Abstract
Birch (Betula) pollen is a major cause of allergy in northern and central Europe. The allergenic potency of products for diagnosis and therapy of birch pollen allergy is adjusted nearly exclusively to the major birch pollen allergen Bet v 1. Although every fifth patient is additionally sensitized to Bet v 4, both content and variability of this minor allergen in birch allergen products remain unclear due to a lack of simple and cost-effective quantitative methods. This study aimed to develop and in-house validate the first Bet v 4-specific sandwich enzyme-linked immunosorbent assay (ELISA). Based on a murine monoclonal antibody in combination with a polyclonal rabbit antiserum, the ELISA proved to be highly sensitive, with a lower limit of quantification of 30 pg/ml Bet v 4. After confirmation of satisfactory accuracy, reproducibility, and robustness, the ELISA was utilized to quantify Bet v 4 in 30 authorized birch allergen products. The allergen was detected in all samples tested, ranging from 0.2 to 4.4 μg/ml. No significant correlation of Bet v 4 was found with the respective amount of Bet v 1. In contrast to Bet v 1, also no correlation of Bet v 4 with total protein content or total allergenic activity could be observed. Thus, it seems presently unfeasible to base birch allergen product standardization additionally on Bet v 4. In light of these results, the continuous monitoring of Bet v 4 in birch allergen products with the presented ELISA will provide a basis for the understanding of the clinical relevance of minor allergens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To date, authorized allergen products for in vivo diagnosis and allergen immunotherapy (AIT) almost exclusively consist of extracts prepared from natural source material such as pollen. These source materials as well as the resulting products are heterogeneous mixtures containing multiple nonallergenic and allergenic components. In combination with the natural variability in protein expression, substantial variation in the absolute amount and relative ratios of allergens in extracts seems unavoidable, a problem already described not only for products from different manufacturers but also between batches of the same product [1–4]. However, potential consequences on the performance of test allergens as well as on the safety and efficacy of AIT products are unknown, partly due to missing data on single allergen content. The content of so-called minor allergens, especially, to which only a limited number of allergic individuals react, is hardly ever assessed.
Testing of allergen products in accordance with the European Pharmacopoeia (Ph. Eur.) comprises the determination of total protein content and a protein profile. For AIT products, also the total allergenic activity has to be determined, either based on allergen-specific human immunoglobulin (Ig) E antibodies or by quantification of each relevant allergen. However, measuring total allergenic activity in IgE antibody-based tests based on human serum pools results in a bias towards the detection of major allergens to which most patients are sensitized [5]. Minor IgE specificities, only present in some patients, are “diluted”, and thus, the allergenicity of minor allergens is commonly underrepresented in total allergenic activity. In addition, the Ph. Eur. and the European Medicines Agency (EMA) guideline on the production and quality of allergen products demand to verify the presence of the respective relevant allergens, where possible [6, 7]. Consequently, methods allowing quantitative determination of individual components in allergen extracts should be performed in addition to IgE-based potency testing. As a result, numerous immunoassays for the quantification of individual allergens, e.g. in pollen extracts, have been published [8–13]. More recently, also mass spectrometry (MS) has been applied for the detection and quantification of individual allergen components in extracts [14, 15].
Compared to other allergen sources, birch pollen allergy represents a special case as approximately 95 % of patients are sensitized to the major allergen Bet v 1, with some variations depending on the geographic region. Consequently, the bias towards this major allergen in the determination of total allergenic activity is particularly strong in birch allergen products. Far less than 50 % of birch-allergic subjects are sensitized to the highly cross-reactive minor allergens Bet v 2 (profilin) and Bet v 4 (polcalcin) [16–20]. Monoclonal and polyclonal antibody-based enzyme-linked immunosorbent assays (ELISAs) have been developed for plant profilins [9, 21] but not for polcalcins like Bet v 4. This 9.3-kDa protein with two EF-hand Ca2+ binding motifs is considered a pan-allergen, sharing amino acid sequence identity of more than 65 % with allergenic polcalcins from many other plant species [16, 17, 22–24]. Such pan-allergens are supposed to be responsible for poly-sensitizations and both chemical and serological cross-reactivity regardless of taxonomic relationship [25–27]. As a consequence, sensitization to pan-allergens such as Bet v 4 is suspected to reflect a more severe disease status accompanied by more severe symptoms [26, 28, 29]. However, the clinical relevance of Bet v 4 for patients, birch pollen allergy diagnosis, and AIT remains unclear. To facilitate a better understanding of the importance of Bet v 4 for the patients, an allergen-specific sandwich ELISA was developed and validated in-house, allowing specific, accurate, and reproducible quantification of Bet v 4 in commercial birch allergen products. Testing of 30 birch allergen products revealed remarkable variation in Bet v 4 content and no interdependence of the minor allergen with those factors monitored in routine batch control of these products. A future combination of knowledge on Bet v 4 content in different birch allergen products on the one hand and clinical observations in birch pollen-allergic subjects on the other hand could clarify the clinical relevance of this minor allergen.
