Abstract
The concentration of benzene in urban air in the Tri-City area of Poland (Gdańsk–Sopot–Gdynia, and Tczew) was assessed using diffusive passive samplers (Radiello). Samples were collected during a four-year monitoring campaign (2007–2010) at selected monitoring stations managed by the Agency of Regional Air Quality Monitoring in the Gdańsk Metropolitan Area (ARMAAG) Foundation. The performance of the passive samplers was investigated in a field study that measured the benzene concentration in urban air. The results obtained by the Radiello samplers were compared with the results obtained using an on-line monitor (Chrompack CP 7001). Statistical analysis of the results obtained by the two different techniques (passive and on-line) was performed by a linear regression method (Student’s t-test). The influence of temperature fluctuations on the uptake rate behavior of the passive samplers was also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to a report published in 2005 by the World Health Organization (WHO), the presence of volatile organic compounds (VOCs) in the ambient air significantly affects its quality [1]. VOCs are emitted into the atmosphere from both natural and anthropogenic sources, and participate in a number of photochemical reactions in the troposphere. The chemical processes that VOCs undergo in the troposphere may lead to their transformation into more harmful secondary organic pollutants, such as secondary organic aerosols (SOA). VOCs are also considered to be precursors of photochemical oxidants such as tropospheric ozone and PAN. The degradation of VOCs occurs as a result of reactions with the hydroxyl radical ·OH during the daytime, with NO3 during the night, and with Cl· mainly in coastal areas [2].
According to the reports of the American Agency for Toxic Substances and Disease Registry (ASTDR), long-term exposure to hazardous compounds like VOCs can strongly affect humans health and increase the risk of serious disease. Therefore, the monitoring of VOCs in the ambient air as well as the identification of their main anthropogenic emission sources have become matters of particular concern [3].
Benzene is one compound that requires special attention and systematic control in terms of its presence and concentration. According to the legislation adopted by the European Union, the ambient concentration of benzene cannot exceed 5 μg m−3 (EU directive 2000/69/EC for 2010) [4]. This is due to the fact that benzene is considered a human carcinogen, and long-term exposure to even low concentrations of benzene may increase the risk of leukemia. According to the WHO, lifetime exposure of urban populations to benzene concentrations of 1.7 μg m−3 results in an average of ten cases of leukemia per million inhabitants [5]. To reinforce this, benzene was listed in Group I (human carcinogens) by the International Agency for Research on Cancer (IARC) [6].
According to the data in the literature, vehicle traffic is among the most significant sources of anthropogenic benzene emissions in urban areas. This is due to the fact that benzene, like other BTEX compounds, is a natural component of fuel, and is therefore emitted into the ambient air during the consecutive stages of operation of a vehicle’s engine (e.g., fuel combustion, vaporization) [7]. However, due to the carcinogenic potential of benzene, restrictions have been recently enforced in order to reduce the content of benzene in gasoline. According to the most resent regulations adopted by the US EPA, all refiners are required (from the beginning of July 2012) to import or to produce gasoline with a maximum annual average gasoline benzene content of 1.3 vol%. This signifies that the actual annual average gasoline benzene level may not exceed this maximum average standard. Moreover, credits cannot be used to meet the 1.3 vol% standard [8].
From an analytical point of view, monitoring organic compounds in ambient air requires the application of an appropriate sampling technique. It has recently been reported that passive sampling could represent a promising alternative to active techniques at the sample collection stage. This is due to the fact that passive samplers do not require a power supply, which in turn means that electrical devices (e.g., pumps) are not needed. Moreover, the small sizes of passive samplers together with their simple design enables monitoring to be performed in remote areas without highly qualified supervision. Passive sampling allows average time-weighted concentrations to be obtained, and these can serve as an important source of information in terms of long-term exposure to VOCs. Results obtained in this way can be used to generate a long-term overview of pollutant concentration levels in ambient air. This is particularly useful in epidemiological and toxicological studies. An undoubted advantage of passive sampling is also the ability to operate at both regional and global scales [9].
The Tri-City region is listed among the most industrialized areas of Poland. As this is the center of the shipbuilding industry, there are transshipment terminals located in both Gdansk and Gdynia. The quality of the ambient air in this area is also strongly influenced by the emissions from numerous industrial companies, such as an oil refinery (Grupa Lotos S.A.), a plant processing sulfur compounds (Siarkopol S.A.), and one processing phosphorus compounds (Zakłady Nawozów Fosforowych). Strong vehicle traffic is also considered a significant source of emissions of organic compounds in the Tri-City area.
