of two products, 63 % of the 4H-BDMC molecule (a sum of 34 % of the enol form 4Ha, and 29 % of the keto isomer 4Hb, as displayed in Fig. 2), and a 37 % of 8H-BDMC, which is BDMC reduced with eight hydrogen atoms. These percentages were estimated from the integral values of the 1H-NMR signals of the reduced BDMC in DMSO-d6. The H that was monitored for calculating the relative integral values is marked as “a–d” in the corresponding chemical structures in Fig. 2, which appear at the following chemical shifts (δ/ppm): Ha (s, 2H) 3.70, Hb (s, 1H) 5.74, Hc (quin, 2H) 3.47, and Hd (t, 2H) 1.09. The reduction of BDMC was also confirmed by mass spectrometry; the MS-ESI with negative ion detection showed the presence of two compounds: 4H-BDMC (C19H19O4) [M-1]−: 311.1 (calcd.), 310.8 (exp.), and 8H-BDMC (C19H23O4 + CH3OH) [M′-1 + MeOH]−: 347.2 (calcd.), 346.8 (exp.), with the relative peak intensities of 55 % for 4H-BDMC and 45 % for 8H-BDMC (quite in agreement with the NMR results). Since both products are structurally very similar and suitable molecular surrogates for our imprinting purposes, we proceeded with the synthesis of MIP-2 using the mixture of reduced BDMCs without further purification.
Reaction scheme for the reduction of the BDMC molecule with H2 over 10 % Pd-C catalyst. A mixture of two compounds is obtained as shown by the MS-ESI results: 4H-BDMC ([M-1]−: 310.8) and, 8H-BDMC ([M-1 + MeOH]−: 346.8). As predicted from the 1H-NMR data, the mixture was formed by 63 % of 4H-BDMC + 37 % 8H-BDMC
UV-Vis evaluation of the interaction of CUR with EAMA
UV-Vis studies were carried out to investigate the interaction between CUR and the functional monomer selected for MIP synthesis. As deduced from Job’s plot (Fig. S1 in the Electronic Supplementary Material, ESM), a 1:2 CUR-EAMA complex is formed in MeCN. The two phenol groups in the CUR molecule can interact by hydrogen bonding with the amine group of two molecules of functional monomer. The association constants, calculated from the titration experiments applying Eqs. 1 and 2, were K 11 = 36,644 M−1 and K 12 = 7,110 M−1 in MeCN. From these results, EAMA was expected to be a good selection as a functional monomer for MIP synthesis. However, as discussed previously, it was decided not to use CUR as template to prevent its copolymerization during polymer synthesis.
Both HPB and the mixture of reduced BDMCs (4H-BDMC and 8H-BDMC, Figs. 1 and 2) also possess phenol groups in their structure that can interact via hydrogen bonding and/or electrostatic interactions with the EAMA monomer in a similar way to CUR. All MIPs were prepared in MeCN, a hydrogen bond-preserving porogenic solvent; however, the addition of a small amount of DMSO (10 %) was necessary to facilitate the complete dissolution of the templates.
Chromatographic evaluation of the polymers
The polymers were first tested for their ability to retain CUR and BDMC using mobile phases based on binary mixtures of H2O and MeCN ranging from 100 % H2O to 100 % MeCN. A summary of the calculated retention factors (k) and IF are given in Tables 1 and 2. No elution of CUR or BDMC was observed using 100 % H2O as the mobile phase, either in the MIPs or in the NIP (t MIP > 60 min). In these conditions, the functional monomer is positively charged (pK a ca. 9.2 (calculated using Marvin Sketch software V5.12 from Chem Axon, www.chemaxon.com)) and CUR is neutral (pK a1 = 8.38, pK a2 = 9.88, pK a3 = 10.51 [28]); thus, ionic interactions are not to be expected, and hydrogen bonding and hydrophobic interactions will be responsible of the retention of the target compounds in the imprinted cavities. Increasing the MeCN content in the mobile phase resulted in a decrease in the retention factors in the NIP. The same behavior was observed in MIP-1 and MIP-2, but in such polymers, a minimum retention was reached for 50 % H2O in MeCN, both for CUR and BDMC. In 100 % MeCN, an imprinting effect was observed in both MIPs with IFs of 2.6 (MIP-1) and 2.8 (MIP-2) for CUR, and slightly lower values for BDMC (MIP-1: IF = 1.5; MIP-2: IF = 2.6, n = 3).