Materials and methods
Recombinant allergens
The Holo recombinant Bet v 4 (rBet v 4) used for immunization, as a reference standard, and for standard-addition samples, was produced and kindly provided by Prof. Dr. Paul Rösch, University of Bayreuth, Bayreuth, Germany [30]. Other recombinant birch allergens used for cross-reactivity testing of monoclonal antibodies (mAbs) were commercially available (rBet v 2 and rBet v 1 from Biomay, Vienna, Austria; purity >98 %). rBet v 6 (AF135127) and rBet v 7 (AJ311666) were in-house expressed in Escherichia coli BL21 (DE3) pLysS cells using the vector pET23b and NdeI/XhoI restriction sites and prepurified via metal chelate affinity chromatography. Apart from slight modifications, this procedure has been reported previously by Karamloo et al. and Cadot et al. [31, 32]. After elution and dialysis against MOPS buffer, the proteins were further purified by centrifugation for approx. 30 min at 20,000g. Birch pollen extract containing native Bet v 4 (nBet v 4) was purchased from Laboratoire Stallergenes (Antony Cedex, France) and used for sample preparation.
Antibodies and antisera
The primary capture antibody for the ELISA, the rat anti-mouse IgG2a mAb, was purchased from Becton Dickinson Pharmingen (Heidelberg, Germany), while the secondary detection antibody, HRP-conjugated goat anti-rabbit IgG, was purchased from Jackson ImmunoResearch Europe Ltd (Newmarket, UK). For immunoblotting, AP-labeled anti-mouse or anti-rabbit IgG was used (Sigma-Aldrich, Taufkirchen, Germany).
For the production of Bet v 4-specific mAbs, C3H/HeJ mice (Charles River, Sulzfeld, Germany) were immunized with aluminum hydroxide-adsorbed rBet v 4 via intraperitoneal injection administered three times in intervals of 14 days. Each shot contained 10 μg of antigen in a 2-mg/ml aluminum hydroxide suspension. Following a booster injection with 10 μg rBet v 4 after a total period of 4 months, the mice with satisfyingly high IgG titers (determined by ELISA according to Reese et al. [33], using a microplate coated with 50 ng rBet v 4 per well) were sacrificed for spleen cell preparation. Hybridoma cell lines were generated following a standard protocol. In brief, the spleen cells were mixed with X63-Ag8.653 myeloma cells (DSMZ, Brunswick, Germany) in a 10:1 ratio and fused by centrifugation in the presence of 50 % polyethylene glycol 1500 (Roche Diagnostics GmbH, Mannheim, Germany) in 75 mM HEPES buffer. Selection of a hybridoma was performed in RPMI 1640 (Biochrom AG, Berlin, Germany) supplemented with 1 % streptomycin, 0.7 % penicillin, 1 mM l-glutamine (all from Merck, Darmstadt, Germany), 20 % fetal calf serum (FCS) (Invitrogen, Darmstadt, Germany), 1× HAZ (Sigma-Aldrich), and 10 % Condimed H1 hybridoma cloning supplement (Roche Diagnostics GmbH). Clones were generated by limiting dilution and screened for specificity towards both rBet v 4 as well as birch allergen extract by indirect ELISA using a microplate coated with 50 ng rBet v 4 or 10 μg birch pollen extract per well [33]. For clonal expansion, RPMI 1640 (Biochrom AG) was supplemented with 1 % streptomycin, 0.7 % penicillin, 1 mM l-glutamine (all from Merck), 10 % FCS (Invitrogen), and decreasing concentrations of murine IL-6 from Roche Diagnostics GmbH (maximum 100 U/ml; minimum 0 U/ml). For sandwich ELISA development, the cell culture supernatant containing the IgG2a “mP49” (isotype determination via the IsoStrip Mouse mAb Isotyping Kit, Roche Diagnostics GmbH) was applied. The mAb was quantified using anti-mouse IgG2a (Becton Dickinson Pharmingen)-coated microplates. Relative to a purified mouse IgG2a standard (Becton Dickinson Pharmingen), mP49 concentration was assessed using a biotinylated anti-mouse IgG2a for detection (Becton Dickinson Pharmingen) and streptadivin-HRP/3,3′,5,5′-tetramethylbenzidine (TMB) for visualization at 450 with 630 nm as reference.