The main objective of this study was to examine the performance of Radiello passive samplers, when used as reference passive samplers for the long-term characterization of benzene concentration in a given environmental compartment. An attempt was made to investigate the influence of temperature fluctuations on passive sampler uptake behavior. The database created as a result of the monitoring campaign could serve as a source of information on the seasonal and spatial distribution of benzene in the atmosphere, which is relevant when modeling air pollution transport.
Materials and methods
Passive samplers
Radial symmetry diffusive passive samplers (Radiello) developed by the Fondazione Salvatore Maugeri (Padova, Italy) were applied for the sampling and enrichment of benzene from atmospheric air [10]. Graphitized carbon (Carbograph 4) with a medium-strength adsorbing surface (200 m2 g−1) that is suitable for thermal desorption was applied as an adsorbent to retain analytes from the gas phase. The Radiello sampling system consists of a cylindrical cartridge housed inside a microporous polyethylene diffusive body (5 mm thick, 1 μm porosity) [11, 12]. The adsorbing cartridge, which is made of stainless steel, is supplied with a glass tube (commonly used for short-term storage). During the exposure, Radiello passive samplers are fixed onto a triangular supporting plate. In contrast to previously applied axial passive samplers, where the diffusion path is perpendicular to the sampler axis, Radiello is a coaxial sampling system with a diffusion path that is parallel to the cartridge radius. This novel, improved radial–coaxial geometry results in a larger diffusive surface, which makes it possible to obtain higher sampling rates (Q) [12–15]. According to the most recent studies, Radiello can be promising alternative to active sampling techniques, especially for VOC sampling. To confirm this, the Radiello passive sampler has been evaluated, according to the European standard EN 13528–2, as a reference passive sampler of BTEX in ambient air [15].
Sampling sites
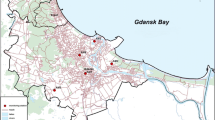
In order to obtain reliable results, appropriate sampling sites need to be selected. This determines whether the collected sample is representative of the current state of environment. Ten monitoring stations managed by the Agency of Regional Air Quality Monitoring in the Gdańsk Metropolitan Area (ARMAAG) Foundation and located in the Tri-City area, were selected as sampling sites during the monitoring campaign (Fig. 1). The sampling sites were selected based on the results of long-term studies, and the locations of the stations were determined by such factors as weather conditions, human population density, and existing databases on pollutant emissions from point and surface sources. All monitoring stations were considered urban background stations. The monitoring campaign was carried out from January 2007 until December 2010.
Locations of the ten monitoring stations in the Tri-City area [13]
During each exposure, two passive samplers were placed 20 cm apart at each monitoring station. This was done in order to avoid the risk of depleting analytes in the boundary layer near the surface of each passive sampler. The decrease in analyte concentration extends the diffusion zone, which in turn reduces analyte uptake rate. This phenomenon, often described as passive sampler “starvation,” results from the high sampling rates obtained by Radiello passive samplers as well as insufficient air velocities around the passive samplers [16].
Passive samplers were placed in specially designed stainless steel shelters to protect them from the precipitation and wind. The shelters were located at a height of 3 m above ground level (Fig. 2). After 14 days of exposure, the sorption medium was removed from the cylindrical diffusive body and placed into a glass tube that was tightly sealed with a polyethylene stopper. The tubes were brought to the laboratory and stored frozen until analysis. The cartridges were analyzed within a week. During the four-year monitoring campaign, two samples were exposed at the each of nine sampling sites during the 24 14-day exposures per year. This gave a total of 432 samples per year.
Applying the passive sampling technique as a tool for monitoring benzene concentrations under real conditions requires a knowledge of the parameters responsible for the uptake of analytes by a particular type of sampler, as well as meteorological conditions that influence the performance of passive samplers. Sampling rate values of the passive samplers (Q) were provided by the manufacturer, while meteorological data were supplied by the Agency of Regional Air Quality Monitoring in the Gdansk Metropolitan Area (ARMAAG) Foundation.
