Abstract
Even though carrot allergy is common in Europe, the amount of different allergens in carrots is still unknown due to a lack of methods for quantitative allergen measurements. The current study aimed at the development of quantitative ELISA tests for the known carrot allergens, namely Dau c 1.01, Dau c 1.02, and Dau c 4 in pure carrot extracts. Monoclonal antibodies targeting the major carrot allergen isoforms Dau c 1.01 and Dau c 1.02 were generated and combined in sandwich ELISA with rabbit antisera against Api g 1, the celery homologue of Dau c 1. A competitive ELISA for the carrot profilin Dau c 4 was based on a polyclonal rabbit antiserum. The three ELISA tests were allergen-specific and displayed detection limits between 0.4 and 6 ng allergen/ml of carrot extract. The mean coefficient of variation (CV) as a means of intraassay variability of the Dau c 1.01, Dau c 1.02 and Dau c 4 ELISA tests was 8.1%, 6.9%, and 11.9%, and the mean interassay CV 13.3%, 37.1% and 15.6%, respectively. Target recovery ranged between 93 and 113%. In conclusion, the specific, accurate and reproducible quantification of three important carrot allergens may help to identify less allergenic carrot varieties, as well as to standardize the amount of allergens in extracts used for carrot allergy diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The structural homology between allergenic pollen proteins and specific food proteins often results in pollen-associated food allergies, caused by cross-reactive IgE antibodies that are primarily induced by the pollen allergens. In fact, 50–80% of birch pollen allergic patients also develop food allergy to fruits, nuts, and vegetables [1].
A population study that investigated the prevalence of adverse reactions to foods in Berlin, Germany, showed by means of skin prick testing that most allergic subjects are sensitized to apple, followed by hazelnut, and carrot [2]. Based on the results of this study, a general food allergy prevalence of 3.6% among the German population was extrapolated [2]. Nearly 55% of these food allergic patients reacted to vegetables, mainly to carrots and celery, which both belong to the Umbelliferae family (Apiaceae) and are phylogenetically closely related. Others reported a 30% rate of sensitization of food allergic subjects to celery in France [3] and around 40% to celery and up to 20% for carrots in Switzerland [4].
For Central Europe, carrot allergy was estimated to occur in approximately 25% of food allergic individuals [5]. The most frequent symptoms belong to the so-called oral allergy syndrome, merely mild allergic reactions, such as pruritus, tickling, and swelling of the oral mucosa. However, also cases of more severe allergic reactions to carrots have been described, displaying symptoms such as urticaria, angioedema, and even dyspnea and anaphylaxis [6–14].
In the majority of cases, Dau c 1, the major carrot allergen, was identified as the causative allergen. It belongs to the pathogenesis related protein 10 (PR-10) family, showing high sequence identity and structural homology to the birch pollen major allergen Bet v 1 [15]. Two isoforms of the homologous carrot allergen are known, namely Dau c 1.01 and Dau c 1.02, that only share around 50% amino acid sequence identity to each other and around 40% with Bet v 1, respectively. In contrast, the isoform Dau c 1.01 is 81% identical with the major allergen of celery, Api g 1.01, and Dau c 1.02 shows approximately 95% identity with its homologue in celery, Api g 1.02.
Another important carrot allergen is Dau c 4, a member of the pan-allergenic protein family of profilins [16], which has been shown to bind IgE of around 20% of carrot-allergic individuals [6]. Dau c 4 shows a high level of amino acid sequence identity with many other plant profilins, including the celery profilin Api g 4 (91%) and the birch pollen profilin Bet v 2 (81%), respectively.
A limited number of methods for the detection and quantification of individual allergens in pollen [17, 18] or food extracts [19, 20] have been developed and published within the last years.
Despite the large number of carrot-allergic subjects in Europe, there is still a lack of quantitative methods to determine the amount of the known allergens in carrot. Consequently, the portion and ratio of single allergens in carrots or extracts thereof is largely unknown.
Thus, this paper deals with the development and validation of ELISA tests for the quantification of the carrot allergens Dau c 1 and Dau c 4, respectively. Previously, it was shown that the isoforms of Dau c 1 [21] or its homologues in celery [22] display a distinct pattern of IgE reactivity. Hence, we further aimed at developing specific ELISAs to distinguish in a quantitative manner between the two isoforms Dau c 1.01 and Dau c 1.02 of the carrot major allergen Dau c 1.