Bet v 4-specific rabbit antiserum was produced by CE-Immundiagnostika (Eschelbronn, Germany) via immunization with aluminum hydroxide-adsorbed rBet v 4 following a standard schedule. The sera IgG titers were assessed by ELISA titration, applying dilution series of the sera. Both the mAb mP49 and the sera were further evaluated via protein immunoblot.
Gel electrophoresis and staining
Separation of birch pollen extract and recombinant allergen preparations was done via 14 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the protocol of Schägger and Jagow [34] using a Mini-PROTEAN II cell (Bio-Rad, Munich, Germany). The recombinant allergens were loaded with 0.5 μg/cm (Bet v 6, Bet v 7) or 1 μg/cm (rBet v 1, rBet v 2, rBet v 4), while birch pollen extract was loaded with a concentration of 10 μg/cm. Subsequently, the gels were subjected either to immunoblot as described below or to Brilliant Blue G staining following the manufacturer’s instructions (Sigma-Aldrich).
Immunoblotting
After electrophoresis, protein transfer on nitrocellulose membranes was performed with the semidry system PEGASUS (PHASE, Lübeck, Germany). Tris-buffered saline (TBS) with 0.05 % Tween 20 and 5 % milk powder (Roth) was used for subsequent blocking (two times for 15 min) and TBS with 0.05 % Tween 20 to wash the membranes (three times for 10 min). Immunodetection by mAbs or sera (1:10 or 1:5000–1:10,000 dilutions in TBS with 0.05 % Tween 20, respectively) took place overnight at 4 °C. Subsequent to three wash steps, AP-labeled IgG was diluted 1:10,000 in TBS with 0.05 % Tween 20 and incubated for 2 h at RT for secondary detection, followed by three washes. NBT (Sigma-Aldrich) was mixed 1:30 with BCIP (Roth) and used for the incubation of the membranes. After an adequate staining intensity had been reached, the membranes were washed with water and dried in the dark.
Total protein quantification
For the estimation of total protein concentrations in cell culture supernatants, recombinant protein, and extract preparations, the method according to Bradford [35] was applied using bovine serum albumin (BSA) (Thermo Fisher Scientific, Bonn, Germany) as calibrator and the Roti-Nanoquant solution (Roth, Karlsruhe, Germany). In allergen extract batches, protein concentration was determined by the Bradford Coomassie brilliant blue method with BSA as standard (Bio-Rad).
Reference and sample preparation
Incubation buffer (0.5 % BSA from Thermo Fisher Scientific, 0.5 % Tween 20 in PBS pH 7) was used as dilution medium. For the standard curve, a 1:2 dilution series starting from 4 ng/ml to 3.9 pg/ml rBet v 4 was prepared. Samples contained birch pollen extract and rBet v 4 either alone or in combination. For the evaluation of robustness towards potentially interfering chemicals, phenol and glycerol were added to the incubation buffer in varying ratios.