Exposure
The exposure period for the Radiello passive samplers was evaluated on the basis of the individual properties of the sampler (e.g., type of sorbent, construction parameters, etc.). Due to the high sampling rates attained by Radiello passive samplers (26.8 ml min−1 for benzene), special care was taken not to saturate the sorbent bed with the compounds of interest. Throughout the whole monitoring campaign, the optimal exposure for the Radiello passive samplers was evaluated as 14 days, which corresponds to two exposure periods per month [16]. During this period of time, the gain in the mass of analytes on the sorbent bed was reported to be linear.
Sample analysis
Liberation of analytes and chromatographic analysis
Analytes retained on the sorption bed in the Radiello passive samplers were liberated by two-step thermal desorption (Unity v.2, Markes Int. Ltd., Pontyclun, UK). During the first desorption step, carrier gas (helium, flow rate 45 ml min−1) was passed through the cartridge (labeled 1 in Fig. 3) for 20 min, and the analytes were then transferred to a cold trap (2) and cooled to 1 °C. The second desorption step started with trap heating to 300 °C, before transferring the analytes directly onto a chromatographic column. This scheme for thermal desorption is presented in Fig. 3. Compared to solvent extraction, thermal desorption is considered to be a precise and environmentally friendly extraction technique, mainly because it eliminates the use of organic solvents. During thermal desorption, the analytes are injected directly onto the chromatographic column, thus avoiding sample manipulation and dilution. Additionally, two-step desorption ensures that narrow bands are formed [17].
Chromatographic analysis was carried out using an Agilent Technologies 6890 GC coupled with a 5873 Network mass spectrometer (MS). A DB-1 (J&W) 30 m × 0.25 μm capillary column with a stationary phase film thickness of 1 μm was applied. The temperature of the transfer line (GC-MS) was 300 °C. The concentration of benzene in ambient air was calculated according to Eq. 1, provided by the manufacturer of Radiello, correcting for exposure, the sampling rate, and the average air temperature.
Chemical standards
External calibration was carried out with a mixture of volatile organic compounds (“Mix 8”) containing 13 VOCs (including benzene) at 2000 μg/mL (Supelco, USA). Further information on the calibration procedure can be found elsewhere [18]
Quality control/quality assurance
Quality assurance and quality control procedures included the analysis of field blanks and parallel duplicate samples. For field blanks, additional passive samplers were taken to nine monitoring stations and left unexposed during the exposure period (14 days). These field blanks were then analyzed under the same conditions applied during the analysis of samplers exposed to the atmospheric air. The measurement uncertainty evaluated by the manufacturer was 25%.
Results and discussion
The time-weighted average benzene concentration was calculated according to Eq. 1, based on knowledge of the exposure of the passive samplers, the masses of the analytes trapped by the sorption medium, and sampling rates. As the Q values supplied by the manufacturer were evaluated under standard conditions, 25 °C and 1013 hPa, an adjustment to account for the mean temperature was performed using Eq. 2.
- C :
-
concentration of analyte (μg m−3)
- M :
-
mass of analyte (ng)
- Q :
-
sampling rate (ml min−1)
- t :
-
exposure time (min)
- Q :
-
sampling rate (ml min−1)
- Q 298 :
-
sampling rate at 298 K (ml min−1)
- T :
-
temperature (K)
Seasonal distribution
The monitoring campaign carried out in years 2007–2010 allowed us to obtain seasonal concentration profiles for benzene in ambient air in the Tri-City area, which are presented in Fig. 4. The error bars plotted in Fig. 4 reflect the standard deviations (SD) of the obtained results. The average monthly concentrations of benzene plotted in the seasonal profiles show significant fluctuations depending on the month and season of the year. Results obtained during the campaign confirmed the strong influence of meteorological conditions, especially temperature, on benzene concentration. Figure 5 presents the variation of the benzene concentration with the fluctuations in temperature.
Higher concentrations of benzene were reported in winter to spring months (from November to April), when the ambient temperature slightly exceeded 5 °C (Fig. 5). This was due to increased emissions from heating devices (the cold season in Poland stretches from October until March), as well as emissions from vehicle traffic. It has also been reported that a lower average ambient temperature aids the accumulation of pollutants in the atmosphere, due to limited movement of air masses in the vertical plane [2]. Benzene concentrations obtained in January and February 2010 significantly exceeded the threshold value of 5 μg m−3. This may have been due to the low average ambient temperatures reported for these months compared to the same months in previous years.