Material and methods
Recombinant carrot allergens for ELISA standardization
The isoforms of the major carrot allergen Dau c 1, i.e., Dau c 1.0104 (GenBank accession No. Z81362) and Dau c 1.0201 (accession No. AF456481), as well as the carrot profilin Dau c 4 (GenBank accession No. AF456482), were expressed in Escherichia coli and purified as previously described [21]. All three recombinant allergens were highly pure and, for each protein, circular dichroism spectroscopy indicated a characteristic content of secondary structure elements (data not shown).
Antibodies
Production of Dau c 1 specific mouse mAbs
Five- to six-week-old female mice (BALB/c, Harlan–Winkelmann, Borchen, Germany) were immunized six times i.p. biweekly with 1.0 μg/dose of an equimolar mixture of rDau c 1.0104 and rDau c 1.0201 using alum as adjuvant. At week 12, sera were collected, and the IgG response was determined as previously described [23]. Four mice with high IgG-titres were sacrificed, spleen cells were collected and hybridomas were generated by conventional hybridoma technology using X63Ag8.653 cells (DSMZ, Germany) as fusion partner and peritoneal macrophages of Balb/c mice as feeder cells [24]. Antibody production by hybridomas was screened by ELISA and clones were selected according to their ability to distinguish between the two Dau c 1 isoforms. Briefly, microtitre plates were coated with either 0.5 μg/ml rDau c 1.0104 or rDau c 1.0201. Hybridoma cell culture supernatants were applied at a dilution of 1:3 and isoform-specific IgG was detected with a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Sigma A3673) and 3,3′,5,5′- tetramethylbenzidine (TMB) as the colorimetric substrate (details below).
Finally, two out of six isolated monoclonals were used for the development of sandwich ELISA tests specific for Dau c 1.01 and Dau c 1.02, respectively (Table 1).
Rabbit antibodies
For the immunological detection of Dau c 1, two previously generated rabbit antisera against Api g 1, the celery homologue of Dau c 1, one specifically recognizing isoform Api g 1.01 and the other isoform Api g 1.02, were available. Furthermore, a polyclonal rabbit antiserum, raised against a mixture of recombinant Api g 1.01 and Api g 1.02, that recognizes both isoforms of Api g 1, as well as a polyclonal rabbit antiserum generated against Lol p 12, the profilin of perennial ryegrass, were applied in this study (Table 1).
Extraction of carrot samples
Two independent carrot samples (A and B) of the variety “Fuellhorn” were purchased at a local German retailer. Using a vegetable processor (“Cuocojet”, GAM S.G.I., Igea Marina, Italy), carrots from each sample were cut into 3 mm slices and immediately frozen in liquid nitrogen. The quick-frozen carrot disks were ground under liquid nitrogen to a powder by an analytical mill (Grindomix GM 200, Retsch GmbH&Co.KG, Haan, Germany) in a pre-cooled beaker.
Five grams of frozen carrot powder was suspended in 10 ml PBS buffer (10 mM, pH 7) and extracted by overhead rotation for 1 h at 4 °C. Extracts were clarified by 30-min centrifugation at 10,000×g and 4 °C, and by additional supernatant filtration through 5- and 1.2-μm cellulose acetate filters. Instantly shock-frozen aliquots were either stored at −80 °C or lyophilized and stored at −20 °C.
Protein quantification of allergen standards and carrot extracts
Total protein of carrot extracts or recombinant allergen standards was quantified according to the method of Bradford [25] with a commercial ready-to-use reagent (Roti-Nanoquant protein assay solution, Carl Roth, Karlsruhe, Germany).
Sandwich ELISA for Dau c 1.01
Unless otherwise noted, all incubations were carried out for 1 h at room temperature using 100 μl volumes per microtitre plate (MTP) well. All dilutions following the coating step were done with incubation buffer (PBS, 0.05% Tween 20, 0.1% BSA) and all washings between the incubation steps with PBST (PBS, 0.05% Tween 20) at ambient temperature.