A comparison of the retention factors of both analytes in MIP-1 and MIP-2 reflects a stronger retention of BDMC than for CUR in the imprinted polymers (Tables 1 and 2) as well as in the NIP. On the other hand, the polymers prepared with a mixture of reduced BDMCs (MIP-2), which shows a closer structural resemblance to the target curcuminoids, displayed a higher affinity for both analytes than those prepared with HPB (MIP-1). The highest IF values for CUR and BDMC, IF = 3.51 and IF = 2.68 respectively, were obtained with MIP-2 using a H2O/MeCN (65 %, v/v) mobile phase, improving the results previously reported in the literature [19, 20]. Therefore, MIP-2 was selected for MISPE optimization.
The effect of pH on the retention of both compounds in MIP-2 was evaluated using a mixture of MeCN/NH4Ac, 50 mM, pH 8.8 (30:70 %, v/v) as mobile phase The IFs considerably increased both, for CUR (k NIP = 2.3; k MIP-2 = 11.4; IF = 4.96) and BDMC (k NIP = 4.2; k MIP-2 = 19.0; IF = 4.52) using such mobile phase. This behavior could be explained considering that the recognition mechanism is based on the ionic interactions between the deprotonated curcuminoids and the positively charged polymer network combined with a size and shape molecular fitting.
Evaluation of the MIP cross-selectivity
The specificity of the MIP-2 was evaluated by comparing the retention behavior of CUR, BDMC, and other structurally related and nonrelated compounds. The mobile phase used for these experiments was H2O/MeCN 65:35 (v/v), and the results are summarized in Table 3. The polymer exhibited a marked affinity for CUR and BDMC. Other phenolic compounds such RESOR, 4-MetPOH, HPB, BPA, and α-ZOL, with similar functional groups but different sizes and/or shapes than the target analytes, were retained neither in the MIP nor in the NIP. In view of these results, it can be concluded that the use of a mixture of reduced BDMCs as templates for polymer synthesis generates binding cavities in the polymer network suited to accommodate the target compounds, as they show a close structural and functional resemblance to the surrogates.
Determination of binding site distribution and affinities
The binding affinity and heterogeneity of the binding sites in the polymers were assessed in equilibrium rebinding experiments in H2O/MeCN (70 %, v/v). The selection of this solvent was based on the higher retention factors obtained for CUR, both in MIP-1 and MIP-2, in comparison to those calculated using H2O/MeCN (65 %, v/v), although the IFs were slightly lower.
The binding isotherms showed a good fitting to the Freundlich model (Fig. 3), and the fitting results are summarized in Table 4. The apparent weighted average affinity was higher for the MIPs than for the NIP in the measured concentration range [27]. The total number of binding sites was significantly higher in MIP-2 (\( {\overline{N}}_{\mathrm{MIP}\hbox{-} 2} \): 145 ± 17 μmol g−1) than in MIP-1, which showed just marginally higher values than for the NIP (\( {\overline{N}}_{\mathrm{MIP}\hbox{-} 1} \): 115 ± 12 μmol g−1, \( {\overline{N}}_{\mathrm{NIP}} \): 110 ± 10 μmol g−1). Therefore, the polymer synthesized with a mixture of reduced BDMCs presents higher capacity and binding affinity for CUR than that prepared using HPB. However, both imprinted networks show higher affinity constants than the nonimprinted material.
Equilibrium binding isotherms for the uptake of CUR by MIP-1 (green triangles), MIP-2 (blue circles), and NIP (red squares) in H2O/MeCN 30:70 (v/v). F, concentration of free CUR; B, specific amount of bound CUR. The experimental data were fitted to a Freundlich (FI) isotherm model (see text for details)
Molecularly imprinted solid-phase extraction optimization
Several factors have been evaluated to establish the optimum conditions for the MISPE procedure, including the study of the composition and volume of the eluting solvent, the composition of the washing solvent, and the breakthrough volume.
