Abstract
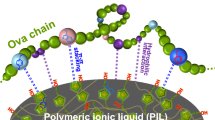
A nanocomposite is prepared by encapsulating silica nano-spheres with polymerized ionic liquid in aqueous medium without use of any organic solvents. Vinyl groups are covalently introduced on to the surface of silica nano-spheres, which are then encapsulated by copolymerization of 1-vinyl-3-ethylimidazolium bromide (monomer) and 1,4-butanediyl-3,3′-bis-l-vinylimidazolium dibromide (cross-linker) at room temperature. The derived nanocomposite, PIL@SiO2, provides a green adsorbent for protein sorption. PIL@SiO2 is selective toward acidic proteins, and its selectivity can be controlled via varying the amount of monomer used in the copolymerization process. At pH 6.0, use of 5 mg PIL@SiO2 nanocomposite results in a sorption efficiency of up to 95 % for 200 mg L−1 ovalbumin in 1 mL sample solution. Electrostatic and hydrophobic interactions between PIL@SiO2 and protein species dominate the adsorption process. The ovalbumin adsorption behavior is consistent with the Langmuir model, giving a sorption capacity of 333.3 mg g−1. The retained ovalbumin is recovered by elution with 0.2 % SDS solution. Circular dichroism spectra reveal virtually no change to the α-helix content of ovalbumin after elimination of SDS by use of dialysis. In summary, high-purity ovalbumin is isolated from chicken egg-white by use of the PIL@SiO2 nanocomposite as adsorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquids (ILs) have been the focus of a variety of investigations because of their distinctive properties of wide liquid range, low volatility, good thermal stability, electrolytic conductivity, and adjustable miscibility [1, 2]. Polymeric or polymerized ionic liquids, e.g. PILs carrying an IL moiety in each of the repeating units of the polymer, are an emerging class of material with promising applications. PILs usually combine the above-mentioned properties of ILs with macromolecular architecture, and have new properties and functions including enhanced mechanical stability, improved processability, durability and spatial controllability, and great potential as green materials [3–5]. By polymerization and/or copolymerization of ionic liquids in the presence or absence of solid supports, different kinds of PIL have been prepared and used for a variety of purposes, including ionic conductive materials [6–8], stationary phases in chromatography [9, 10], catalytic membranes [11, 12], adsorbents [13, 14], fluorescent conjugate polymers [15, 16] and precursors for nanostructures [17, 18].
Alongside the development of polymeric ionic liquids, nanoparticles (NPs) with a variety of constituents and structures or configurations have attracted even more attention. The functionalization and/or engineering of NPs have been widely used in the design of novel functional materials with desirable electronic, optical, and magnetic properties. By manipulation of the surface modifier, NPs with a wide range of potential applications can be created [19]. Recently, immobilization of PILs on a variety of nanostructures, e.g. SWNT, graphene, and silica, has produced multi-functional nanocomposite materials [20–22]. The NPs substantially increase the dynamic hardness of the PIL–NPs composite, and their large surface area provides more functional groups. The incorporation of PILs prevents the coagulation of NPs caused by electrostatic repulsion between the nanoparticles, and the characteristics of the ionic liquid moieties provide the PIL–NPs composite with favorable thermal stability and tunable hydrophilicity or hydrophobicity [23, 24]. In production of PIL–NPs composites, the PILs are usually prepared by use of conventional free radical polymerization of IL monomers [20, 25, 26]. There are also a few reports on preparation of PILs via controlled or “living” radical polymerization, which is expected to enable precise design and control of the macromolecular architecture [27]. However, these polymerization approaches require heat sources and dispersive organic solvents, which might have an adverse environmental impact and could lead to irreversible deactivation of biomacromolecules. For this reason, it is highly desirable to develop eco-friendly or green procedures to produce nano-objects with fine-tuning particle size and dispersibility.