A sheep anti-mouse Ig secondary antibody (Chemicon AP 302, Millipore GmbH, Schwalbach, Germany) diluted in PBS was coated at 180 ng per well to a flat-bottom microtitre plate (Maxisorb, NUNC, Wiesbaden, Germany). Afterwards, a 1:10 dilution of the Dau c 1.01 specific monoclonal antibody (mAb) m3 supernatant (corresponding to an amount of 230 ng mAb) was transferred to the plate and incubated over night at 4 °C. Following a threefold serial dilution and 1 h incubation of the standards (rDau c 1.0104 starting from 700 ng/ml) and of the carrot extracts, the Dau c 1.01 specific sandwich was established by a combination with the polyclonal rabbit anti-Api g 1.01 + 1.02 serum diluted 1:10.000. An HRP-conjugated goat anti-rabbit IgG antibody (Sigma A0545; 1:20.000 diluted) was used for detection. Colorimetric staining with TMB diluted in citrate buffer (210 mM, pH 3.95) was stopped after 10 min by the addition of 50 μl 3 M H2SO4. Optical densities (ODs) were read at 450 nm wave length in a SpectraMAX 340PC microtitre plate reader and the data calculated by Softmax Pro software version 2.4.1 (Molecular Devices, Munich, Germany). Between incubation steps, plates were washed four times for 5 min with 300 μl PBST per well.
Sandwich ELISA for Dau c 1.02
The Dau c 1.02 ELISA was performed as described for the Dau c 1.01 ELISA, except that the Dau c 1.02 specific mAb m9 supernatant (diluted 1:20) was used as catching antibody, rDau c 1.0201 as standard (starting from 2000 ng/ml) and the specific rabbit anti-Api g 1.0201 serum (Phadia-2), diluted 1:10,000 as detector antibody, respectively.
Competitive ELISA for Dau c 4
Each MTP well (Maxisorp, NUNC) was coated with recombinant Dau c 4 at 10 ng per 100 μl of PBS for 1 h at RT. The wells were then blocked for 1 h with 200 μl of PBST containing 1% BSA. Except for the buffer blanks, 50 μl of threefold serial dilutions of rDau c 4 (starting from 1000 ng/ml) and carrot extracts were simultaneously transferred to the plates. The competitive reaction was started by the immediate addition of 50 μl rabbit anti-Lol p 12 serum (diluted 1:10,000) to all wells, and the MTP was incubated over night at 4 °C. Detection of bound rabbit IgG was done as described for the Dau c 1-specific ELISA tests. All dilution and washing steps were done as described above for the Dau c 1.01-specific ELISA.
Specificity testing of the ELISA
The specificity of each ELISA was tested by comparing a threefold serial dilution of the specific allergen applied for the standard curve, and of the other two carrot allergens. Serial dilutions started from 2000 ng/ml in case of Dau c 1.01 or Dau c 1.02, and from 1,000 ng/ml in case of Dau c 4, respectively. Specificity was analyzed at concentrations between 3 ng/ml and up to 2,000 ng/ml, respectively.
Furthermore, a purified glycopeptide with the glycan structure M0XF [Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc], known to be common on many plant glycoproteins [21, 26, 27], was additionally tested for cross-reactivity in the above-mentioned range.
Determination of LOD, LOQ, and precision
For the determination of the limits of detection (LOD) and quantification (LOQ) of non-competitive ELISA, the mean concentration of target allergen was calculated that corresponded to the average OD of multiple blanks plus three and six times the standard deviation of the mean OD of the blanks, respectively. For the competitive ELISA, we calculated these limits of target allergen from the OD at zero inhibition (B 0) reduced by threefold or sixfold the standard deviation of the mean B 0 value. Moreover, the LOQ required a precision for duplicate quantification with a coefficient of variation (CV) of ≤20%, which was met at an OD difference of six times the standard deviation in comparison to the blank OD. The working range of the ELISA tests was defined between the LOQ and the highest concentration of the standards allowing duplicate quantification at ≤20% CV.
