Abstract
A simple and efficient method, based on ultrasound-enhanced surfactant-assisted dispersive liquid–liquid microextraction (UESA-DLLME) followed by high-performance liquid chromatography (HPLC) has been developed for extraction and determination of ketoconazole and econazole nitrate in human blood samples. In this method, a common cationic surfactant, cetyltrimethylammonium bromide (CTAB), was used as dispersant. Chloroform (40 μL) as extraction solvent was added rapidly to 5 mL blood containing 0.068 mg mL−1 CTAB. The mixture was then sonicated for 2 min to disperse the organic chloroform phase. After the extraction procedure, the mixture was centrifuged to sediment the organic chloroform phase, which was collected for HPLC analysis. Several conditions, including type and volume of extraction solvent, type and concentration of the surfactant, ultrasound time, extraction temperature, pH, and ionic strength were studied and optimized. Under the optimum conditions, linear calibration curves were obtained in the ranges 4–5000 μg L−1 for ketoconazole and 8–5000 μg L−1 for econazole nitrate, with linear correlation coefficients for both >0.99. The limits of detection (LODs, S/N = 3) and enrichment factors (EFs) were 1.1 and 2.3 μg L−1, and 129 and 140 for ketoconazole and econazole nitrate, respectively. Reproducibility and recovery were good. The method was successfully applied to the determination of ketoconazole and econazole nitrate in human blood samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ketoconazole and econazole nitrate are azole antifungal pharmaceuticals used for systemic and local infections. They inhibit ergosterol biosynthesis and change the composition of cell membrane lipoid substances [1–4]. Clinical studies have revealed that, in the event of substantial amounts of the residues in the human body, azoles participate in interactions with many drugs [5–7] and thus pose a possible threat to human health. It is, therefore, necessary to perform therapeutic drug monitoring of azoles in human blood, for which rapid, accurate, and simple methods are indispensable.
Many analytical methods have been used to determine azole antifungal pharmaceuticals, including high-performance chromatography [8–10], spectrofluorimetry [11], capillary zone electrophoresis [12], and high-performance thin-layer chromatography (HPTLC) [13]. Because of the complexity of biological matrices and the low levels of the target analytes in the samples, sample pretreatment is required. Numerous sample-preparation methods have been proposed for extraction and preconcentration of azole residues, including liquid–liquid extraction (LLE) [14], ultrasonic extraction (UE) [15], and solid-phase extraction (SPE) [16, 17]. However, most of these require large amounts of hazardous solvents, which are potentially harmful to human health. Hence, to solve the problem, much effort is being devoted to development of miniaturized extraction techniques, for example liquid-phase microextraction (LPME) [18] and solid-phase microextraction (SPME) [19, 20], which not only reduce organic solvent consumption but also improve extraction efficiency. However, the fiber in SPME is quite expensive and the polymer coating is fragile, so LPME techniques, for example single-drop microextraction [21], solvent-bar microextraction [22], continuous-flow LPME [23], and dynamic LPME [24] have recently attracted increasing interest.
Very recently, a novel LPME technique, termed dispersive liquid–liquid microextraction (DLLME), was developed and introduced by Assadi et al. [25]. The method is based on a ternary component solvent system in which an appropriate mixture of organic extractant and dispersive solvents is rapidly injected into the aqueous sample by means of a syringe, resulting in a cloudy solution. In fact, the aforementioned ternary component solvent system consists of fine particles of extraction solvent which are dispersed throughout aqueous phase. This method has many advantages (simplicity of operation, speed, low cost, and high extraction efficiency), and many researchers have attempted to explore alternatives to further improve it. This led Huang et al. to develop the ultrasound-assisted dispersive liquid–liquid microextraction method (UDLLME) [26] on the basis of DLLME, shortening the extraction time by use of ultrasonic radiation. Yamini et al. used surfactant-assisted dispersive liquid–liquid microextraction (SA-DLLME) for sample preparation in the analysis of chlorophenols in water samples [27]. Surfactants are organic compounds that contain both hydrophobic and hydrophilic groups. Therefore they are soluble in both organic solvent and water. From the standpoint of solubility in both organic and aqueous phases, there is a similarity between the disperser solvent in DLLME and surfactant. Furthermore, surfactants can reduce interfacial tension and, hence, increase the contact area between the organic and water phases. So extraction efficiency can be greatly improved.