The low benzene concentrations reported during the summer to autumn (May to October) months throughout the monitoring campaign resulted from the meteorological conditions typical of this time of year: high average ambient air temperatures, high levels of insolation, low relative humidities, all of which accelerate the degradation of organic compounds in the atmosphere through reactions with hydroxyl radicals. As the lifetime of benzene in ambient air is significantly shorter in summer months, the concentration of benzene decreases with increasing ambient air temperature (Fig. 4) [2, 19]. Seasonal concentration profiles obtained for benzene during the four-year monitoring campaign demonstrate that fluctuations in benzene concentrations in ambient air result from a combination of meteorological conditions, atmospheric chemistry, and emissions from anthropogenic sources.
Gdansk is considered the most industrialized region of the Tri-City area. There are large companies in this region, including the Wybrzeże CHP refinery, the energy company Energa S.A., and loading terminals within the port area. Gdansk is also known as a center of the shipbuilding industry. Increasing numbers of vehicles and petrol stations can also be significant sources of a wide range of hazardous compounds (e.g., benzene) in the ambient air, which significantly affect its quality.
The highest average annual benzene concentrations in the Tri-City area were reported in Gdansk, at monitoring stations am1 and am8 (Table 1). This may result from the high levels of vehicle traffic close to Kartuska St. and J. Hallera St. (>10 bus lines reported). Lower concentrations of benzene were in turn reported at the monitoring stations located in Sopot and Gdynia (am6, am10, and am4), which are considered to be less industrialized areas than Gdansk. In particular, Sopot is known to be a popular tourist resort, so there is no heavy industry in this area. Moreover, as it is a health resort, Sopot must abide by stricter legislations regarding the concentrations of particular compounds in ambient air. The elevated benzene concentrations reported for winter months (from November to March) at the monitoring stations located in Sopot and Tczew may result from the use of tiled stoves in central heating. It has been reported that emissions from traditional tiled stoves include a wide range of organic compounds and particulate matter.
Benzene concentration profiles plotted with seasonal resolution for Gdansk, Gdynia, Sopot, and Tczew are presented in Fig. 6a, and these confirm the tendency for higher concentrations to occur in more industrialized areas: Gdansk and Tczew. Local spatial differences in benzene concentration occur, especially during the autumn to winter months (October to March), as can be observed in Fig. 6b.
Average annual concentrations of benzene obtained in the urban air of the monitored cities during 2007–2010 are given in Table 1. According to the literature, these values correspond well with the annual average concentrations of benzene measured in the atmospheric air of other European cities, such as Copenhagen (2.9 μg m−3), Coruna (3.4 μg m−3), Bari (1.7 μg m−3), Rome (2.4 μg m−3), Milan (2.4 μg m−3), and Antwerp (1.6 μg m−3) [20–22]. The minimum and maximum annual concentrations of benzene obtained during year 2007–2010 are presented in Table 2. It should be noted that the monitoring stations at which the maximum annual concentrations were reported were located in Gdansk and Tczew, while the minimum annual concentrations were obtained in Gdynia.
Correlation analysis
One of the aims of this study was to identify sources of benzene emissions into the ambient air in the Tri-City area. Therefore, the correlation coefficients between benzene and toluene (B/T) in atmospheric air were studied. According to the literature, if analytes originate from the same emission source, the correlation is linear [20–22].
The benzene and toluene (B/T) correlation coefficients (r 2) obtained during the four-year monitoring campaign that used Radiello passive samplers are presented in Table 4 with seasonal resolution.
It is clear that the studied compounds tend to be better correlated during the autumn to winter months (September to February). This is probably due to the greater observed homogeneity of the air as well as the increased emissions from heating appliances during the cold season. However, compared to other European cities with dense street traffic, such as Antwerp—where the correlation coefficient between benzene and toluene was close to unity [21], r 2 values in the Tri-City area were significantly lower, and ranged between 0.14 and 0.61 (Table 3). This suggests that street traffic is not the usual emission source for the studied compounds; other origins of the BTEX in urban air are possible.
The BTEX emissions from vehicle exhaust depend on the type of fuel used by the vehicle as well as the technology and type of catalyst system employed by the vehicle, and its speed. B/T emission ratios are often used to investigate the contribution of vehicle traffic to the total emission of BTEX into the ambient air.
A common method of determining the emission ratios of organic compounds is to calculate the slope of the linear fit to a scatter plot of the emissions of the two compounds. According to the literature, B/T emission ratios in vehicle exhaust range from 2 to 8 [23].