Further, Dau c 1.01, Dau c 1.02, and Dau c 4 were quantified repeatedly either on the same MTP (intraassay precision) or on different days (interassay precision) from aliquots of one carrot total protein extract that was used as in-house reference (sample B). To ensure stability throughout the investigations, each aliquot was lyophilized, stored at −80 °C and only thawed and analyzed once. As a measure of precision, the inter- and intraassay variability was expressed as %CV that was determined as the ratio of the standard deviation and the average of the repeated quantifications, and expressed as percentage.
Reproducibility testing of the extraction method
The reproducibility of the extraction method was investigated by the simultaneous analysis of 4 independent sample grindings of one carrot sample (sample A, trade name “Fuellhorn”). From each ground carrot sample, total protein was extracted twice and the concentration of Dau c 1.01, Dau c 1.02, and Dau c 4 was measured for each extract in duplicate by the specific ELISA tests. For each grinding (nos. 1–4) the average of quantified allergen was calculated from the corresponding duplicate extracts.
Allergen recovery from carrot extracts in ELISA
For determination of the accuracy of the quantification of carrot extracts, extracts were spiked with increasing amounts of the recombinant carrot allergens. For identification of the optimal measuring range, the allergen concentrations of these extracts were pre-quantified and suitable steps of dilution were chosen prior to spiking with the corresponding allergens.
In the case of the Dau c 1.01-ELISA, the extract had to be diluted 1:80 and the spiking was done by adding 3.3, 6.6, or 9.9 ng/ml of rDau c 1.0104 to the carrot extract. For the Dau 1.02-ELISA, the recombinant allergen was added in concentrations of 80, 160, or 240 ng/ml to the 1:30 diluted carrot extract, and the recovery study in the Dau c 4-ELISA was done by spikings of 3, 6, or 9 ng/ml rDau c 4, respectively, into a 1:120 diluted carrot extract. The mean recovery over the spiking range was calculated as the ratio between quantified and spiked allergen, represented by the slope of the corresponding linear regression curve, and expressed in percent.
Electrophoresis and immunoblotting
Carrot extract (20 μg total protein/cm preparative gel slot) was separated by SDS-PAGE [28] and transferred to nitrocellulose membranes by semi-dry electrotransfer [29]. For total protein detection, the membrane was stained with Ponceau S (Sigma P3504) according to the manufacturer’s instructions, and afterwards blocked with blocking buffer (Tris-buffered saline (TBS), 0.3% Tween 20) two times 15 min. The preparative part of the membrane was cut into identical strips of 3 mm width prior to the detection of the carrot allergens by the specific antibodies. The blocked membrane strips were incubated overnight either with mAb (1:10 diluted in incubation buffer [TBS, 0.05% Tween 20, 0.1% BSA]) or with the rabbit antisera (diluted 1:10,000). Following 1 h of incubation with biotinylated rabbit anti-mouse IgG (SIGMA B8520, diluted 1:2,500) or biotinylated goat anti-rabbit IgG (DAKO E0432, diluted 1:2,500), the colorimetric detection was done by 30 min incubation with AP-labeled streptavidin (CALTAG SA1008, diluted 1:3000) using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium as substrate. Between incubation steps, membrane stripes were washed three times 10 min with TBST (TBS, 0.05% Tween 20).
Results and discussion
Characterization of antibodies in immunoblot analysis
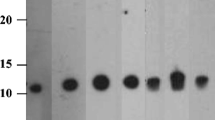
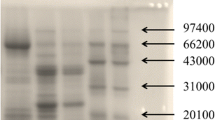
The immunoblot analysis (Fig. 1) with antibodies specific for carrot allergens (Table 1) showed that the extraction method was suitable for the detection of the known carrot allergens, namely Dau c 1.01, Dau c 1.02 and Dau c 4. The mouse mAb m3 (lane 3) and mAb m9 (lane 4) clearly detected a single protein at the expected molecular mass range of around 16 kDa. Also, the rabbit antisera raised against the mixture of Api g 1.01 and Api g 1.02 (lane 7), against Api g 1.01 (lane 8) or Api g 1.02 (lane 9) demonstrated specific binding to proteins in the range of the molecular mass of Dau c 1.