The objective of this work was to develop a simple and efficient method called ultrasound-enhanced surfactant-assisted dispersive liquid–liquid microextraction (UESA-DLLME) by combining the advantages of UDLLME and SA-DLLME. We applied the method to the extraction and determination of ketoconazole and econazole in blood samples by using high-performance liquid chromatography–diode array detection (HPLC–DAD). To the best of our knowledge, this is the first report of the development and application of UESA-DLLME for simultaneous determination of two azole antifungal pharmaceuticals in blood samples.
Experimental
Chemical and materials
Ketoconazole and econazole nitrate were obtained from Baijingyu Pharmaceutical (Nanjing, China). Chloroform, dichloromethane, chlorobenzene, and tetrachloromethane of analytical-reagent grade were purchased from Tokyo Chemical Industry (Tokyo, Japan). Tween 80, Triton X-100, sodium dodecyl sulfate (SDS), and cetyltrimethylammonium bromide (CTAB) were chemically pure and purchased from Sinopharm Chemical Reagent (Shanghai, China). HPLC-grade methanol and acetonitrile were obtained from Tedia, USA. Sodium chloride was procured from Zhanyun Chemical (Shanghai, China). The water used was ultrapure (Millipore Simplicity 185, Billerica, MA, USA).
Preparation of standard solutions
A mixed stock solution containing ketoconazole and econazole nitrate at 100 μg mL−1 was prepared in methanol. A series of standard solutions was obtained by further diluting the stock solution with diluted blank serum (300 μL blank serum was diluted to 5 mL with ultrapure water ). All solutions were stored at 4°C.
Instruments
HPLC analysis was performed with an Agilent 1200 liquid chromatograph (Agilent Technologies, USA) equipped with an online degasser, a quaternary pump, a diode array detector (DAD), a 20-μL sample loop, and an analytical ChemStation. An Agilent HC-C18 column (250 mm × 4.6 mm, 5 μm) was used for separation of the analytes. The mobile phase was a mixture of acetonitrile–water (75:25, v/v) and the flow rate was 1.0 mL min−1. The column temperature was 25°C and the detection wavelength was 220 nm. The injection volume was 10 μL. Ultrasonic instrument KQ-100DE was purchased from Kunshan Ultrasonic Instrument (Jiangsu, China) and an 80–2 centrifuge used for phase separation was produced by Changzhou Guohua Electric Appliance (Jiangsu, China).
UESA-DLLME procedure
In the procedure, 5 mL sample solution was placed in a 10-mL glass conical tube. The pH of the sample solution was adjusted to an appropriate value (pH 6) by use of NaH2PO4–NaOH buffer solution. Chloroform as extraction solvent (40 μL) and CTAB as disperser (the concentration of CTAB in the 5 mL sample solution was 0.068 mg mL−1) were then added to the sample solution. The mixture was sonicated for 2 min at 25 ± 2°C. An emulsion was formed in the conical tube. The tube was then centrifuged for 3 min at 1,500 g and the organic phase sedimented to the bottom of the centrifuge tube. The sedimented organic phase was entirely transferred into another conical tube by use of a 20-μL HPLC syringe, and then evaporated to dryness under a stream of nitrogen (99.999%). The residue was dissolved in 40 μL acetonitrile and 10 μL was injected for HPLC analysis.
Blood sample preparation
Blood samples from five healthy people and ten patients treated with the two azole antifungal pharmaceuticals were obtained from Wuhan Eleventh Hospital. (Hubei, China). Ethical approval for the study was obtained from the Ethics Committee of Wuhan Eleventh Hospital before collection and analysis of human blood samples. The blood samples were stored at 4°C and were not further pretreated before use. In the serum analysis, 500 μL acetonitrile was added to 100 μL serum to remove the protein and some impurities. The serum supernatant (300 μL) was diluted to 5 mL with ultrapure water as the sample solution for simultaneous determination of ketoconazole and econazole. The extraction procedure was the same as that described above for UESA-DLLME.
Results and discussion
In optimization of UESA-DLLME method, different experimental conditions were investigated, for example type and volume of extraction solvent, type and concentration of surfactant, pH, ultrasound extraction time, and the salt effect. All the experiments were performed in triplicate.