In order to select the best eluting solvent for quantitative analyte recovery, 2 mL of an aqueous solution of CUR (0.25 mg L−1) was percolated through the MIP cartridges. The analyte was eluted with 3 × 1 mL volumes of different solvents, and the concentration of the CUR was measured in each fraction. The eluting solvents tested were MeOH, EtOH, mixtures H2O/MeCN (50:50, v/v), THF, and 0.05 M tetra-n-butyl ammonium hydrogen sulfate (TBA), an ion-pairing reagent, in MeOH. The results are collected in ESM Table S1.
The best recoveries in MIP-2 were achieved with MeOH (CUR, 98 %, relative standard deviation (RSD) < 6 %; n = 3) and with THF; however, the use of this solvent was discarded due to the limited stability of the SPE cartridges in this solvent after repeated use. MeOH volumes lower than 3 mL did not provide quantitative recoveries neither for CUR nor for BDMC (data not shown); therefore, 3 × 1 mL of MeOH was selected as eluent solvent for the MISPE method.
As concluded from the chromatographic experiments, the nonspecific interactions between CUR and BDMC and the imprinted polymers can be minimized in the presence of a mixture of MeCN/NH4Ac, 50 mM, at pH 8.8 (30:70 %, v/v), that would also facilitate washing out the less polar nonrelated compounds from the sample matrix. To evaluate this effect, a sample (1 mL NH4Ac, 50 mM, pH 8.8) containing 0.25 mg L−1 of each curcuminoid was loaded into the MIP-2 and NIP cartridges, washed with 1 mL of the hydro-organic mixture and the analytes were eluted with (3 × 1 mL) of MeOH. The extracts were analyzed by HPLC-FLD. Recoveries for CUR and BDMC were 91 % (RSD 5 %) and 93 % (RSD 4 %) in MIP-2, and 37 % (RSD = 6 %) and 41 %, (RSD = 7 %) in the NIP, respectively. Thereby, a volume of 1 mL of MeCN/NH4Ac 50 mM, at pH 8.8 (30:70 %, v/v), was selected as washing solvent for further experiments.
The breakthrough volume (V B) can be defined as the largest volume of a sample, which can be preconcentrated without significant loss of analyte and for which recovery, after elution for all sample volumes less than the breakthrough volume, will be 100 % [29]. In order to evaluate this parameter of the MIP-2/NIP cartridges, the sorbents were equilibrated with 5 mL of MeOH and 10 mL of NH4Ac (50 mM, pH 8.8). Then, increasing loading volumes (1, 5, 10, 25, and 50 mL) of a sample containing 0.75 mg L−1 of CUR and BDMC were percolated on the MIP-2/NIP cartridge flow rate of 0.5 mL min−1; a washing step of 1 mL of MeCN/NH4Ac, 50 mM, at pH 8.8 (30:70 %, v/v) was used, and finally, the analytes were eluted with 3 × 1 mL of MeOH followed by chromatographic analysis. As it can be observed in Table 5, percolation of sample volumes lower than 10 mL allowed recoveries higher than 90 % (RSD ≤ 5 %, n = 3) for both curcuminoids in MIP-2. The nonspecific retention in the NIP was significantly lower in such conditions, in the range of 33–39 % (RSD 3–6 %, n = 3). The MIP capacity towards CUR and BDMC was estimated at 0.27 mg g−1 of the polymer, while the corresponding values for the NIP were 0.10 and 0.12 mg g−1 for CUR and BDMC [21], respectively, further supporting the imprinting effect for both curcuminoids in MIP-2. As the percolated volume was increased to 50 mL, the extraction recoveries decreased in the MIP-2 (CUR, 53 %; BDMC, 63 %) but especially in the NIP (CUR, 7 %; BDMC, 14 %).
For calibration purposes, 10-mL NH4Ac (50 mM, pH 8.8) solutions containing increasing concentration of both CUR and BDMC (0–500 μg L−1) were preconcentrated using the optimized MISPE method. Good linearity was obtained for both compounds in the spiked range (R 2 > 0.991). Extraction recoveries of 87–92 % were obtained at all fortification levels for both analytes, with RSDs < 5.3 % (n = 3). The detection limits, based on a signal-to-noise ratio of 3:1, were 19 μg L−1 for CUR and 9 μg L−1 for BDMC. Quantification limits (signal-to-noise ratio of 10:1) were 63 and 30 μg L−1 for CUR and BDMC, respectively.