Immunoblot analysis of carrot extract with specific antibodies derived from monoclonal mouse hybridoma (lanes 1–4) and polyclonal rabbit antisera (lanes 5–10). MP: total protein stain (Ponceau S) of marker (M) and carrot extract (P), lane 1 buffer control, lane 2 irrelevant mouse mAb PN-t (anti-peanut Ara h 1), lane 3 mAb anti-Dau c 1.0104 (“m3”), lane 4 mAb anti-Dau c 1.0201 (“m9”), lane 5 buffer control, lane 6 rabbit normal serum, lane 7 rabbit anti-Api g 1.01 and 1.02, lane 8 rabbit anti-Api g 1.01, lane 9 rabbit anti-Api g 1.02, lane 10 rabbit anti-Lol p 12
Some weaker reactivities to other carrot proteins may result from minor unspecificities of the rabbit antisera. However, because the highly specific mouse mAbs are used as catching antibodies in sandwich assay design, weak unspecific binding to other carrot proteins is unlikely to affect the final assay performance (see below).
More unspecific binding was observed for the rabbit anti-Lol p 12 antiserum which was used for the Dau c 4 inhibition ELISA. This serum strongly detected proteins at the molecular mass range of carrot profilin (14 kDa), but also at least two other bands at higher molecular masses (Fig. 1, lane 10).
Selection and combination of antibodies in ELISA
In preliminary investigations, the mouse and rabbit antibodies were tested for sensitivity to the recombinant reference proteins. Besides the ability to detect low amounts of allergen, also the signal intensity of the colorimetric detection was considered for the reason of assay robustness. In the case of Dau c 1.01 and Dau c 1.02, mouse and rabbit antibodies were combined to develop specific sandwich ELISA tests. For the immunological detection of isoform Dau c 1.01, the rabbit antiserum against celery Api g 1.0101 turned out to be less qualified than the polyclonal rabbit antiserum anti-Api g 1.01 + 1.02 against both isoforms (data not shown), and thus the latter (Table 1) was used in the Dau c 1.01-specific sandwich ELISA.
Despite testing of two other profilin-specific antibodies, it was not possible to find a suitable pair of antibodies for a Dau c 4-specific two-site ELISA (data not shown). Hence, the highly profilin reactive rabbit anti-Lol p 12 antiserum was used for the development of a competitive ELISA test.
High specificity of the developed ELISA tests
As shown in Table 2, all three ELISA tests are highly specific for their particular target allergen and did not show any cross-reactivity above the limit of detection to the other two carrot allergens or to the carbohydrate determinant M0XF, that is frequently found in the plant kingdom,. Because of its known occurrence at many high molecular weight glycoproteins of various plants [26, 27] the recognition of this glycan would otherwise lead to false positive results of the ELISA.
There was no need for further testing of other potential (cross-) reactive food or pollen allergens because these ELISA tests are designed for the investigation of carrot extracts only.
LOD, LOQ, and precision of the developed ELISA tests
By repeated measurements of recombinant reference curves in combination with 12–80 blank or B 0 buffer (PBS) control samples the mean LOD was determined at 0.4 ng/ml for Dau c 1.01, 6 ng/ml for Dau c 1.02, and 0.7 ng/ml for Dau c 4, and the mean LOQ at 0.8 ng/ml for Dau c 1.01, 11 ng/ml for the Dau c 1.02, and 1.3 ng/ml for Dau c 4, respectively. For quantification of Dau c 1.01 the average working range was between 0.8 and 100 ng/ml, for Dau c 1.02 between 11 and 1800 ng/ml, and for Dau c 4 between 1.3 ng/ml and 1,000 ng/ml, respectively.
The reproducibility of the ELISA measurements was tested by repeatedly quantifying one single carrot extract (sample B) used as in-house reference either on the same microtitre plate or on different days. Furthermore, various pre-defined extract dilutions, ranging from 2 to 2,200, were repeatedly measured with all three assays. From these results, the intraassay variabilities were calculated to be 8.1%, 6.9%, and 11.9% for the Dau c 1.01-, Dau c 1.02- and the Dau c 4-specific ELISA, respectively.
Table 3 summarizes the interassay variation, expressed as percent of coefficient of variation, for all 3 ELISA tests. In more than 20 repetitions, the quantification of Dau c 1.01 and Dau c 4 was precise with variation coefficients of 13–16%. In the case of the Dau c 1.02 quantification, the interassay precision was somewhat lower (Table 3). Nonetheless, this precision was still considered sufficient to clearly discriminate between substantial differences in the allergen content in carrot extract samples.