Effect of type and volume of extraction solvent
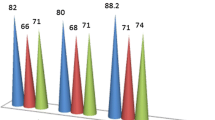
Selecting an appropriate extractant is essential for UESA-DLLME. It should have low solubility in water, higher density than water, high affinity for the analytes, and should form a stable emulsion with the emulsifier under the action of ultrasound. Four solvents with these properties were tested: chlorobenzene, chloroform, dichloromethane, and tetrachloromethane. The extraction solvent creates a cloudy solution when the container is shaken in the presence of cationic surfactant. The compatibility of these solvents with the UESA-DLLME technique was studied by adding 50 μL of each solvent to 5 mL sample solution containing 200 μg L−1 azoles and 0.102 mg mL−1 CTAB as dispersing agent. The mixture was sonicated for 2 min to complete the extraction process. After centrifugation of the cloudy solutions, 20 μL of the separated organic phase was collected for subsequent analysis. The enrichment factors (EFs) obtained by use of different solvents are shown in Fig. 1. The results showed that chloroform has the highest EFs. Therefore, chloroform was selected as extraction solvent in subsequent experiments.
Effect of extraction solvent (chloroform) on extraction efficiency. Extraction conditions: sample volume 5.0 mL, 0.102 mg mL−1 CTAB, room temperature, no salt addition, ultrasonic extraction time 2 min, spike level 200 μg L−1. Error bars represent the standard deviation of the mean peak area for n = 3 replicates
The effect of chloroform volume on extraction efficiency for ketoconazole and econazole nitrate was also investigated. In the experiment, different volumes of chloroform (30, 40, 50, 60, or 70 μL) were added to 5 mL sample solution containing 0.102 mg mL−1 CTAB. As shown in Fig. 2, the EFs increased with increasing extractant volume from 30 to 40 μL, and then decreased with increasing extractant volume from 40 to 70 μL. As a result, 40 μL chloroform was selected for subsequent work.
Effect of extraction solvent (chloroform) volume on extraction efficiency. Extraction conditions: sample volume 5.0 mL, 0.102 mg mL−1 CTAB, other conditions as for Fig. 1
Effect of type and concentration of surfactant
The choice of surfactant is of great importance to achieving a satisfactory preconcentration and extraction of analytes. Surfactant, which serves as an disperser, could accelerate dispersion of the water-immiscible extraction solvent into the aqueous solution under ultrasound irradiation. After dispersion, the extraction solvent is dispersed as fine droplets in the sample solution, which favors mass transfer of the analytes from the aqueous phase to the organic phase. The effect of different surfactants (Tween 80, Triton X-100, SDS, CTAB) on the EFs is shown in Fig. 3. Among the surfactants investigated, SDS gave the lowest EFs for the analytes. Tween 80 and Triton X-100 gave comparable results for extraction of the analytes, and 0.102 mg mL−1 CTAB resulted in the highest EFs for both ketoconazole and econazole nitrate. This might be because ketoconazole and econazole nitrate are acidic and are most compatible with the cationic surfactant (CTAB). SDS is an anionic surfactant with greater hydrophilicity. Tween 80 and Triton-100 are polyoxyethylene-type nonionic surfactants. The higher hydrophilic–lipophilic balance (HLB) value means greater hydrophilicity. When the HLB value of a surfactant is between 8 and 18, the surfactant can be used as a disperser. The HLBs of Tween 80, Triton X-100, CTAB, and SDS are 15.0, 13.4, 15.8 and 40, respectively. So, SDS is not suitable for use as disperser. On the basis of the experimental and analytical results, CTAB was chosen as surfactant for further studies.
Effect of surfactant on extraction efficiency. Extraction conditions: sample volume 5.0 mL, extraction solvent volume 50 μL, surfactant (CTAB, SDS, Tween 80, TX-100) volume 300 μL, other conditions as for Fig. 1
The concentration of the surfactant is another important conditions that could affect extraction efficiency. The experimental results showed that if the concentration of CTAB was higher than 0.202 mg mL−1 (its critical micelle concentration is 0.337 mg mL−1 [27]), almost no phase separation occurred after centrifugation and no settled droplet appeared. This result occurred because the surface tension between the aqueous and organic phases decreased with increasing surfactant concentration, and the extraction droplet was soluble in the sample solution. Thus, the effect of CTAB was investigated at concentrations of 0.034, 0.068, 0.102, 0.136, and 0.17 mg mL−1. As shown in Fig. 4, the EFs increased with increasing CTAB concentration from 0.034 to 0.068 mg mL−1 then decreased with increasing CTAB concentration from 0.068 to 0.17 mg mL−1. As a result, the most suitable surfactant concentration was 0.068 mg mL−1, which was chosen for further studies.