In this paper, we report a novel hybrid nanocomposite material for protein isolation, prepared by encapsulating silica nano-spheres with polymeric ionic liquids—i.e. polymerization of ionic liquids, with 1-vinyl-3-ethylimidazolium bromide as monomer and 1,4-butanediyl-3,3′-bis-l-vinylimidazolium dibromide as cross-linker—at room temperature, without use of organic solvents. The aqueous medium facilitates homogeneous dispersion of silica NPs. The prepared nanocomposite PIL@SiO2 is of uniform size and has good selectivity for adsorption of acidic proteins. Ovalbumin has been successfully isolated from chicken egg white, as demonstrated by SDS-PAGE. The circular dichroism (CD) spectra of the recovered protein clearly reveal preservation of the natural structure of the isolated protein species.
Experimental
Materials
Lysozyme from chicken egg white (Lys, L2879, isoelectric point pI 11), cytochrome c from horse heart (cyt-c, C7752, pI 10.3), bovine hemoglobin (Hb, H2500, pI 6.9), immunoglobulin G from human serum (IgG, I4506, pI 5.8), and ovalbumin (Ova, A5503, pI 4.7) were purchased from Sigma (St Louis, MO, USA) and used without further purification. N-Vinylimidazole, bromoethane, 1,4-dibromobutane, tetraethoxysilane (TEOS), γ-methacryloxypropyltrimethoxysilane (γ-MAPS), N,N,N′,N′-tetramethylethylenediamine (TEMED) and sodium dodecylsulfate (SDS) were obtained from Sinopharm Chemical Reagent (Shenyang, China) and used as received. Dialysis membrane (21 MM, MW: 8,000–14,400) was the product of Biosharp (Hefei, China). Other chemicals used, e.g. acetonitrile, ethyl acetate, diethyl ether, phosphoric acid, acetic acid, and sodium hydroxide, were of at least analytical-reagent grade. Deionized water of 18 MΩ cm−1 was used throughout the experiments.
Instrumentation
UV–visible absorption spectra were recorded on an Hitachi (Japan) U-3900 spectrophotometer at room temperature (293 K). A 1.0-cm quartz cuvette was used for quantifying proteins in aqueous medium, and an integrating sphere setup was used for characterizing the solid composite. FT-IR spectra in the range 4000–400 cm−1 were obtained by use of a Nicolet-6700 FT-IR spectrometer (Thermo, USA). 1H NMR spectra were recorded on a Bruker ARX-600 spectrometer operated at 600 MHz. Circular dichroism (CD) spectra in the wavelength range 200–260 nm were obtained on a MOS-450 (Bio-Logic, France) automatic recording spectropolarimeter at 293 K. The spectra were recorded with nitrogen protection by use of a 5 mm cell length with a scan rate of 200 nm min−1. The morphology and structure of the nanocomposite were examined by use of a scanning electronic microscope (SEM, S-3400 N; Hitachi) and transmission electronic microscope (TEM, H-7650; Hitachi). Zeta potential measurement for surface charge analysis of the PIL@SiO2 nanocomposite was performed by use of a Zetasizer Nano ZS90 (Malvern, UK).
Preparation of the PIL@SiO2 nanocomposite
Monomer
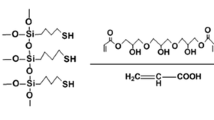
1-Vinyl-3-ethylimidazolium bromide (VeimBr) was prepared as follows. First, bromoethane (50 mL, 0.67 mol) was gradually added to N-vinylimidazole (40 mL, 0.44 mol) in a 250 mL round-bottomed flask at 70 °C. The mixture was then stirred for 12 h. After phase separation, the product was obtained as a viscous liquid and was dissolved in 20 mL acetonitrile, then re-crystallized by adding three drops ethyl acetate at −22 °C. After filtration and washing with ethyl acetate, the monomer powder was dried in a vacuum.