Bet v 4-specific sandwich ELISA
The development and validation of the Bet v 4 ELISA was performed in accordance with common guidelines and recommendations [36–38] and according to the rules of the quality assurance system of the Paul-Ehrlich-Institut (PEI). Nunc MaxiSorp microtiter plates were coated with 200 ng of the isotype-specific primary capture antibody rat anti-mouse IgG2a (Becton Dickinson Pharmingen) in 100 μl/well PBS, pH 7, and incubated overnight at 4 °C. After three washes (300 μl/well, 0.05 % Tween 20 in PBS), plates were blocked with 1.5 % BSA (Thermo Fisher Scientific) in PBS for 30 min at RT and washed again. Five nanograms of Bet v 4-specific mAb was immobilized by incubating 100 μl/well mP49 cell culture supernatant in the incubation buffer for 1 h at RT under agitation. The reference and samples diluted in incubation buffer (100 μl) were added and incubated for 1 h under agitation. Anti-Bet v 4 rabbit serum diluted 1:16,000 (100 μl) in incubation buffer was added and incubated for 1 h under agitation. Detection was performed with 100-μl/well HRP-conjugated anti-rabbit IgG diluted 1:30,000 in incubation buffer for 1 h at RT. Three washes were performed between all incubations.
Substrate solution (100 μl) containing 2.5 % TMB (21 mM stock, Sigma-Aldrich) and 0.034 % H2O2 in potassium citrate buffer, pH 4, was added and incubated for 10 min at RT. The reaction was stopped by adding 50 μl/well of 20 % sulfuric acid. Optical density (OD) was measured at 450 with 630 nm as reference using a SpectraMax 340 reader provided with SoftMax Pro 5.2 rev C software (Molecular Devices Corp., Sunnyvale, USA).
Bet v 1-specific sandwich ELISA
Bet v 1 was determined using the kit from INDOOR Biotechnologies Inc. (Cardiff, UK) according to the manufacturer’s instructions.
Birch allergen products
All products and batches used in this study were authorized for the German market by the PEI. Prick test solutions (PTS) and products used in sublingual immunotherapy (SLIT), all containing glycerol and phenol, were included. Samples were taken from lots submitted to official batch release. Consent was obtained from all marketing authorization holders concerned (ALK-Abelló Arzneimittel GmbH, Allergopharma GmbH & Co. KG, Bencard Allergie GmbH, HAL Allergy GmbH, Leti Pharma GmbH, Stallergenes GmbH (listed in alphabetical order)). Results are presented in a blinded fashion.
Total allergenic activity (potency)
For determination of total allergenic activity, an IgE inhibition assay was performed using commercial birch pollen ImmunoCAP® (Thermo Fisher Phadia, Sweden) as the solid phase. A serum pool from birch pollen-allergic patients (n = 15) was prepared in accordance with the EMA guideline on production and quality issues of allergen products [6]. In brief, the serum pool was diluted 1:4 in physiologic sodium chloride solution and mixed with serial dilutions of allergen and in-house reference, respectively. Serum pool allergen mixtures were incubated for approx. 30 min. Finally, free specific IgE binding to immobilized birch pollen extract was determined using ImmunoCAP® according to the instruction of the manufacturer. Relative potency was calculated with the software CombiStats 4.0 (EDQM, Council of Europe).
Statistics
All samples were measured in dilution series allowing for robust curve analysis. Primary ELISA data (OD values) were processed via SoftMax Pro 5.2 rev C software (Molecular Devices) by four-parameter logistic fitting of the reference curve y = (A − D) / [1 + (x / C)B] + D where A = ODmin, B = slope, C = EC50, and D = ODmax, and interpolation of the sample values after background subtraction. R 2 = coefficient of determination was calculated. Parallelism and linearity of curves as well as potency assessment were estimated with the parallel lines model using CombiStats 4.0. Precision (total variability, intra- and inter-assay variability including the variables day and operator) was estimated via a mixed ANOVA model with the SAS/STAT Software, version 9.3, MIXED Procedure (SAS Institute Inc., Heidelberg, Germany). Accuracy was calculated via the formula spike recovery = (1 + bias / [spike]) × 100 %, where bias = [mean of spiked sample] − ([mean of matrix] + [spike]) and [] indicates concentrations [39]. The limit of detection (LOD) was determined based on blank measurements and the formula LOD = 2.6 × SD + mean, relating to a confidence limit of 99 % [40]. The lower and upper limits of quantification (LLoQ, ULoQ) were defined as the limits of the linear and parallel section of the reference curve [38, 41]. Measurements using birch pollen extract preparations were conducted as dilution series with parallelism and linearity of samples as well as reference curves (assessed via CombiStats) as precondition for quantitative evaluation. Differences between product classes were assessed for statistical significance using Student’s t test. For calculation of Pearson’s correlation coefficient (r) as well as the respective p values controlled with the Holm-Bonferroni method [42], the software R, version 3.0.1, was used [43]. Ninety-five percent confidence intervals of r were estimated by repeated (n = 1000) sampling with replacement (bootstrapping). GraphPad Prism version 5.04 (GraphPad Software, La Jolla, USA) was used for the generation of graphs.