The B/T emission ratios obtained during the monitoring campaign by applying the Radiello passive samplers for sample collection ranged between 0.47 and 12.9 (Table 4), which is consistent with the B/T emission ratios reported in the literature (1.7–9.3 [24], 3–12 [25], and 0.7–3.8 [26]). However, except from the year 2008 (when B/T emission ratios were between 1.67 and 12.99), the B/T ratios were lower than unity. This suggests that the benzene in the ambient air of the Tri-City did not originate from the vehicle traffic. As confirmation of this statement, according to the literature, lower B/T emission ratios are typical of “background” areas with less dense street traffic. This corresponds well with the fact that all monitoring stations in the Tri-City were considered urban background stations [23].
Comparison of the concentrations of benzene obtained using the passive sampling technique and on-line monitoring
To validate the suitability of passive samplers for ambient air studies, a comparison of on-line monitoring and passive sampling was carried out.
The results (for monthly benzene concentration) obtained by an on-line monitor, the Chrompack CP 7001, employed at monitoring station number 10 (am10), were supplied by the ARMAAG Foundation. The Chrompack CP 7001 on-line monitor combines a sampling and an analytical system. Air is pumped through a cold trap filled with a sorbent to trap VOCs. The trap is heated and the analytes are transferred to a gas chromatograph coupled with FID detector for analysis. The resolution time is 1 h.
A comparison of the monthly benzene concentration profiles obtained using the passive sampling technique and the Chrompack CP 7001 on-line monitor are presented in Fig. 7 graphically.
The results obtained by passive sampling technique were slightly higher than those obtained using the on-line monitor (apart from in 2008), especially for the months from November to March. However, the seasonal profiles obtained for both techniques were similar. Good agreement between the results obtained with both of these techniques (active on-line monitoring and passive sampling), in terms of monitoring VOCs, has been found in several studies [27, 28]. However, due to the fact that meteorological conditions (e.g., temperature, wind, and humidity) can affect the behavior of passive samplers, the performance of this sampling technique should be investigated continuously (especially under real conditions) and compared to other methods.
In accordance with this statement, a statistical comparison of the monthly benzene concentrations determined with two different techniques—passive sampling and on-line monitoring—was realized via linear regression (Student’s t-test). The monthly benzene concentrations determined with the Radiello diffusive sampler [y(C passive)] were plotted versus those determined with the Chrompack CP 7001 on-line monitor (x(C on-line)] during 2008–2010. The obtained plot is presented in Fig. 8. Additionally, the y = x line (C passive = C on-line) representing the ideal scenario where there are no differences between the monthly benzene concentrations determined with the two different methods is also presented in Fig. 8. In the ideal scenario, the regression line should pass through the origin of the coordinate system (a = 0), and the slope b should be equal to unity (b = 1).
The statistical parameters obtained from the correlation analysis are presented in Table 4 separately for each monitoring year as well as for the whole three-year monitoring period.
It was found that at the probability level P = 95%, the condition t acalc a/S a ≤ t cr was fulfilled for the intercept a. Thus, it can be stated that the intercept a was not significantly different from the expected value of zero. This was observed for the years 2008–2010. In the case of the slope b, the dependence t acalc(1 − b)/S b ) ≤ t cr was not fulfilled for the regression line for the year 2010, which means that the slope b was significantly different from 1.
To summarize, the monthly benzene concentrations determined with the Radiello diffusive sampler and the Chrompack CP 7001 on-line monitor were not statistically different for the years 2008–2009. Taking the on-line technique as the reference technique, these results show that passive sampling is relatively useful as a tool for environmental studies. However, the differences between the monthly benzene concentrations determined with the Radiello diffusive sampler and the Chrompack CP 7001 on-line monitor obtained for 2010 were statistically significant due to the value of the slope b.
The influence of temperature on passive sampler performance
The lack of a strong statistical relationship between the results obtained by passive sampling and those given by the on-line monitor in our study may result from the strong dependence of diffusive passive sampler performance on the meteorological conditions. This strong influence of the temperature fluctuations as well as the varying wind speed on passive sampler uptake bahaviour is often described in the literature as being a huge drawback of this technique. However, quantification errors (of up to 50%) may also occur due to the nonideal behavior of the sorbents applied to sample the VOCs (e.g., Tenax TA) [27].