Cross-linker
To prepare 1,4-butanediyl-3,3′-bis-l-vinylimidazolium bromide (BVD), 1,4-dibromobutane (12 mL, 0.10 mol) was added to N-vinylimidazole (18 mL, 0.19 mol) and methanol (30 mL) in a 100 mL round-bottomed flask. The mixture was stirred at 60 °C for 15 h, then cooled to room temperature and added drop-by-drop to 1 L diethyl ether. The white precipitate was filtered and then dried at room temperature until constant weight was obtained.
Modification of silica nano-spheres with vinyl groups
First, 6 mL tetraethoxysilane (TEOS) was added to a mixture of 100 mL ethanol, 11 mL deionized water, and 6 mL ammonia (25 %). The reaction mixture was then vigorously stirred for 24 h at 30 °C. γ-Methacryloxypropyltrimethoxysilane (γ-MAPS, 750 μL; used as the source of the vinyl group) diluted with 10 mL ethanol was then added to the reaction system over 30 min. The mixture was then stirred for a further 24 h to facilitate coating of the silica nano-spheres with MAPS. The MAPS-modified silica nano-spheres (SiO2–MAPS) were collected by centrifugation and then re-suspended in ethanol under ultra-sonication followed by centrifugation. Finally, the product was dried to constant weight at room temperature under vacuum conditions.
Encapsulation of silica nano-spheres with polymerized ionic liquid
A series of suspensions was prepared by dispersing 0.200 g of the previously obtained SiO2–MAPS in 40 mL phosphate buffer (0.02 mol L−1, pH 6.2). Then 0.100, 0.200, 0.300, 0.400, 0.600, 0.800, and 1.000 g VeimBr monomer (corresponding to 0.5, 1, 1.5, 2, 3, 4, and 5 mmol) and amounts of cross-linker BVD corresponding to 5 % of the monomer mass were added to the suspensions. These were then stirred for 30 min before addition of the ammonium persulfate (10 %, m/v) initiator in volumes of 150, 300, 450, 600, 900, 1200, or 1500 μL, with higher volumes of initiator added to higher masses of monomer. The reaction mixtures in the flasks were homogenized under magnetic stirring and purged with nitrogen to remove residual oxygen. Polymerization was then initiated by addition of 50, 100, 150, 200, 300, 400, or 500 μL catalyst (TEMED (5 %, m/v)). After reaction overnight at room temperature, the product was centrifuged and washed with deionized water. Finally the nano-spheres derived from different masses of monomer, termed PIL@SiO2-1, PIL@SiO2-2, PIL@SiO2-3, PIL@SiO2-4, PIL@SiO2-5, PIL@SiO2-6, and PIL@SiO2-7, were freeze-dried to remove any residual water.
Protein adsorption
PIL@SiO2 nanocomposite (5.0 mg) in 1 mL of aqueous solution (at pH 6.0, Britton–Robinson buffer) was used to extract 200 μg mL−1 protein. The mixture was shaken vigorously for 30 min to facilitate protein adsorption, followed by phase separation by use of centrifugation for 5 min at 7000 rpm. Protein concentrations in the aqueous phase (supernatant) before and after adsorption were determined by measuring absorbance at the proteins’ characteristic absorption wavelengths: 408 nm for Hb and cyt-c, 280 nm for the other proteins. Recovery of proteins retained on PIL@SiO2 nano-spheres was performed by use of SDS (0.2 %, m/v) as stripping reagent. Potential conformational changes of the proteins were elucidated by use of circular dichroism spectra analysis. To avoid carry-over of proteins, a new sample of PIL@SiO2 nanocomposite was used for the next adsorption operation.
As a model protein, ovalbumin was isolated from chicken egg-white by use of PIL@SiO2 nano-spheres as adsorbent. The egg white was diluted 100-fold with Britton–Robinson buffer at pH 6.0, and the mixture was gently stirred to form a homogeneous suspension. The supernatant was then collected by centrifugation at 8000 rpm for 20 min at 4 °C. To extract ovalbumin from the supernatant, 20.0 mg PIL@SiO2-6 nanocomposite was shaken with 1 mL supernatant. After the isolation process, the PIL@SiO2-6 was separated from the reaction mixture and washed with deionized water to eliminate any loosely retained components on the surface of the nano-spheres. Finally, the retained Ova was recovered by elution with 0.2 % SDS followed by assay with SDS-PAGE.