Statement of human and animal rights
The institutional and national guidelines for the care and use of laboratory animals were followed.
Results
Development and validation of the Bet v 4 ELISA
Highly specific murine monoclonal antibody and rabbit antiserum recognize recombinant and native Bet v 4 equally
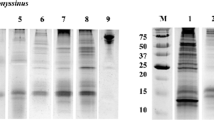
The development of the Bet v 4-specific sandwich ELISA was based on the generation of a rabbit antiserum raised against Bet v 4 and a murine monoclonal anti-Bet v 4 antibody. Testing of the binding capacity to both rBet v 4 and nBet v 4 by indirect ELISA led to the selection of the IgG2a-producing clone mP49. The specificity of mP49 and the rabbit antiserum was analyzed by immunoblots, showing strong binding to two independently produced batches of rBet v 4 as well as clear binding to a band at approx. 7 to 9 kDa in birch pollen extract (Fig. 1). Additional bands visible in the lanes loaded with rBet v 4 in the immunoblots are most likely oligomers of the recombinant minor allergen. Other recombinant birch allergens were not detected by either the antiserum or mP49 in immunoblot analysis. In contrast, the monoclonal antibody and the antiserum were still able to detect rBet v 4 when the protein was diluted to 320 and 64 pg/cm, respectively (data not shown). For specificity assessment in the highly sensitive ELISA test format, the LOD was determined at OD = 0.146 based on six blank replicates, corresponding to approx. 15 pg/ml Bet v 4. Subsequently, all available recombinant birch allergens in high concentrations of 40 ng/ml and a birch pollen extract were tested in the ELISA for cross-reactivity. As expected, only rBet v 4 and the birch pollen extract resulted in a positive signal above the LOD (Table 1).
Purity and specificity of Bet v 4 ELISA components. a SDS-PAGE followed by Coomassie blue staining was utilized for visualization of recombinant birch allergens and birch pollen extract (BPE) relative to a molecular weight marker (MWM). Immunoblot analysis using b Bet v 4-specific rabbit antiserum and c Bet v 4-specific monoclonal antibody mP49 showed antibody specificity for two batches of recombinant Bet v 4 as well as detection of native Bet v 4 in birch pollen extract
Figure 2a shows the dilution series of rBet v 4 and birch pollen extract, revealing a typical sigmoid curve course in the semi-log plot. The four-parametric nonlinear regression resulted in very good correlations (R 2 = 1) and comparable, satisfyingly high slopes. As estimated via the parallel lines model, the curves obtained with recombinant reference and extract were parallel for concentrations between 0.0039 and 0.5 ng/ml Bet v 4. Most accurate measurements are possible in the linear section ranging from 0.031 to 0.25 ng/ml Bet v 4, defining the LLoQ and ULoQ, respectively. The signal-to-noise ratio of >5:1 at the LLoQ was sufficiently high (LLoQ: OD = 0.47/blank mean: OD = 0.083). According to the precision profile, the intermediate variability of four replicates of rBet v 4 and birch pollen extract, respectively, did not exceed a CV of 10 % in the quantitation range (Fig. 2b).