For a direct comparison of the ELISA tests that were performed at different days, the OD values of the reference curves were normalized against the maximum OD value, which again was calculated by the reader software as one characteristic curve parameter. Doing so, the comparison of various reference curves showed only minor deviations and thus demonstrated assay robustness (Fig. 2a–c). Moreover, both the recombinant reference protein and the natural allergen from carrot extract demonstrated parallel dilution curves (Fig. 2), i.e., comparable binding properties, for all three specific ELISA tests. This was also demonstrated by the overall good recovery results (below).
Representative curves for the quantification of a Dau c 1.01, b Dau c 1.02, and c Dau c 4, using the recombinant allergens as standards (left set of curves) and the in-house reference carrot extract B (right set of curves). All curves were normalized against their individual maximum OD (%ODmax) to allow visual comparison of the curve features
The preparation of carrot extracts was highly reproducible
The amount of allergen present in one carrot sample was determined by averaging the results of two independent protein extracts simultaneously derived from one grinding. The average of four independent grindings is displayed in Table 4: the mean value for Dau c 1.01 was 2,336 ng/ml, having a variability of 8.4% CV. The average quantified amount of Dau c 1.02 was 1314 ng/ml, with a variability of 14.7% CV. Dau c 4 was extracted and quantified at a mean of 905 ng/ml with a variability 14% CV. Thus, all three carrot allergens were quantified with less than 15% inaccuracy. Since part of the observed inaccuracy would be due to variations in the ELISA method, the results strongly suggest that the allergen extraction method was highly reproducible.
Recovery studies
Co-extracted components of the extract could influence the accuracy of the quantifications of the target proteins. This potential influence of the carrot matrix on the allergen quantification in extracts was investigated by spiking experiments with increasing amounts of the recombinant allergens and repeated quantification.
Based on the method of standard addition, the amount of quantified allergen was plotted against the amount of spiked allergen and the linear response calculated by linear regression. Thereby, the slopes of the graphs represented the average recovery within the range of spiked allergen.
The regression results from the standard addition methods of the Dau c 1.01, Dau c 1.02 and Dau c 4 ELISA tests are shown in Fig. 3a and b.
For all three ELISA tests, recoveries of 93–113% percent were obtained in the low concentration range (Table 5). This demonstrates the comparably good detection of both the natural and the recombinant allergens and the accurate quantification of individual allergens in carrot extracts with the specific ELISA tests.
Concluding remarks
So far, several antibodies have been generated with specificities to pollen or food allergens. Due to the recognition of different epitopes, they were used either in sandwich ELISAs for allergen quantification or for the detection of homologous proteins or even isoforms in the various plants [17, 18, 30, 31].
To our knowledge, this is the first study dealing with the development of specific assays for the quantification of two different isoforms, Dau c 1.01 and Dau c 1.02, of a major food allergen, Dau c 1, from carrot.
The need for quantification of allergens in carrot is clearly given by several cases of severe allergic symptoms [8–12] or even life-threatening reactions [7, 13] after contact or ingestion of carrots.
The major carrot allergen, Dau c 1, is recognized by the IgE of more than 85% of carrot-allergic patients [6, 21]. Besides the dominant isoform Dau c 1.01, also the less abundant isoform Dau c 1.02 is known to bind IgE of many carrot-allergic patients. Furthermore, IgE to Dau c 1.0201 has been found to be highly specific for clinically relevant sensitization [21].
Taking into account the high specificity, good allergen recovery, and precision of these newly developed ELISA tests, it is now possible to reproducibly and accurately quantify the amount of Dau c 1.01, Dau c 1.02, and Dau c 4 in carrot extracts. As an example, we quantified Dau c 1.01, Dau c 1.02, and Dau c 4 in two independent commercial carrot samples at 467 and 559 μg, 68 and 263 μg, and 127 and 181 μg per 100 g of carrot, respectively. Between the two carrot samples, the amount of Dau c 1.01 and Dau c 4 was similar, but differences were observed in Dau c 1.02 concentration. The amount of isoform Dau c 1.02 was clearly below that of isoform Dau c 1.01. The limited number of independent sample data does not allow further conclusions but demonstrated applicability of the ELISA tests for quantification of individual allergens and even isoforms. Thus, these ELISA tests are of potential value for the quantification of carrot allergens in a more systematic approach. This includes the standardization of in vitro diagnostic carrot extracts, as well as the quantification of allergens in different carrot varieties, enabling the identification of ones with reduced allergen content which may be less likely to cause allergic reactions in sensitized individuals.