Results and discussion
Preparation and characterization of the PIL@SiO2
A schematic diagram of the on-surface polymerization of ionic liquids and in-situ encapsulation on silica nano-spheres used to derive the PIL@SiO2 nanocomposite is depicted in Fig. 1. First, colloidal silica nanoparticles (NPs) with highly uniform size and good dispersibility were obtained via the Stöber method, using tetraethoxysilane (TEOS) as sole precursor [28]. Then vinyl groups were introduced on to the surface of silica nano-spheres via chemical modification, with the objective of producing a homogeneous and stable coating. Because silica NPs disperse better in an aqueous medium than in an organic phase, polymerization of ILs on the surface of silica nano-spheres was performed in aqueous solution.
The developed PIL@SiO2 nanocomposite was characterized by use of 1H NMR, FT-IR and UV–visible spectroscopy. Details, and corresponding discussion, are provided in the Electronic Supplementary Material (Figs. S1–S3).
The SEM images in Fig. 2a reveal that the silica nano-spheres were perfectly spherical, of uniform size and mono-dispersed, with an average diameter of 273 nm. After modification with MAPS and covalent encapsulation with polymerized ionic liquid, the average diameters of SiO2–MAPS (Fig. 2b) and of PIL@SiO2 nanocomposite (Fig. 2c) changed to 276 nm and 286 nm, respectively. In contrast with the TEM images of pure silica nano-spheres and SiO2–MAPS in Fig. 2d–e, the TEM image of PIL@SiO2 in Fig. 2f clearly reveals that a core–shell structure was formed, with a silica nano-sphere as the core and thin polymerized ionic-liquid layer as the shell. The thickness of the shell was approximately 10 nm.
The PIL@SiO2 nanocomposite was further characterized by surface charge analysis (measurement of its Zeta potential) in the range pH 3–9. As a result of the dissociation of surface silanol groups, both pure silica and SiO2–MAPS nano-spheres were negatively charged across the whole pH range (Fig. 3a, b) [29, 30]. After encapsulation with polymerized ionic liquid via the vinyl group, protonation of surface imidazolium groups meant that the surface of the PIL@SiO2 nanocomposite became positively charged at pH 3–9 (Fig. 3c) [31].
Adsorption of proteins with PIL@SiO2 as sorbent
As previously described, preparation of PIL@SiO2 nanocomposite was performed in an aqueous medium with no use of organic solvent. In this respect, PIL@SiO2 nanocomposite can be regarded as a green interaction medium. Its potential use as a sorbent for the isolation of biomacromolecules, specifically proteins, was therefore investigated.
Selective adsorption of acid proteins
The charge properties of protein species in an aqueous medium can be readily manipulated by varying the pH of the solution. A protein is positively charged at a pH below its isoelectric point (pI), becoming negatively charged when the pH exceeds its pI. In this study, we tested the adsorption behavior of the PIL@SiO2 nanocomposite toward acid proteins Ova and IgG, neutral protein Hb, and basic proteins cyt-c and Lys. The protein (200 μg mL−1 in 1 mL aqueous solution) was extracted by use of 5.0 mg PIL@SiO2-6 at pH 6.0; each protein species was extracted separately. The adsorption efficiencies achieved were 86 % for Ova, 65 % for IgG, 50 % for Hb, 4 % for cyt-c, and 3 % for Lys. The PIL@SiO2 nanocomposite had obvious selectivity for the acidic protein species, but virtually no adsorption of basic protein species was observed. This was attributed to the fact that acidic proteins are negatively charged at pH 6.0: the electrostatic interaction between the protein species and the cationic imidazolium moiety in the polymerized ionic liquid has a vital function in protein adsorption. However, the electrostatic repulsion between the cationic ionic liquid moiety and the positive protein species is not favorable for protein adsorption.
