Abstract
Acrylonitrile (AN), a widely used industrial chemical also found in tobacco smoke, has been classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer. AN can be detoxified by glutathione S-transferase (GST) to form glutathione (GSH) conjugates in vivo. It can be metabolically activated by cytochrome P450 2E1 to form 2-cyanoethylene oxide, which can also be detoxified by GST to generate GSH conjugates. The GSH conjugates can be further metabolized to mercapturic acids (MAs), namely, N-acetyl-S-(2-cyanoethyl)cysteine (CEMA), N-acetyl-S-(2-hydroxyethyl)cysteine (HEMA), and N-acetyl-S-(1-cyano-2-hydroxyethyl)cysteine (CHEMA). This study developed an ultraperformance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) method to quantitatively profile the major AN urinary metabolites (CEMA, HEMA, and CHEMA) to assess AN exposure, as well as analyze urinary cotinine (COT) as an indicator for tobacco smoke exposure. The limits of quantitation were 0.1, 0.1, 1.0, and 0.05 μg/L for HEMA, CEMA, CHEMA, and COT, respectively. This method was applied to analyze the three AN-derived MAs in 36 volunteers with no prior occupational AN exposure. Data analysis showed significant correlations between the level of COT and the levels of these MAs, suggesting them as biomarkers for exposure to low levels of AN. The results demonstrate that a highly specific and sensitive UPLC-MS/MS method has been successfully developed to quantitatively profile the major urinary metabolites of AN in humans to assess low AN exposure.

Simultaneous quantitation of 3 acrylonitrile-derived mercapturic acids and cotinine in human urine samples with on-line solid-phase extraction LC-MS/MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
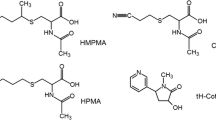
Acrylonitrile (AN) is a monomer widely used to manufacture various household articles such as artificial fibers, resins, rubbers, and plastics. The number of AN-exposed workers was estimated to be 35,000 in Europe and 80,000 in the USA [1]. AN is reported to be present in tobacco smoke, a major source for nonoccupational AN exposure, at about 3-15 μg per cigarette [2] and was classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer in 1999 [1]. Upon absorption, AN can be detoxified by glutathione S-transferase (GST) to form glutathione (GSH) conjugates, which can be further metabolized to N-acetyl-S-(2-cyanoethyl)cysteine (CEMA). Otherwise, AN can be metabolized by cytochrome P450 2E1 to 2-cyanoethylene oxide (CEO), which can also be detoxified by GST to generate CEO–GSH conjugates. The GSH conjugates can be further metabolized to mercapturic acids (MAs), namely, N-acetyl-S-(2-hydroxyethyl)cysteine (HEMA) and N-acetyl-S-(1-cyano-2-hydroxyethyl)cysteine (CHEMA) [3] (Fig. 1).
Analysis of three MAs can be used to confirm AN exposure and to serve as a chemically specific biomarkers for AN exposure [4–9]. The presence of urinary CEMA in exposed human subjects suggests detoxification of AN by GST [6]. An online solid-phase-extraction high-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry (MS/MS) method was developed to analyze CEMA in urine samples collected from the general public with a limit of detection (LOD) of 1 μg/L [10]. HEMA could be derived from ethylene oxide, vinyl chloride, or AN exposure [11]. Several liquid chromatography (LC)–MS/MS methods have been established to analyze urinary HEMA with excellent selectivity and sensitivity [12–15]. CHEMA was identified as a specific urinary metabolite of AN in an animal study [9]; nevertheless, a method to analyze CHEMA has not been reported.
Quantitatively profiling these MAs will shed light on AN metabolic mechanisms in detail and interindividual differences in AN metabolism [16]. Cotinine (COT) has frequently been analyzed to serve as a biological marker for tobacco smoke exposure [17] and can be applied to evaluate the relationships between the levels of MAs and exposure to AN in tobacco smoke. This study thus developed an ultraperformance LC (UPLC) coupled with MS/MS method for simultaneously and quantitatively profiling urinary CEMA, HEMA, CHEMA, and COT. Urine samples collected from volunteers were analyzed to validate whether these urinary MAs can serve as biomarkers for AN exposure.