Determination of CUR and BDMC in medicinal herbal extracts
To evaluate the applicability of the MISPE method, a medicinal herbal extract containing curcuminoids was analyzed using the optimized protocol. Direct analysis of the sample revealed the presence of both CUR and BDMC at concentrations of 80 μg L−1 (RSD = 2.5 %, n = 3) and 260 μg L−1 (RSD = 6.3 %, n = 3), respectively. As observed in Fig. 4, cleaner HPLC chromatograms were obtained for the herbal extract submitted to the MISPE protocol in comparison to the SPE using NIP.
The accuracy and precision of the whole analytical method was assessed analyzing the herbal extract sample fortified with CUR and BDMC at four concentration levels in the range of 100–500 μg L−1 for CUR and 30–150 μg L−1 for BDMC (n = 3). The results are reported in Table 6. Good linearity was obtained for both compounds in the spiked range (R 2 > 0.995), and no significant differences (confidence level 95 %) were observed between the slope of the standard addition curve and that obtained after the preconcentration of standard solutions of the curcuminoids (data not shown) confirming the lack of matrix effect using the optimized MISPE conditions. Therefore, CUR and BDMC concentrations were calculated by interpolation of the peak area values in the calibration curve obtained after preconcentration of standard curcuminoid solutions as described in the “Experimental” section. Satisfactory accurate results were achieved for both curcuminoids at the all the fortification levels tested with recoveries between 97 and 101 % (RSD 2.5–5.0 %, n = 3) for CUR and between 99 and 101 % (RSD 2.5–6.3 %, n = 3) for BDMC. To determine the within-laboratory reproducibility, the sample was analyzed by triplicate on three different days obtaining RSD values of 3.8 and 7.2 % for CUR and BDMC (Table 6), respectively, which confirm the good reproducibility of the optimized methodology and its applicability for the analysis of herbal extracts. The cartridges have been used more than 25 times without losing their recognition properties.
Conclusions
This work demonstrates the usefulness surrogate templates for the preparation of molecularly imprinted polymers selective to CUR and BDMC. A mixture of reduced BDMCs was the best choice for the generation of binding cavities with the right shape and functionality to achieve the efficient recognition of the target curcuminoids in aqueous samples. The application of this approach avoids the problems associated to CUR copolymerization during MIP preparation. A poly(EAMA-co-EDMA) molecularly imprinted polymer has been synthesized and applied to the optimization of a MISPE procedure followed by HPLC with fluorescence detection for the simultaneous extraction of CUR and BDMC from herbal extracts with good recoveries and precision, improving the performance of previously reported MIPs for these analytes. Cartridge capacity could be improved using imprinted microbeads, and work is in progress to explore such approach. In any case, the novel MIPs represent a cost-effective alternative to traditional SPE sorbents for the analysis of curcuminoids in complex matrices.