Selectivity manipulation by varying the monomer concentration
When preparing the PIL@SiO2 nanocomposite, the concentration of the 1-vinyl-3-ethylimidazolium bromide (VeimBr) monomer is a crucial variable, controlling the selectivity of the nanocomposite to proteins. To investigate this, Ova and Lys were chosen as models of acidic and basic proteins. The amount of monomer was varied within the range 0.5–5 mmol, with a monomer-to-cross-linker ratio of 20 (VeimBr/BVD, m/m). Nanocomposite selectivity for Ova and Lys obtained by use of a variety of monomer concentrations is depicted in Fig. 4. The pure silica modified with vinyl groups, SiO2–MAPS, clearly adsorbed both protein species, without selectivity. When the amount of monomer was increased within the range 0.5–4 mmol, adsorption selectivity for Ova was significantly improved. The sorption efficiency for acidic protein Ova remained high at >86 %, whereas that for Lys was well controlled, being below 5 % for PIL@SiO2-3, PIL@SiO2-4, PIL@SiO2-5, PIL@SiO2-6, and PIL@SiO2-7 when >1.5 mmol monomer was used. A sorption efficiency for Lys of <1 % was achieved by use of PIL@SiO2-6, and this was used for the ensuing experiments. Further increases of the VeimBr amount to above 5 mmol are not recommended, because polymerization of monomer and cross-linker on the surface of the silica nano-spheres at higher monomer concentrations causes aggregation of the nano-spheres.
The effect of the amount of monomer (VeimBr) on adsorption selectivity for Ova and Lys. 200 μg mL−1 Ova or Lys in 1 mL Britton–Robinson buffer (pH 6.0) is adsorbed by 5.0 mg PIL@SiO2-1 (0.5 mmol VeimBr), PIL@SiO2-2 (1 mmol VeimBr), PIL@SiO2-3 (1.5 mmol VeimBr), PIL@SiO2-4 (2 mmol VeimBr), PIL@SiO2-5 (3 mmol VeimBr), PIL@SiO2-6 (4 mmol VeimBr), and PIL@SiO2-7 (5 mmol VeimBr)
The dependence of Ova/Lys adsorption on pH and ionic strength
The effect of pH on the efficiency of Ova and Lys adsorption by PIL@SiO2-6 is illustrated in Fig. 5a. Variation of pH changes the extraction efficiency for both protein species within a small range. At pH 4.0 Ova is positively charged in aqueous medium, and the surface of the PIL@SiO2-6 is also positively charged. The adsorption efficiency of 99 % for Ova was therefore obviously not caused by electrostatic interaction, meaning there must be other interactions between the protein species and the surface of the PIL@SiO2-6 nanocomposite. It is known that ionic strength is an important variable for controlling the partitioning of a protein species. The dependence of Ova and Lys adsorption on ionic strength was therefore investigated. The ionic strength was altered by varying the NaCl concentration within the range 0.2–3 mol L−1. The results are illustrated in Fig. 5b. Increased adsorption efficiency for Lys with increased ionic strength was observed, and approximately 45 % of the Lys was retained at an ionic strength of 3 mol L−1 NaCl. Increased ionic strength causes electrostatic hindrance, which weakens electrostatic interaction and makes the hydrophobic interaction more evident [32]. Figure 5b also reveals that the adsorption efficiency of Ova remained high, at >82 % within the range of 0.2–3 mol L−1 NaCl, demonstrating that hydrophobic interaction is among the mechanisms responsible for the adsorption of proteins by the PIL@SiO2 nanocomposite.