Materials and methods
Chemicals and reagents
AN (LC/mass spectrometry grade, 100%) was purchased from J.T. Baker (USA). Formic acid (98-100%) and ammonium formate (minimum 98%) were obtained from Riedel-de Haën (Germany). HEMA [N-acetyl-S-(2-hydroxyethyl)-l-cysteine dicyclohexylammonium salt, 98%], CEMA [N-acetyl-S-(2-cyanoethyl)-l-cysteine, 98%], d 4-HEMA [N-acetyl-S-(2-hydroxyethyl-d 4)-l-cysteine dicyclohexy)ammonium salt, 98%], d 3-CEMA [N-acetyl-d 3-S-(2-cyanoethyl)-l-cysteine, 98%], and CHEMA [N-acetyl-S-(1-cyano-2-hydroxyethyl)-l-cysteine dicyclohexylamine salt, 98%] were bought from TRC (Canada). COT and d 3-COT were supplied by Sigma (USA). Deionized water was prepared with a Milli-Q system supplied by Millipore (USA). The stock solution (500 μg/mL) of each standard was prepared in 50% acetonitrile in water and stored at -20 °C. The working solutions were prepared by diluting the stock solutions with ammonium formate buffer (50 mM) to 50 μg/L, and were then subjected to serial dilutions to their calibration curve solutions. The stock solution of each isotope-labeled standard was also prepared in 50% acetonitrile in water at a concentration of 100 μg/L for d 4-HEMA and d 3-CEMA, and 500 μg/L for d 3-COT, and the stock solutions were then subjected to dilution to 50 μg/L with ammonium formate buffer to their corresponding working solutions.
Sample collection and preparation
Spot urine samples were collected randomly in the morning from 36 volunteers and stored at -20 °C until analysis. All participants were graduate students of the National Taiwan University and none had previous history of occupational AN exposure. Information regarding age, gender, and smoking status was collected using questionnaires. The study was approved by the Institutional Review Board committee of the College of Public Health, National Taiwan University. All subjects provided written informed consent prior to their participation.
Creatinine levels were determined as the creatinine–picrate complex according to the Jaffe [18] method using an automated method. Urine samples with a creatinine level less than 30 mg/dL or greater than 300 mg/dL were excluded from further data analysis. Three subjects were excluded because their creatinine levels were out of the range. The final study population consisted of 31 nonsmokers and two smokers from 22 to 26 years old.
Sample preparation
Spot urine samples from nine nonexposed nonsmokers were pooled to serve as the urine blank solution and were stored at -20 °C until analysis. The urine blank was thawed and mixed rigorously with a vortex mixer. One hundred microliters of the urine was transferred into an Eppendorf tube (1.5 mL), and 100 μL of the internal standard was then added to each sample. Twenty microliters of formic acid (98-100%) was then added and mixed rigorously. The sample was diluted with ammonium formate buffer (50 mM) to a final volume of 1,000 μL. The solution was mixed, allowed to stand for 5 min, then centrifuged at 10,000 rpm for 5 min. The supernatant was filtered through a 0.22-μm poly(vinylidene difluoride) membrane filter, and was transferred to a 1.5-mL sample vial. The pH of each sample was adjusted to 2.5 with formic acid (98-100%) before analysis.
UPLC-MS/MS analysis
The UPLC-MS/MS system was equipped with a Thermo Scientific Accela pump, an Accela autosampler, and a TSQ Quantum Access system with a heated electrospray ionization (H-ESI) interface. The spray voltage was set at 2,500 V, the vaporizer temperature was set at 200 °C, the capillary temperature was set at 203 °C, the sheath gas (N2) pressure was set at 40 (arbitrary units), the auxiliary gas (N2) pressure was set at 5 (arbitrary units), and the collision gas (Ar) pressure was set at 1 (arbitrary unit). The H-ESI ion source was operated in negative mode for HEMA, d 4-HEMA, CEMA, d 3-CEMA, and CHMEA and in positive mode for COT and d 3-COT. The LC-MS/MS parameters were optimized with an infusion system, with each working solution being delivered at a flow rate of 10 μL min-1, and the mobile phase (50% acetonitrile solution in water) being delivered at a flow rate of 200 μL min-1 with a Hamilton 500-μL syringe. The mass-to-charge ratio (m/z) range for full scan was set at 50-500 for each standard. The parameters for quadrupole 1 were optimized for precursor ions and those for quadrupole 3 were optimized for product ions automatically. Pairs of precursors and product ions with maximum intensities were selected for monitoring in selected reaction monitoring (SRM) mode. The ion pairs were monitored at 205.88→77.18 m/z for HEMA, 209.88→81.18 m/z for d 4-HEMA, 214.84→162.28 m/z for CEMA, 217.84→165.28 m/z for d 3-CEMA, 231.30→213.10 m/z for CHEMA, 177.04→80.39 m/z for COT, and 180.04→80.39 m/z for d 3-COT. The collision energy for each ion pair was optimized to -14 V for HEMA and d 4-HEMA, -12 V for CEMA and d 3-CEMA, -10 V for CHEMA, and 24 V for COT and d 3-COT. The tube lens offset parameter was set at -61 (arbitrary units) for HEMA and d 4-HEMA, -57 for CEMA and d 3-CEMA, -41 for CHEMA, and +64 for COT and d 3-COT.