According to literature data, the sampling rate (Q) is the main factor influencing the uncertainty of the obtained results [29]. This is due to the fact that sampling rates are usually calculated in the laboratory, in exposure chambers, for concentrations that are different from those generally found in the environment, and without the interference of other organic compounds present in the air [23, 24].
According to Eq. 3, the sampling rate Q T, which determines the uptake rate of the passive sampler, depends on the diffusion coefficient of the organic compound in the gas phase:
- Q :
-
sampling rate (ml min−1)
- D :
-
diffusion coefficient (cm s−1)
- A :
-
surface of diffusion (cm2)
- L :
-
path length of diffusion (cm)
As diffusion coefficient depends on the temperature,
- D :
-
diffusion coefficient (cm s−1)
- D 298 :
-
diffusion coefficient for 298 K (cm s−1)
- T :
-
temperature (K),
the same dependence is observed for Q T (Eq. 2).
The effects of both the temperature and the wind speed effect on Radiello sampling rate values have been widely investigated by different scientists. According to French scientists, an increase in the sampling rate of the sampler is observed when the wind velocity increases in a logarithmic manner. The magnitude of this effect was evaluated as ±13% when the wind velocity ranged from 0.2 to 1.4 ms−1 [27, 30].
As the Q T values supplied by the manufacturer were evaluated for 25 °C, the appropriate adjustment for the actual temperature must be made. It is also important to note that Q T values were calculated for each exposure period based on the average monthly temperature.
The average monthly temperature supplied by the ARMAAG Foundation differed significantly from the daily temperature. Figure 9a presents the daily fluctuations in temperature noted in January 2010. The horizontal line in Fig. 9a 7reflects the average monthly temperature used to calculate Q T.
As presented in Fig. 9a, the average monthly temperature reported for January which was then applied to calculate Q T values was −5.6 °C, while the amplitude of the temperature fluctuation for this month was −13.6 °C, with a maximum temperature of 0 °C and a minimum temperature of −13.6 °C. Such strong monthly fluctuations in temperature may influence the performances of passive devices, affecting the response rate (response time) to changes in analyte concentration in the sampling zone. The fluctuations in Q T observed during the month of January 2010, which resulted from daily fluctuations in temperature, are presented in Fig. 9b. The horizontal line in Fig. 9b reflects the average Q T value obtained when using the average monthly temperature of −5.6 °C. The large amplitude of the Q T fluctuation [ranging from 21.8 (−13.6 °C) to 23.5 (0 °C)] obtained for daily values in January, when compared to the average Q T value, suggest that incorporating the average Q T value into the calculations of benzene concentration may lead to inaccurate results. This may explain the differences between the monthly benzene concentrations determined with the on-line monitor and the Radiello passive samplers (Fig. 7).
Due to the fact that benzene concentrations obtained by passive sampling were calculated using Q T values evaluated on the basis of the average monthly temperature and taking into account the monthly amplitude of the temperature fluctuation reported in January 2010, we made an attempt to empirically determine Q values by using concentrations obtained by the on-line analyzer as reference values. These Q on-line values were calculated according to the equation provided by the manufacturers of Radiello:
where:
- M :
-
mass of analytes retained on the sorbent bed (μg)
- C on-line :
-
concentration obtained by the on-line analyzer (μg m−3)
- t :
-
exposure time (s)
Additionally, by comparing the concentrations obtained by the passive sampling technique and the on-line technique (with the on-line technique used as the reference), we calculated corrected Q values (Q corrected) from the equation
Both Q on-line and Q corrected are higher than Q T, as expected from the concentrations obtained by the passive sampling technique. A comparison of the three values Q on-line, Q corrected, and Q T is given in Fig. 10.
The results obtained for 2009 correspond well with those obtained in other studies, where Q T values were calculated for benzene (for 14 days exposure) and ranged between 26.4 and 33.0 ml min−1 [24].
As a confirmation, some attempts to determine Q T values in exposure chambers have been reported. The results obtained under steady conditions, close to the standard conditions (25 °C and 1013 hPa), did not differ significantly from the values provided by the manufacturer [26–28]. A different situation occurred when the values were obtained under ambient conditions. The most significant difference was reported for when temperature fell below 0 °C, and Q T tended to differ significantly from the values evaluated under standard conditions. The correction for temperature performed for Q T each month does not fully reflect the monthly fluctuation in temperature and thus the fluctuation in Q T. This confirms that applying the average temperature to calculate Q T may lead to significant inaccuracy [28, 30–35].