Adsorption capacity of Ova by PIL@SiO2 nanocomposite
Adsorption of Ova by the PIL@SiO2-6 nanocomposite was performed at different initial concentrations of Ova, ranging from 200–5500 μg mL−1. The amount of Ova adsorbed on PIL@SiO2-6 increased rapidly as Ova concentration was increased from 200 to 4500 μg mL−1, after which a plateau was reached and further increases of Ova concentration had no effect. The adsorption behavior of Ova by the PIL@SiO2-6 nanocomposite fits the Langmuir adsorption model, characterized by the equation:
where C e is the equilibrium concentration of Ova, Q e is the adsorption capacity for Ova by PIL@SiO2 at equilibrium concentration, Q max is the theoretical maximum adsorption capacity, and b is the adsorption equilibrium constant.
The Langmuir regression equation is 1/Q e = 0.862/C e + 0.003 (r 2 = 0.990), from which is derived an adsorption capacity of 333.3 mg g−1 for Ova by the PIL@SiO2-6 nanocomposite. PIL@SiO2-6 has a diameter of approximately 280 nm, with a 100 % grafting ratio of the ionic liquid moiety (an imidazolium ring is included in each repeating unit). In a previous study of the adsorption of hemoglobin, a sorption capacity of 26.5 mg g−1, achieved by use of a methylimidazolium ionic-liquid-coated poly(vinyl chloride) (PVC) hybrid, was reported. The hybrid had a diameter of 150 μm, with a 15.1 % grafting ratio of the ionic liquid moiety [33]. The nanocomposite produced by encapsulating silica nano-spheres with polymerized ionic liquid thus has a significantly improved adsorption capacity, attributed partly to the nano-size of the composite and partly to the interactions between the nanocomposite and the protein species.
Recovery of Ova from PIL@SiO2 nanocomposite
For some biological applications it is preferable to use an aqueous solution of the protein species. Therefore, recovery of the retained Ova from the surface of the PIL@SiO2 nanocomposite is highly desirable. The fact that, as discussed in the previous section, variations of pH and of ionic strength have virtually no effect on adsorption of Ova by the PIL@SiO2 nanocomposite, explains the observation that no Ova is recovered by use of citrate, phosphate, and carbonate buffers as stripping reagent or eluent. Because the surfactant SDS has favorable protein solubilization, it should be a suitable stripping reagent for use in recovery of Ova. Experiment revealed that >0.2 % SDS aqueous solution enables a 60 % recovery of Ova (Fig. 6). Protein denaturation is frequently encountered with SDS at a high level [34], and the concentration of SDS solution should therefore be kept at a minimum: in this study, 0.2 % SDS aqueous solution was used to recover the adsorbed Ova from the surface of the PIL@SiO2 nanocomposite.
It was important to evaluate whether there was denaturation of the Ova recovered from the PIL@SiO2 nano-spheres by use of SDS as stripping reagent. This was done by investigating the conformational change of Ova by use of far-UV circular dichroism (CD) spectra (Fig. 7). In deionized water (Fig. 7a) and buffer solution (Fig. 7b), pure Ova has two clear negative bands at 210 nm and 218 nm. These are characteristic of the α-helical structure of proteins, and are attributed to n–π* transition of the α-helix peptide bond [35]. After treatment with the PIL@SiO2 nanocomposite, the Ova stripped into 0.2 % SDS had expanded bands at 207 nm and 225 nm (Fig. 7c). This indicates structural and/or conformational change of the Ova in the SDS solution. It was not known whether this change is caused by the PIL@SiO2 nanocomposite during adsorption, or by the SDS itself in the elution process; to determine this, the CD spectra of Ova stripped into 0.2 % SDS (Fig. 7c) and of pure Ova directly dissolved in 0.2 % SDS were recorded for comparison (Fig. 7d). The spectra of both Ova solutions appear almost identical, indicating that the conformational change of Ova is most probably caused by the SDS, and indirectly demonstrating that the PIL@SiO2 nanocomposite is biocompatible. To determine whether the conformational change is reversible, the eluate was subjected to dialysis to remove the SDS, and the corresponding CD spectrum was recorded. Figure 7e illustrates that after SDS elimination, the CD spectrum observed was the same of that of pure Ova dissolved in deionized water without treatment by the PIL@SiO2 nanocomposite, with the same two characteristic bands at 210 nm and 218 nm (Fig. 7a).