Liquid-chromatographic separation was performed with a Waters Acquity UPLC high-strength silica (HSS) T3 1.8 μm (2.1 mm × 100 mm) column with a Waters Acquity VanGuard HSS T3 1.8 μm (2.1 mm × 5 mm) precolumn. The mobile phase consisted of 0.1% formic acid in acetonitrile (solvent A) and 0.1% aqueous formic acid (solvent B) and was delivered as follows: 0.00-1.23 min (3% solvent A, 250 μL min-1), 1.24-1.80 min (3% solvent A, 200 μL min-1), 1.81-2.00 min (3% solvent A, 100 μL min-1), 2.01-3.00 min (3-30% solvent A, 100-150 μL min-1), 3.01-5.00 min (30-80% solvent A, 150-100 μL min-1), 5.01-6.00 min (80% solvent A, 100-250 μL min-1), 6.01-8.00 min (3% solvent A, 250 μL min-1). The total run time was 8 min, including the last 2 min for column conditioning.
Validation and quality control
For quality assurance and quality control, four sets of calibration curves were prepared to evaluate the accuracy, precision, recovery, matrix effect, and effect of the pH of the sample solution for this method. The first set (set 1) of solutions was prepared in deionized water. The second set (set 2) of solutions was prepared in the blank urine, and the standards were added before the sample preparation. The third set (set 3) of solutions was prepared similarly to the second set, but formic acid was not added and the pH was not adjusted during the sample preparation to evaluate the pH effect. The fourth set (set 4) of solutions was also prepared similarly to the second set, but the standards were added after sample preparation to evaluate the recovery during the sample preparation procedure. Linear calibration curves were constructed by plotting the quotients of the peak areas for HEMA, CEMA, and COT and those of their corresponding d 3- or d 4-labeled internal standards versus the concentrations of these standards. For CHEMA, the calibration curve was established by plotting the peak area versus concentration, and its correlations with d 4-HEMA and d 3-CEMA were also checked.
The matrix effect was evaluated by comparing the slope of set 2 with that of set 1 [19], and the recovery was determined by comparison of the slope of set 2 with that of set 4 [20]. The lower limit of quantitation (LLOQ) was estimated by following US Food and Drug Administration (FDA) [21] guidelines for bioanalytical method validation with a signal-to-noise ratio of 10 or greater, and the LOD was defined as half of the LLOQ. Samples for quality control were also prepared according to the FDA’s guidelines [21]. The accuracy and precision of this method were assessed by repeated analysis of the second set of calibration standard solutions. Intraday (n = 30) and interday (n = 18) standard solutions at low (10 μg/L), medium (100 μg/L), and high (1,000 μg/L) concentrations were prepared. Another six duplicates of the low, medium, and high concentration samples were also prepared and underwent a freeze–thaw cycle on the first, third, seventh, and 14th days for analysis to evaluate sample stability [21, 22].
Results and discussion
Optimization of UPLC-MS/MS conditions
The MS/MS parameters were optimized manually, and the ion pairs monitored for quantitation in SRM mode were the fragments with maximum intensities. The precursor-ion scan showed the most abundant fragment of HEMA was at m/z 205.88, and the product-ion scan showed the most abundant fragment ion was at m/z 77.18 (2-mercaptoethanol) [10]. For CEMA, the precursor ion was at m/z 214.84, and the product ion was at m/z 162.28 by loss of one sulfur-bonded AN molecule [15]. For CHEMA, the most abundant ion appeared at m/z 231.30 for the precursor ion and at m/z 213.10 for the product ion by loss of one molecule of H2O. The ion pair at m/z 231.30→213.10 was monitored for CHEMA in SRM mode, as shown in Fig. 2. To save instrumentation time, COT was simultaneously analyzed by previous methods [23–30]. The ion pair at m/z 177.04→80.39 was monitored for COT in SRM mode to achieve the maximum sensitivity [24, 28].