Abbreviations
- AP:
-
Alkaline phosphatase
- HRP:
-
Horseradish peroxidase
- Ig:
-
Immunoglobulin
- mAb:
-
Monoclonal antibody
- MTP:
-
Microtitre plate
- TBS:
-
Tris-buffered saline
- TMB:
-
3,3′,5,5′- Tetramethylbenzidine
References
Vieths S, Scheurer S, Ballmer-Weber BK (2002) Current understanding of cross-reactivity of food allergens and pollen. Ann NY Acad Sci 964:47–68
Zuberbier T, Edenharter G, Worm M, Ehlers I, Reimann S, Hantke T, Roehr CC, Bergmann KE, Niggemann B (2004) Prevalence of adverse reactions to food in Germany—a population study. 59(3):338–345
André F, André C, Colin L, Cacaraci F, Cavagna S (1994) Role of new allergens and of allergens consumption in the increased incidence of food sensitizations in France. Toxicology 93(1):77–83
Wüthrich B (1993) Zur Nahrungsmittelallergie. Häufigkeit der Symptome und der allergieauslösenden Nahrungsmittel bei 402 Patienten - Kuhmilchallergie - Nahrungsmittel und Neurodermitis atopica. (Food allergy. Frequency of symptoms and allergenic foods in 402 patients—lactose intolerance, food and atopic dermatitis). Allergologie 16(7):280–287
Etesamifar M, Wüthrich B (1998) IgE-vermittelte Nahrungsmittelallergien bei 383 Patienten unter Berücksichtigung des oralen Allergie-Syndroms. Allergologie 21(9):451–457
Ballmer-Weber BK, Wüthrich B, Wangorsch A, Foetisch K, Altmann F, Vieths S (2001) Carrot allergy: double-blinded, placebo-controlled food challenge and identification of allergens. J Allergy Clin Immunol 108(2 Pt 1):301–307
Fernandez-Rivas M, Gonzalez-Mancebo E, van Leeuwen WA, Leon F, van Ree R (2004) Anaphylaxis to raw carrot not linked to pollen allergy. 59(11):1239–1240
Gomez M, Curiel G, Mendez J, Rodriguez M, Moneo I (1996) Hypersensitivity to carrot associated with specific IgE to grass and tree pollens. 51(6):425–429
Helbling A, Lopez M, Schwartz HJ, Lehrer SB (1993) Reactivity of carrot-specific IgE antibodies with celery, apiaceous spices, and birch pollen. Ann Allergy 70(6):495–499
Moneo I, Gomez M, Sánchez-Monge R, Alday E, de las Heras M, Esteban I, Bootello A, Salcedo G (1999) Lack of crossreaction with Bet v 1 in patients sensitized to Dau c 1, a carrot allergen. Ann Allergy Asthma Immunol 83(1):71–75
Moreno-Ancillo A, Gil-Adrados AC, Dominguez-Noche C, Cosmes PM, Pineda F (2005) Occupational asthma due to carrot in a cook. Allergol Immunopathol (Madr) 33(5):288–290
Moreno-Ancillo A, Gil-Adrados AC, Cosmes PM, Dominguez-Noche C, Pineda F (2006) Role of Dau c 1 in three different patterns of carrot-induced asthma. Allergol Immunopathol (Madr) 34(3):116–120
Schiappoli M, Senna G, Dama A, Bonadonna P, Crivellaro M, Passalacqua G (2002) Anaphylaxis due to carrot as hidden food allergen. Allergol Immunopathol (Madr) 30(4):243–244
Schwartz HJ, Arnold JL, Strohl KP (1995) Nasal response to carrot and lettuce challenge in allergic patients. Ann Allergy Asthma Immunol 74(2):152–154
Hoffmann-Sommergruber K, O'Riordain G, Ahorn H, Ebner C, da Camara L, Machado M, Pühringer HM, Scheiner O, Breiteneder H (1999) Molecular characterization of Dau c 1, the Bet v 1 homologous protein from carrot and its cross-reactivity with Bet v 1 and Api g 1. Clin Exp Allergy 29(6):840–847
Valenta R, Duchêne M, Ebner C, Valent P, Sillaber C, Deviller P, Ferreira F, Tejkl M, Edelmann H, Kraft D (1992) Profilins constitute a novel family of functional plant pan-allergens. J Exp Med 175(2):377–385