Soret region CD spectra of Ova. (a) 200 μg mL−1 Ova in deionized water; (b) 200 μg mL−1 Ova in Britton–Robinson buffer without treatment by the PIL@SiO2 nanocomposite; (c) Ova stripped into 0.2 % SDS aqueous solution as eluate; (d) Ova directly dissolved in 0.2 % SDS aqueous solution; (e) Ova in the eluate after removal of SDS by use of dialysis
A quantitative estimate of the α-helix content of Ova was made by analysis of the CD spectra. The CD results are expressed in terms of mean residue ellipticity (MRE):
where C p is the protein concentration, n is the number of amino acid residues of the target protein (385 for Ova), and l is the optical length. The α-helical contents of Ova are calculated from the MRE values at 208 nm by use of the equation:
where MRE208 is the observed MRE value, 4000 is the MRE value of the β-form and random coil conformation, and 33000 is the MRE value of a pure α-helix.
The α-helical contents of Ova in different media, i.e. deionized water, buffer solution, Ova stripped into 0.2 % SDS, Ova directly dissolved in 0.2 % SDS, and Ova after removal of SDS from the eluate by use of dialysis, were 46.0 %, 47.2 %, 36.7 %, 35.1 % and 46.0 %, respectively. These results clearly indicate that the PIL@SiO2 nanocomposite has good biocompatibility, and that the structural change of Ova during the adsorption–desorption process is completely reversible.
Real sample pretreatment
The practical applicability of the PIL@SiO2 nano-spheres was demonstrated by selective adsorption of Ova from chicken egg-white. After 100-fold dilution, the sample was subjected to the adsorption procedure as detailed in the Experimental section. The adsorbed Ova in the PIL@SiO2-6 nanocomposite was recovered by elution with 0.2 % SDS solution, and then assayed by use of SDS-PAGE. The results in Fig. 8 indicate that Ova was effectively isolated from chicken egg-white that contained other coexisting protein species and complex sample matrices. Recovery of Ova was approximately 45 %. This clearly reveals the effectiveness of polymerized ionic-liquid-encapsulated silica nano-spheres for pretreatment of complex biological samples.
Conclusions
Encapsulation of silica nano-spheres with polymerized ionic liquid produces a core–shell nanocomposite with a 10 nm thick coating layer. The encapsulation process avoids the use of organic solvents at room temperature, thus providing a green adsorbent for protein sorption. The core–shell nanocomposite has favorable selectivity for adsorption of acidic protein species, with a high sorption capacity. The nanocomposite is also biocompatible. This approach provides an alternative way to develop novel nanocomposites for selective retention of proteins.