References
Jayaprakasha GK, Jaganmohan LR, Sakariah KK (2006) Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem 98:720–724
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278
Hwan LJ, Myoung-Gun C (2011) Determination of curcuminoid colouring principles in commercial foods by HPLC. Food Chem 124:1217–1222
Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H (2003) Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Radic Biol Med 35:475–484
Pana MH, Ho CT (2008) Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev 37:2558–2574
Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemoned V, Fayad R (2012) Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct 3:1109–1117
Regulation (EC) 1333/2008 of the European Parliament and of the Council (2008) On food additives. Off J Eur Union L 354:16–33
http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/ (Accessed 26 Dec 2013)
Mahattanadul S, Nakamura T, Panichayupakaranant P, Phdoongsombut N, Tungsinmunkong K, Booking P (2009) Comparative antiulcer effect of bisdemethoxycurcumin and curcumin in a gastric ulcer model system. Phytomed 16:342–351
Thongcai W, Liawruangrath B, Liawruangrath S (2009) Flow injection analysis of total curcuminoids in turmeric and total antioxidant capacity using 2,2′-diphenyl-1-picrylhydrazyl assay. Food Chem 112:494–499
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB (2008) Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol 76:1590–1611
Gupta AP, Gupta MM, Sushil K (1999) Simultaneous determination of curcuminoids in Curcuma samples using high performance thin layer chromatography. J Liq Chromatogr Relat Technol 22:1561–1569
Jadhav BK, Mahadik KR, Paradkar AR (2007) Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatogr 65:483–488
Heath DD, Pruitt MA, Brenner DE, Rock CL (2003) Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B 783:287–295
Lin XL, Xue L, Zhang HY, Zhu CF (2005) Determination of curcumins in turmeric by micellar electrokinetic capillary chromatography. Can J Anal Sci Spectrosc 51:35–42
Rohman A (2012) Analysis of curcuminoids in food and pharmaceutical products. Int Food Res J 19:19–27
Tóth B, Horvai G (2012) Chromatography, solid-phase extraction, and capillary electrochromatography with MIPs. In: Haupt K (ed) Molecular imprinting, 325th edn. Springer, Berlin, pp 267–306
Wang P, Hu W, Su W (2008) Molecularly imprinted poly(methacrylamide-co-methacrylic acid) composite membranes for recognition of curcumin. Anal Chim Acta 615:54–62
Azizi ES, Ahmad MN, Islam AKMS, Arbain D, Tahir I (2011) Porogen effect towards the quality of curcumin imprinted polymer. J Chem 11:207–211
Kitabatake T, Tabo H, Matsunaga H, Haginaka J (2013) Preparation of monodisperse curcumin-imprinted polymer by precipitation polymerization and its application for the extraction of curcuminoids from Curcuma longa L. Anal Bioanal Chem 405:6555–6561
Piletska EV, Burns R, Terry LA, Piletsky SA (2012) Application of a molecularly imprinted polymer for extraction of kukoamine A from potato peels. J Agr Food Chem 60:95–99
Vasapollo G, Del Sole R, Mergola L, Lazzoi MR, Scardino A, Scorrano S, Mele G (2011) Molecularly imprinted polymers: present and future prospective. Int J Mol Sci 12:5908–5945
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Urraca JL, Marazuela MD, Merino ER, Orellana G, Moreno-Bondi MC (2006) Molecularly imprinted polymers with streamlined mimic for zearalenone analysis. J Chromatogr A 1116:127–134
Chen Z, Alvarez-Perez M, Navarro-Villoslada F, Moreno-Bondi MC, Orellana G (2014) Fluorescent sensing of “quat” herbicides with a multifunctional pyrene-labeled monomer and molecular imprinting. Sens Actuat B 191:137–142
Connors KA (1987) Binding constants: the measurement of molecular complex stability. Wiley, New York
Rampey AM, Umpleby RJ, Rushton GT, Iseman JC, Shah RN, Shimizu KD (2004) Characterization of the imprint effect and the influence of imprinting conditions on affinity, capacity and heterogeneity in molecularly imprinted polymers using the Freundlich isotherm-affinity distribution analysis. Anal Chem 76:1123–1133
Ravindran PN, Babu KN, Sivaraman K (eds) (2007) Turmeric: the genus Curcuma. CRC, Boca Raton, p 302
Poole CF, Poole SK (2002) In: Simpson NJK (ed) In: Theory meets practice. Solid phase extraction: principles, techniques and applications. Marcel Dekker Inc, New York, pp 183–226
Acknowledgments
This work has been funded by I-MHERE B.2c grant for Sandwich Doctoral Program of Institut Teknologi Bandung, Indonesia, and by MINECO (ref. CTQ2012-37573-C02-02). JUR thanks the International Excellence Campus CEI-Moncloa for a postdoctoral contract, and ABD thanks the Spanish Ministry of Economy and Competitiveness for a Ramón y Cajal contract.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection celebrating ABCs 13th Anniversary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 123 KB)
Rights and permissions
About this article
Cite this article
Wulandari, M., Urraca, J.L., Descalzo, A.B. et al. Molecularly imprinted polymers for cleanup and selective extraction of curcuminoids in medicinal herbal extracts. Anal Bioanal Chem 407, 803–812 (2015). https://doi.org/10.1007/s00216-014-8011-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8011-5