Akkerdaas JH, van Ree R, Aalbers M, Stapel SO, Aalberse RC (1995) Multiplicity of cross-reactive epitopes on Bet v I as detected with monoclonal antibodies and human IgE. Allergy 50(3):215–220
Asturias JA, Arilla MC, Aguirre M, Gómez-Bayón N, Martínez A, Palacios R, Sánchez-Gascón F, Martínez J (1999) Quantification of profilins by a monoclonal antibody-based sandwich ELISA. J Immunol Methods 229(1–2):61–71
Duffort OA, Polo F, Lombardero M, Diaz-Perales A, Sánchez-Monge R, Garcia-Casado G, Salcedo G, Barber D (2002) Immunoassay to quantify the major peach allergen Pru p 3 in foodstuffs. Differential allergen release and stability under physiological conditions. J Agric Food Chem 50(26):7738–7741
Werner MT, Faeste CK, Egaas E (2007) Quantitative sandwich ELISA for the determination of tropomyosin from crustaceans in foods. J Agric Food Chem 55(20):8025–8032
Ballmer-Weber BK, Wangorsch A, Bohle B, Kaul S, Kundig T, Fotisch K, van Ree R, Vieths S (2005) Component-resolved in vitro diagnosis in carrot allergy: does the use of recombinant carrot allergens improve the reliability of the diagnostic procedure? Clin Exp Allergy 35(7):970–978
Wangorsch A, Ballmer-Weber BK, Rosch P, Holzhauser T, Vieths S (2007) Mutational epitope analysis and cross-reactivity of two isoforms of Api g 1, the major celery allergen. Mol Immunol 44(10):2518–2527
Reese G, Ballmer-Weber BK, Wangorsch A, Randow S, Vieths S (2007) Allergenicity and antigenicity of wild-type and mutant, monomeric, and dimeric carrot major allergen Dau c 1: destruction of conformation, not oligomerization, is the roadmap to save allergen vaccines. J Allergy Clin Immunol 119(4):944–951
Peters JH, Baumgarten H (1990) Monoklonale Antikörper - Herstellung und Charakterisierung. Springer, Berlin
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Wilson IB, Zeleny R, Kolarich D, Staudacher E, Stroop CJ, Kamerling JP, Altmann F (2001) Analysis of Asn-linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core alpha1, 3-linked fucose and xylose substitutions. Glycobiology 11(4):261–274
Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F (1998) Core alpha1, 3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology 8(7):651–661
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Kyhse-Andersen J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 10(3–4):203–209
Lowenstein H, Sparholt SH, Klysner SS, Ipsen H, Larsen JN (1995) The significance of isoallergenic variations in present and future specific immunotherapy. Int Arch Allergy Immunol 107(1–3):285–289
Son DY, Fahlbusch B, Müller WD, Petersen A, Lee SI, Vieths S (2001) Monoclonal antibodies raised against the major apple allergen, Mal d 1, are useful tools for epitope studies. Food Agric Immunol 13(1):39–49
Acknowledgments
The authors like to thank Heike Hopfenbach for excellent technical assistance for the generation of the monoclonal antibodies. We are grateful to Dr. Friedrich Altmann (Department of Chemistry, University of Agriculture, Vienna) for kindly providing the M0XF-glycopeptide.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foetisch, K., Dahl, L., Jansen, B. et al. Development and in-house validation of allergen-specific ELISA tests for the quantification of Dau c 1.01, Dau c 1.02 and Dau c 4 in carrot extracts (Daucus carota). Anal Bioanal Chem 399, 935–943 (2011). https://doi.org/10.1007/s00216-010-4348-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4348-6