According to the most recent data published on this issue, studies are being carried out to minimize the influence of meteorological conditions on passive sampler performance. Some attempts have been made to develop models for calculating Q T values for VOCs, which depend on the temperature and exposure period [31].
Conclusions
The concentration of benzene in ambient air can vary over time as well as spatially. Therefore, an appropriate analytical procedure for measuring benzene concentration levels must be developed in order to obtain reliable data. In recent years, passive sampling has gained broad attention as a tool that can be applied to monitor a wide range of organic compounds in ambient air.
The four year long monitoring campaign carried out from January 2007 to December of 2010 in the Tri-City area proved that passive sampling can be considered a useful sampling and preconcentration technique for monitoring organic compounds in urban air. This relatively simple and inexpensive technique allowed time-weighted average concentrations of analytes to be obtained, which may serve as a valuable source of long-term information on human exposure to VOCs.
On-line monitoring instruments also have the ability to obtain concentrations of organic compounds as well as short- and long-term air-quality trends in urban air. However, due to practical constraints (e.g., cost of maintaining the equipment, the need for highly qualified supervision, etc.), the use of automatic analyzers is restricted to a limited number of roadside and background locations within a city. In such cases, especially in remote areas, low-cost passive samplers can be used to characterize the spatial variability of air pollution [36].
The research carried out in the four-year monitoring campaign described above confirmed the ability of Radiello passive samplers to show the fluctuations in benzene concentrations in urban air, making them useful tools for monitoring at the local scale. The monitoring campaign yielded seasonal concentration profiles for benzene in ambient air. This information should serve as a useful database for further research, such as modeling the transport of benzene in ambient air.
However, the fact that experimentally determined Q T values were higher than those calculated under standard conditions showed the strong dependence of passive sampler performance on meteorological conditions. This limitation of the passive sampling technique requires further study, so that more knowledge is attained on the issue of passive sampler uptake rate behavior as well as the impact of real environmental conditions (e.g., temperature fluctuations, varying wind speed) on sampling rate values.
Abbreviations
- ARMMAG:
-
Agency of Regional Air Quality Monitoring Foundation in the Gdańsk Metropolitan Area
- ASTDR:
-
American Agency for Toxic Substances and Disease Registry
- BTEX:
-
Benzene, toluene, ethylbenzene, xylenes
- GC:
-
Gas chromatography
- IARC:
-
International Agency for Research on Cancer
- MS:
-
Mass spectrometer
- SR:
-
Sampling rate
- TD:
-
Thermal desorption
- VOC:
-
Volatile organic compounds
- WHO:
-
World Health Organization
References
World Health Organization (2005) The World Health Report 2005: make every mother and child count. World Health Organization, Geneva. http://www.who.int/whr/2005/overview_en.pdf. Accessed 8 Sept 2011
Atkinson R (2000) Atmos Environ 34:12–14
Agency for Toxic Substances and Disease Registry (2011) Toxic Substances Portal: benzene. Agency for Toxic Substances and Disease Registry, Atlanta. http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=14. Accessed 18 Sept 2011
EC (2000) Directive 2000/69/EC of the European Parliament and of the Council of 16 November 2000 relating to limit values for benzene and carbon monoxide in ambient air.Off J Eur Commun L313:12–21. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0069:EN:HTML. Accessed 19 Sept 2011
Wong O, Harris F, Armstrong TW, Hua F (2010) Chem Biol Inter 184:112–128