References
Dupont J, de Souza RF, Suarez PAZ (2002) Chem Rev 102:3667–3691
Davis JH (2004) Chem Lett 33:1072–1077
Burguete MI, Galindo F, Garcia–Verdugo E, Karbass N, Luis SV (2007) Chem Commun 3086–3088
Sans V, Karbass N, Burguete MI, Compan V, García–Verdugo E, Luis SV, Pawlak M (2011) Chem–Eur J 17:1894–1906
Anderson EB, Long TE (2010) Polymer 51:2447–2454
Washiro S, Yoshizawa M, Nakajima H, Ohno H (2004) Polymer 45:1577–1582
Green O, Grubjesic S, Lee S, Firestone MA (2009) Polym Rev 49:339–360
Marcilla R, Alcaide F, Sardon H, Pomposo JA, Pozo–Gonzalo C, Mecerreyes D (2006) Electrochem Commun 8:482–488
Anderson JL, Armstrong DW (2005) Anal Chem 77:6453–6462
Hsieh YN, Horng RS, Ho WY, Huang PC, Hsu CY, Whang TJ, Kuei CH (2008) Chromatographia 67:413–420
Pourjavadi A, Doulabi M, Hosseini SH (2012) Polymer 53:5737–5742
Yang JH, Qiu LH, Liu BQ, Peng YJ, Yan F, Shang SM (2011) J Polym Sci Pol Chem 49:4531–4538
Wanigasekara E, Perera S, Crank JA, Sidisky L, Shirey R, Berthod A, Armstrong DW (2009) Anal Bioanal Chem 396:511–524
Feng JJ, Sun M, Xu LL, Wang S, Liu X, Jiang SX (2012) J Chromatogr A 1268:16–21
Ho HA, Boissinot M, Bergeron MG, Corbeil G, Dore K, Boudreau D, Leclerc M (2002) Angew Chem Int Edit 41:1548–1551
Liu XF, Fan QL, Huang W (2011) Biosens Bioelectron 26:2154–2164
Wu BH, Hu D, Kuang YJ, Liu B, Zhang XH, Chen JH (2009) Angew Chem Int Edit 48:4751–4754
Yuan JY, Giordano C, Antonietti M (2010) Chem Mater 22:5003–5012
De M, Ghosh PS, Rotello VM (2008) Adv Mater 20:4225–4241
Fukushima T (2003) Science 300:2072–2074
Chen H, Zhao G (2012) J Solid State Electr 16:3289–3297
Ho TD, Yu HL, Cole WTS, Anderson JL (2012) Anal Chem 84:9520–9528
Hu BJ, Wu TB, Ding KL, Zhou XS, Jiang T, Han BX (2010) J Phys Chem C 114:3396–3400
Marcilla R, Curri ML, Cozzoli PD, Martínez MT, Loinaz I, Grande H, Pomposo JA, Mecerreyes D (2006) Small 2:507–512
Carrasco PM, Tzounis L, Mompean FJ, Strati K, Georgopanos P, Garcia–Hernandez M, Stamm M, Cabanero G, Odriozola I, Avgeropoulos A, Garcia I (2013) Macromolecules 46:1860–1867
Yan H, Liu S, Gao M, Sun N (2013) J Chromatogr A 1294:10–16
Vijayakrishna K, Jewrajka SK, Ruiz A, Marcilla R, Pomposo JA, Mecerreyes D, Taton D, Gnanou Y (2008) Macromolecules 41:6299–6308
Stober W, Fink A, Bohn E (1968) J Colloid Interf Sci 26:62–68
Wu ZJ, Xiang H, Kim T, Chun MS, Lee K (2006) J Colloid Interf Sci 304:119–124
Palmai M, Nagy LN, Mihaly J, Varga Z, Tarkanyi G, Mizsei R, Szigyarto IC, Kiss T, Kremmer T, Bota A (2013) J Colloid Interf Sci 390:34–40
Markiewicz M, Mrozik W, Rezwan K, Thoming J, Hupka J, Jungnickel C (2013) Chemosphere 90:706–712
Shiomori K, Ebuchi N, Kawano Y, Kuboi R, Komasawa I (1998) J Ferment Bioeng 86:581–587
Shu Y, Chen XW, Wang JH (2010) Talanta 81:637–642
Michaux C, Pomroy NC, Prive GG (2008) J Mol Biol 375:1477–1488
Sahoo BK, Ghosh KS, Dasgupta S (2009) Biopolymers 91:108–119
Acknowledgments
The authors appreciate financial support from the Natural Science Foundation of China (no. 21105008, 21275027, 21235001), the Program of New Century Excellent Talents in University (NCET-11-0071) and the Fundamental Research Funds for the Central Universities (N110305005, N110705002, and N110805001).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 239 kb)
Rights and permissions
About this article
Cite this article
Han, L., Shu, Y., Wang, X. et al. Encapsulation of silica nano-spheres with polymerized ionic liquid for selective isolation of acidic proteins. Anal Bioanal Chem 405, 8799–8806 (2013). https://doi.org/10.1007/s00216-013-7295-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7295-1