An HSS T3 column was applied to perform chromatography. The HSS T3 column is a reversed-phase column coated with a trifunctional C18 ligand, and can be used in the pH range 2-8 to retain and separate polar organic compounds, such as MAs. Since the chemical and physical characteristics of HEMA, CEMA, and CHEMA are very similar, these MAs and COT could be separated by modulating both the composition of the mobile phase and the flow rate. The retention times were 1.7, 3.4, 4.4, and 5.9 min for COT, HEMA, CHEMA, and CEMA, respectively, as shown in Fig. 3.
Method performance
Four sets of calibration curves were prepared to assess the performance of this method. Each calibration curve was calculated by a weighted (1/y 2) linear regression method, and the results are shown in Table 1. By comparing the slope of each set, we evaluated various effects on quantitation of these analytes, which included the matrix effect (set 2/set 1), the pH effect (set 3/set 2), and recovery (set 4/set 2), as shown in Table 2.
The matrix effect was defined as the interference due to the presence of unintended analytes [22], and was assessed by comparing the slope of set 2 with that of set 1. There was no manifest matrix effect for HEMA (104.41%), CEMA (103.09%), and CHEMA (97.33%), but there was one for COT (116.65%). The matrix effects of HEMA, CEMA, and COT slightly higher than 100% might be caused by their presence as a background in the urine of our study subjects.
The effect of pH was evaluated by comparing the slope of set 3 with that of set 2. Adjustment of the pH would not significantly affect quantitation of HEMA (98.20%), CEMA (99.47%), and COT (95.47%), but may influence quantitation of CHEMA (84.75%). This might be due to the absence of an isotope-labeled internal standard for quantitation of CHEMA. On the other hand, the calibration curves for HEMA, CEMA, and COT were established by the relative ratios of an individual standard to its isotope-labeled standard, and the pH would have a similar effect on both synthetic and isotope-labeled standards.
Recovery was defined as the efficiency of an analytical procedure or sample preparation [22], and was determined by comparing the slope of set 2 with that of set 4. The sample preparation procedures for both sets were the same, except that the standard was added before sample preparation for set 2 and after for set 4. The results indicated no apparent difference between the slopes of both calibration curves. The recoveries were 106.51%, 104.91%, 110.00%, and 109.10% for HEMA, CEMA, CHEMA, and COT, respectively. Similarly, recoveries of analytes slightly over 100% might arise from their presence as a background in urine of our study subjects.
The calibration curves were established by analyzing the set 2 standard solution for quantitation of the intended analytes in human samples. The LLOQs for HEMA, CEMA, CHEMA, and COT were estimated as 0.1, 0.1, 1.0, and 0.05 μg/L, respectively, and the LODs were 0.05, 0.05, 0.5, 0.025 μg/L, respectively. These results were compared with those obtained by previous LC-MS/MS methods. Our method for HEMA is not as sensitive as a previous method with an LOD of 0.03 μg/L [15], but is more sensitive than other methods [12–14]. CEMA was first analyzed with HPLC–electrospray ionization–MS/MS with an LOD of 1 μg/L. Our method with an LOD of 0.05 μg/L is more sensitive than previous methods [9–11]. Our method is the first to be used to analyze CHEMA, not to mention to simultaneously quantitate urinary HEMA, CEMA, CHEMA, and COT in one sample preparation and one injection. The relationships between the three AN-derived MAs and COT could be evaluated simultaneously.