Effect of the amount of surfactant on extraction efficiency. Extraction conditions: sample volume 5.0 mL, extraction solvent volume 40 μL, other conditions as for Fig. 1
Effect of pH
Sample pH value is important in the transfer of the target analytes into the organic phase in many LPME methods. Because of the weak alkalinity of azoles. (ketoconazole pK a1 = 2.9, pK a2 = 6.5, [1] and econazole (pK a = 6.6, [28]), and the importance of the effect of pH on their extraction, the effect of pH was investigated in the range 2–10. As can be seen in Fig. 5, the best extraction efficiency was obtained at pH 6. Enrichment factors increased as the pH was increased from 1 to 6, but decreased slightly from pH 6 to 10. It seems that both neutral and ionized azoles were efficiently extracted into the organic phase. Extraction of neutral azoles into the organic phase is reasonable, because of the normal interactions, but the extraction of deprotonated charged species looks like an interesting phenomenon which might be a result of ion-pair formation between cationic surfactant and deprotonated analytes. pH 6 was therefore chosen for subsequent studies.
Effect of pH on extraction efficiency. Extraction conditions: sample volume 5.0 mL, extraction solvent volume 40 μL, 0.068 mg mL−1 CTAB, other conditions as for Fig. 1
Salt effect
In general, addition of sodium chloride to an aqueous solution increases its ionic strength, which reduces the solubility of the analytes in the sample solution and improves EF values. Therefore, the sodium chloride concentration was varied between 0 and 10% (w/v), and the effect on the EF was observed. The results indicated that EFs decreased as the salt concentration increased from 0 to 10%. This might be because addition of salt led to the reduction of the solubility of the extraction solvent in the sample solution and hence reduced the EFs. Thus, no salt was added in this method.
Effect of extraction temperature and ultrasonic extraction time
Temperature can affect both mass transfer and emulsification, thus affecting extraction efficiency. Different temperatures ranging from 25 to 45°C were studied to determine the effect of extraction temperature. No significant differences were found among the results in this range of temperatures, possibly because emulsification and equilibrium of mass transfer were easily achieved under the combined effect of the ultrasound waves and surfactant. For convenience, the extraction procedure was performed at room temperature (25 ± 2°C).
The ultrasound extraction time is important in emulsification and mass transfer. The ultrasound extraction time was defined as the time between addition of the extraction solvent (chloroform) and the end of sonication. The effect of ultrasound extraction time on the EFs was examined by varying the ultrasound time from 0 to 10 min. The EFs increased with increasing ultrasound time in the range 0–2 min and remained almost constant after 2 min. Therefore, 2 min was chosen as the most appropriate ultrasound extraction time.
Method validation
Linearity, repeatability, LODs, and LOQs
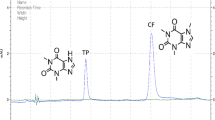
Under the optimum conditions, working solutions containing the two azole pharmaceuticals at seven different concentrations were extracted and analyzed to construct calibration curves. The calibration data obtained are listed in Table 1. Linear calibration plots were obtained in the range 4 to 5000 μg L−1 for ketoconazole and 8 to 5000 μg L−1 for econazole nitrate. The limits of detection (LODs), calculated as three times the signal-to-noise ratio (S/N), were 1.1 and 2.3 μg L−1 for ketoconazole and econazole nitrate, respectively. The limits of quantification (LOQs, S/N = 10) were 3.7 and 7.8 μg L−1 for ketoconazole and econazole nitrate, respectively. When the repeatability of the method was measured by performing five parallel experiments, relative standard deviations (RSDs) ranged from 5.2% to 7.8 %. Recoveries of ketoconazole and econazole nitrate spiked at two concentrations (10 μg L−1, 40 μg L−1) in plasma were calculated; the results are shown in Table 2. Typical chromatograms obtained from blank and spiked blood samples treated by UESA-DLLME are illustrated in Fig. 6.
Application to real samples
UESA-DLLME was used to determine ketoconazole and econazole nitrate in human blood (control samples and treated samples). Blood samples from ten patients treated with the two pharmaceuticals and five control samples were analyzed. The results summarized in Table 3 indicate that the concentrations of ketoconazole and econazole nitrate detected in the blood of patients ranged from 5.6 to 16.4 μg L−1 and from 9.8 to 17.6 μg L−1, respectively. However, ketoconazole concentrations in normal people are in the range 4.3 to 7.6 μg L−1, and econazole nitrate cannot be detected. This implies that ketoconazole is retained more easily than econazole nitrate by human blood.
Comparison with other methods
In Table 4 the performance of the UESA-DLLME method is compared with that of other methods reported for determination of azoles. The linearity of the UESA-DLLME method was comparable, LODs were much lower, and enrichment factors for the analytes were higher. Moreover, less organic extraction solvent is required in the proposed method. In addition, the results showed that surfactant as disperser was a favorable factor for extraction efficiency. So, UESA-DLLME was proved to be suitable for determination of ketoconazole and econazole nitrate in human blood.