IARC (1998) Benzene (Group 1). International Agency for Research on Cancer, Lyon. http://www.inchem.org/documents/iarc/suppl7/benzene.html. Accessed 18 Sept 2011
Christensen JS, Elton J (1996) Soil and groundwater pollution from BTEX. Civil Eng Dept, Virginia Tech, Blacksburg. http://www.cee.vt.edu/ewr/environmental/teach/gwprimer/btex/btex.html. Accessed 19 Sept 2011
US EPA (2010) Summary and analysis of the 2010 gasoline benzene pre-compliance reports. US EPA, Washington, DC. http://www.epa.gov/otaq/regs/toxics/420r10029.pdf. Accessed 18 Sept 2011
Zabiegała B, Kot-Wasik A, Urbanowicz M, Namieśnik J (2010) Anal Bioanal Chem 396:273–96
Fondazione Salvatore Maugeri (2008) Radiello homepage (English version). Fondazione Salvatore Maugeri, Pavia. http://www.radiello.it/english/index_en.html. Accessed 18 Sept 2011
Bruno P, Caputi M, de Gennaro G, de Rienzo M (2005) Atmos Environ 39:1347–1355
Pennequin-Cardinal A, Plaisance H, Locoge N, Ramalho O, Kirchner S, Gallo J-C (2005) Atmos Environ 39:2535–2544
Brooks Mason J, Fujita EM, Campbell DE, Zielińska B (2011) Environ Sci Tech 45:2243–2249
Plaisance H, Leonarids T, Gerboles M (2008) Atmos Environ 42:2555–2568
Indigo Sp. z o.o., Aqurat Sp. z o.o., Geosystems Polska Sp. z o.o. (2011) Targeo.pl Mapa Polski. http://www.targeo.pl. Accessed 20 Sept 2011
Zabiegala B, Urbanowicz M, Namiesnik J, Górecki T (2010) J Environ Qual 39:896–906
Urbanowicz M, Zabiegala B, Namiesnik J (2011) Anal Bioanal Chem 399:277–300
Zabiegała B, Sarbu C, Urbanowicz M, Namiesnik J (2010) J Air Waste Manag Assos 61:260–269
Monod A, Sive BC, Avino P, Chen T, Blake DR, Sherwood Rowland F (2001) Atmos Environ 35:135–149
Casell M, de Gennaro G, Marzocca A, Trizio L, Tutino M (2010) Chemosphere 81:308–311
Buczynska AJ, Krata A, Stranger M, Locateli Godoi AL, Kontozova Teutsch V, Bencs L, Naveau I, Roekens E, van Grieken R (2009) Atmos Environ 43:311–318
Parra MA, Gonzalez L, Elustondo D, Garrigo J, Bermejo R, Santamaria JM (2006) Sci Total Environ 370:157–167
Muren F (2007) J Environ Sci 19:578–583
Lee SC, Chiu MY, Ho KF (2002) Chemosphere 48:375–382
Yamamoto N, Okayasu H, Murayama S (2000) Atmos Environ 34:4441–4446
Pilidis GA, Karakitsios SP, Kasosmenos PA (2005) Atmos Environ 39:6051–6065
Walgraeve Ch, Demesteere K, Dewulf J, van Huffel K, van Langenhove H (2011) Atmos Environ 45:5872–5879
Gallego E, Roca FJ, Perales JF, Guardino X (2011) Talanta 85:662–672
Zabiegała B, Namieśnik J (2007) Use of permeation passive samplers in air monitoring. In: Greenwood R, Mills G, Vrana B (ed) Passive sampling techniques in environmental monitoring (Comprehensive Analytical Chemistry, vol 48). Elsevier, Amsterdam, pp 85–106
Roukos J, Riffault V, Locoge N, Plaisance H (2009) Environ Pollut 157:3001–3009
Oury B, Lhuillier F, Protois J-C, Moréle Y (2006) J Occup Environ Hyg 3:547–557
Pilidis GA, Karakitsios SP, Kassomenos PA (2005) Atmos Environ 39:6051–6065
Barro R, Regueiro J, Llompart M, García-Jares C (2009) J Chromatogr A 1216:540–566
Harper M (2000) J Chromatogr A 885:129–151
Cocheo C, Boaretto C, Pagani D, Quaglio F, Sacco P, Zaratin L, Cottica D (2009) J Environ Monit 11:297–306
Vardoulakis S, Solazzo E, Lumbreas J (2011) Atmos Environ 45:5069–5078
Acknowledgements
The authors are grateful to the Agency of Regional Air Quality Monitoring Foundation in the Gdańsk Metropolitan Area (ARMAAG) for access to the monitoring stations, as well as to Michalina Bielawska for providing meteorological data during the whole monitoring campaign.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Euroanalysis XVI (The European Conference on Analytical Chemistry) with guest editor Slavica Ražić.
Rights and permissions
About this article
Cite this article
Król, S., Zabiegała, B. & Namieśnik, J. Measurement of benzene concentration in urban air using passive sampling. Anal Bioanal Chem 403, 1067–1082 (2012). https://doi.org/10.1007/s00216-011-5578-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5578-y