Quality assurance and quality control
According to the FDA guidelines [21], precision represents the closeness between individual measurements and should not exceed 15% of the coefficient of variation, and accuracy is defined as the deviation of the mean from the true value with an acceptable coefficient of variation within 15%. Table 3 shows the concentrations of standard solutions (10, 100, 1,000 μg/L) prepared to evaluate the accuracy and precision of this method. Accuracy and precision were all within 3% for the four analytes at all concentrations. For the test of sample stability, six duplicates at the three different concentrations of the standard solutions (10, 100, 1,000 μg/L) were also prepared. These samples were analyzed on the first, third, seventh, and 14th days. The results revealed that the four urinary analytes were relatively stable, at least up to 14 days of storage at –20 °C. The variations were within 15% for the three concentrations of each analyte compared with the initial concentrations prepared on the first day. Reproducibility was evaluated by repeated analysis of HEMA, CEMA, CHEMA, and COT in all concentrations in the set 2 calibration standard solutions. For HEMA, CEMA, CHEMA, and COT, the intraday variations were 1.35-1.73%, 0.98-1.54%, 1.03-1.89%, and 0.40-1.29%, respectively, and the interday variations were 1.19-1.78%, 0.86-2.32%, 1.38-1.46%, and 0.58-2.29%, respectively. These results demonstrate that this method simultaneously quantitated three AN-derived MAs and COT with excellent sensitivity, specificity, precision, accuracy, and reproducibility, and these analytes were very stable for 14 days at –20 °C.
Concentrations of urinary metabolites
In total, 36 samples were analyzed, and the creatinine levels in 33 samples fell in the range 30-300 mg/dL. Analysis of all 33 samples showed that HEMA, CEMA, CHEMA, and COT were detectable in 45.45%, 69.70%, 96.97%, and 100% of the samples, respectively. For those samples with urinary metabolites below the LOD, their contents were designed as half of the LOD. The data are summarized in Table 4 as the percentage of detectable HEMA, CEMA, CHEMA, and COT and their corresponding geometric mean and range concentrations. Compared with the geometric mean (range) of urinary HEMA and CEMA levels, our results with a geometric mean (range) of 1.04 (not detectable to 61.83 μg/g creatinine) for HEMA and 2.59 (not detectable to 111.70 μg/g creatinine) for CEMA are comparable with those of previous studies [8, 10, 14]. To assess the relationships between COT and MAs, the values for the MAs were log-transformed and analyzed with linear regression. There was high correlation between CEMA concentration and COT concentration (r p = 0.801, p < 0.001), and this correlation was consistent with that in previous studies [10, 31]. These results suggest that CEMA can serve as a biomarker for very low AN exposure in the general population and indicate that tobacco smoke could be the major source of nonoccupational AN exposure for our study subjects. The correlation between the summation of the HEMA and CHEMA concentrations and the COT concentration was highly significant (r p = 0.780, p < 0.001), but COT concentration was not significantly correlated with either HEMA concentration or CHEMA concentration. These results demonstrated that AN is indeed metabolized to CEO in humans. The presence of HEMA could also represent the internal dose of coexposure to AN, ethylene, and ethylene oxide in tobacco smoke. Our study showed that there was insignificant correlation between HEMA concentration and COT concentration, and this was inconsistent with the findings of a previous study [32]. One of the reasons could be that our study subjects were mainly nonsmokers and included only two smokers. In contrast, there were 40 smokers in the previous study, and HEMA data of smokers and nonsmokers were combined for statistical analysis to achieve a significant correlation. Another reason could be that HEMA was not detectable in a great portion of samples, and the true correlation between HEMA concentration and COT concentration might not be obtained by using statistical analysis in this study. Interpretation of no significant correlation between CHEMA concentration and COT concentration would need further study of the kinetics. However, the summation of the HEMA and CHEMA concentrations was significantly correlated with the COT concentration in urine, and this suggests that the summation of the HEMA and CHEMA concentrations may serve as an indicator for exposure to tobacco smoke. Although CEMA is a good biomarker of AN exposure, our data show that CHEMA is a poor AN biomarker. However, the combined analysis of CEMA, HEMA, and CHEMA could be useful in estimating the dose of alkylating agents in cigarette smoke.
Conclusions
A method for simultaneous quantitation of three AN-derived MAs (CEMA CHEMA, and HEMA) and COT has successfully been developed with excellent reliability, sensitivity, and specificity with easy sample preparation procedures. To our knowledge, the method presented is the first to simultaneously quantitate the three AN-derived MAs in one sample preparation. Additionally, CEMA can serve as a biomarker for AN exposure, and the summation of the HEMA and CHEMA concentrations may be a biomarker for exposure to tobacco smoke. This method can be used to analyze the three AN-derived MAs in urine collected from AN-exposed workers to study occupational AN exposure from multiple routes.