Conclusions
In the work discussed in this paper, a simple UESA-DLLME method combined with HPLC–DAD was developed for rapid determination of ketoconazole and econazole nitrate in complex blood samples. An ultrasound-enhanced surfactant-assisted microextraction process was used to shorten the extraction time and accelerate dispersion of the extraction solvent into the sample solution, which led to enhanced extraction efficiency. The experimental results indicated that the method could provide low LOD, a good linear range, and good enrichment factors within a very short time. In addition, recovery of the analytes spiked at two levels was >80%. Therefore, the method proved to be a useful tool for rapid determination of the two azoles in clinical pharmaceutical analysis.
References
Beggs WH (1991) Mycopathologia 116:3–4
Zarn JA, Bruschweiler BJ, Schlatter JR (2003) Environ Health Perspect 111:255–261
Abu-Elteen KH, Hamad M, Kavanagh K (ed) (2005) Fungi Biology and Applications, Wiley, UK, pp 191–217
Gaona-Galdosa AA, Filho LAZ, Tavares MFM, Aurora-Pradoa MS, Santoro MIRM, Kedor-Hackmanna ERM (2008) J Chromatogr A 1192:301–305
Levêque D, Nivoix Y, Jehl F, Herbrecht R (2006) Int J Antimicrob Agents 27:274–284
Farhadi Kh, Maleki R (2002) J Pharm Biomed Anal 30:1023–1033
Gordien JB, Pigneux A, Vigouroux S, Tabrizi R, Accoceberry I, Bernadou JM, Rouault A, Saux MC, Breilh D (2009) J Pharm Biomed Anal 50:932–938
Vertzoni MV, Reppas C, Archontaki HA (2006) J Chromatogr B 839:62–67
Gagliardi L, De Orsi D, Chimenti P, Porrá R, Tonelli D (2003) Anal Sci 19:1195–1197
Kim SS, Im HT, Kang IM, Lee HS, Lee HW, Cho SH, Kim JB, Lee KT (2007) J Chromatogr B 852:174–179
Khashaba PY, El-Shabouri SR, Emara KM, Mohamad AM (2000) J Pharm Biomed Anal 22:363–376
Crego AL, Marina ML, Lavandera JL (2001) J Chromatogr A 917:337–345
Parmar P, Mehta A (2009) J Pharm Sci 71:451–454
Brodie RR, Chasseaud LF, Walmsley LM (1978) J Chromatogr A 155:209–213
Chao L (2001) Int J Cosmetic Sci 23:183–188
Chiap P, Hubert Ph, Crommen J (2002) J Chromatogr A 948:151–161
Abdel-Moety EM, Khattab FI, Kelani KM, AbouAl-Alamein AMO (2002) Il Farmaco 57:931–938
Guo XM, Mester Z, Sturgeon RE (2002) Anal Bioanal Chem 373:849–855
Wen Y, Wang Y, Feng YQ (2007) Anal Bioanal Chem 388:1779–1787
Saraji M, Bakhshi M (2005) J Chromatogr A 1098:30–36
Jiang XM, Lee HK (2004) Anal Chem 76:5591–5596
Liu WP, Lee HK (2000) Anal Chem 72:4462–4467
Hou L, Lee HK (2003) Anal Chem 75:2784–2789
Berijani S, Assadi Y, Anbia M, Hosseini MRM, Aghaee E (2006) J Chromatogr A 1123:1–9
Rezaee M, Assadiab Y, Hosseinia MRM, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1–9
Wu QH, Chang QY, Wu CX, Rao H, Zeng X, Wang C, Wang Z (2010) J Chromatogr A 1217:1773–1778
Morteza M, Yadollah Y, Ali E, Shahram S (2010) Talanta 82:1864–1869
http://www.druginfosys.com/Drug.aspx?drugCode=271&drugName=Econazole (Nitrate)&type = 0
Acknowledgements
The project was supported by the National Natural Science Foundation of China (no. 30971948) and the China Scholarship Council (no. 2010677504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Y., Zhi, X., Wang, X. et al. Ultrasound-enhanced surfactant-assisted dispersive liquid–liquid microextraction and high-performance liquid chromatography for determination of ketoconazole and econazole nitrate in human blood. Anal Bioanal Chem 402, 1241–1247 (2012). https://doi.org/10.1007/s00216-011-5508-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5508-z