References
IARC (1999) Summary of data reported and evaluation. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide, vol 71. IARC, Lyon, France
Hoffmann D, Hoffmann I, El-Bayoumy K (2001) Chem Res Toxicol 14:767–790
Léonard A, Gerber GB, Stecca C, Rueff J, Borba H, Farmer PB, Sram RJ, Czeizel AE, Kalina I (1999) Mutat Res 436:263–283
National Research Council (1995) Biologic markers in urinary toxicology. National Academies Press, Washington
Sumner SC, Selvaraj L, Nauhaus SK, Fennell TR (1997) Chem Res Toxicol 10:1152–1160
Jakubowski M, Linhart I, Pielas G, Kopecký J (1987) Br J Ind Med 44:834–840
Vermeulen NP, de Jong J, van Bergen EJ, van Welie RT (1989) Arch Toxicol 63:173–184
Calafat AM, Barr DB, Pirkle JL, Ashley DL (1999) J Expo Anal Environ Epidemiol 9:336–342
Linhart I, Smejkal J, Novák J (1988) Arch Toxicol 61:484–488
Schettgen T, Musiol A, Alt A, Ochsmann E, Kraus T (2009) Anal Bioanal Chem 393:969–981
De Rooij BM, Commandeur JNM, Vermeulen NPE (1998) Biomarkers 3:239–303
Barr DB, AshleyDL J (1998) Anal Toxicol 22:96–104
Haufroid V, Merz B, Hofmann A, Tschopp A, Lison D, Hotz P (2007) Cancer Epidemiol Biomarkers Prev 16:796–802
Schettgen T, Musiol A, Kraus T (2008) Rapid Commun Mass Spectrom 22:2629–2638
Ding YS, Blount BG, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL (2009) Chem Res Toxicol 22:1018–1025
Schulte PA, Waters M (1999) Ann N Y Acad Sci 895:101–111
Benowitz NL (1996) Epidemiol Rev 18:188–204
Jaffe M (1886) Hoppe-Seylers Z Physiol Chem 10:391
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Matuszewski BK, Constanzer ML, Chavez-Eng CM (1998) Anal Chem 70:882–889
Food and Drug Administration (2001) Guidance for industry bioanalytical method validation. CVM & CDER, FDA, US DOH
Chung CC (2004) Analytical method validation and instrument performance verification. John Wiley & Sons, Inc., Publication, Hoboken
Kataoka H, Inoue R, Yagi K, Saito K (2009) J Pharm Biomed Anal 49:108–114
Fan Z, Xie F, Xia Q, Wang S, Ding L, Liu H (2008) Chromatographia 68:623–627
Chadwick CA, Keevil B (2007) Ann Clin Biochem 44:455–462
Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM (2007) Am J Clin Pathol 126:880–887
Heavner DL, Richardson JD, Morgan WT, Ogden MW (2005) Biomed Chromatogr 19:312–328
Xu X, Iba MM, Weisel PC (2004) Clin Chem 50:2323–2330
Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD (2002) Clin Chem 48:1460–1471
Tuomi T, Johnsson T, Reijula K (1999) Clin Chem 452:2164–2172
Minet E, Cheung F, Errington G, Sterz K, Scherer G (2011) Biomarkers 16:89–96
Eckert E, Schmid K, Schaller B, Hiddemann-Koca K, Drexler H, Goen T (2011) Int J Hyg Environ Health 214:196–204
Acknowledgements
The authors gratefully appreciate the technical support and assistance with the UPLC-MS/MS analyses provided by Dr. Wei-Chung Shih. This study was supported in part by grants from the Institute of Occupational Safety and Health, Council of Labor Affairs (grant no. IOSH98-A313), the National Sciences Council (no. NSC98-2314-B-002-082-MY3), and the Environmental and Occupational Health Center at National Taiwan University, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chia-Fang Wu and Shi-Nian Uang are co-first authors
Rights and permissions
About this article
Cite this article
Wu, CF., Uang, SN., Chiang, SY. et al. Simultaneous quantitation of urinary cotinine and acrylonitrile-derived mercapturic acids with ultraperformance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 402, 2113–2120 (2012). https://doi.org/10.1007/s00216-011-5661-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5661-4