Comparison of curve progression and precision profile for rBet v 4 standard and nBet v 4 in extract. a Four ELISA tests were performed with rBet v 4 and birch pollen extract, respectively, in concentrations as indicated. The OD was measured and depicted as the mean value with the respective standard deviation (SD). The curves follow a four-parametric nonlinear curve fitting. The grey striped section indicates parallelism of the curves, whereas in the blank grey section, parallelism and linearity of the curves are given. b Four ELISA tests were performed with rBet v 4 and birch pollen extract, respectively, in concentrations as indicated. The OD was measured and the coefficient of variation (CV) calculated for each concentration to create a precision profile
High accuracy and precision of Bet v 4 determination in birch pollen extract matrix
Accuracy and precision of the Bet v 4 ELISA were determined by the standard addition method. The experimental setup comprised two different concentrations of birch pollen extract (=matrix) containing nBet v 4, spiked with four different concentrations of rBet v 4. In total, 54 replicates measured in nine experiments with six replicates each and performed by two operators on 5 days were evaluated. The calculation of recovery showed a clear linear relationship between the expected versus measured Bet v 4 concentrations (Fig. 3). The overall recovery was nearly 100 % without a significant influence of matrix or spike concentrations, indicating accurate results in terms of absolute quantity.
Estimation of accuracy and precision based on standard addition experiments. Nine ELISA tests were performed with six internal replicates each. Two concentrations of nBet v 4 in the extract (=matrix) as indicated were spiked with four different rBet v 4 concentrations (S1–S4 = 0.02, 0.05, 0.1, 0.5 ng/ml). The mean measured total Bet v 4 quantities with the respective SD were plotted against the expected total Bet v 4 concentrations. Slopes and determinants of correlation (R 2) are given for the resulting linear regression lines
The precision of an ELISA is generally defined by its degree of variability. Using a mixed linear model, the total variability can be estimated from replicate values from the same experiment (intra-assay variability) in combination with replicate values from several independent tests following an identical procedure (inter-assay variability). In general, the Bet v 4 ELISA showed overall high precision, not exceeding a CV of 25 % at any point both for unspiked as well as spiked matrices (Table 2). Intra-assay variability was found to be low, and the inter-assay parameter “day” had a significantly higher impact compared to “operator.”
The Bet v 4 ELISA is robust to changes in matrix composition
In many allergen products, the extract is dissolved in a glycerol/phenol buffer. Consequently, for successful characterization of PTS and SLIT products with the Bet v 4 ELISA, these potentially interfering components have to be taken into account. By measuring serial dilutions of rBet v 4 supplemented with different concentrations of glycerol and phenol, it turned out that quantification remained unaffected up to 5 % glycerol plus 0.05 % phenol compared to a matrix without the two components (Table 3). However, higher concentrations caused a dose-dependent decline of detectable Bet v 4 content. This level of robustness towards glycerol and phenol is sufficient for Bet v 4 quantification in PTS and SLIT products as these are diluted at least 200-fold to fit the measurement range of the assay.
Quantification of Bet v 4 in birch allergen products
In total, 30 batches (15 PTS and 15 SLIT products) from six different manufacturers were analyzed (Fig. 4). These extract preparations were controlled by the manufacturers with respect to total protein and total allergenic activity, which were both redetermined. In addition, the content of Bet v 1 and Bet v 4 was assessed using two allergen-specific sandwich ELISAs. The range of Bet v 1 in different PTS was approx. eightfold (6–45 μg/ml), while a larger range of approx. 17-fold was observed in the examined SLIT products (14–241 μg/ml) (Fig. 4a). Interestingly, variability in Bet v 4 content in the two product types was similar, ranging approx. 15-fold both in PTS (0.2–3.1 μg/ml) and in SLIT products (0.3–4.4 μg/ml). According to the correlation coefficient r and the broad confidence interval, the amount of Bet v 4 did not correlate with the respective Bet v 1 content in birch pollen products. In contrast, a highly significant correlation with a narrow confidence interval was found between total allergenic activity and total protein content (Fig. 4b). Total allergenic activity ranged from 20 to 139 U/ml in PTS and from 18 to 228 U/ml in SLIT products, while total protein content was found to be 27–267 and 44–568 μg/ml in PTS and SLIT products, respectively. Note that four PTS batches had to be excluded from the analysis of total protein content because they contained human serum albumin for stabilization. As expected, a highly significant correlation was found for the content of the major birch allergen Bet v 1 with the total allergenic activity and the total protein content, respectively (Fig. 4c, d). In contrast, no correlation between the concentration of the minor allergen Bet v 4 and total allergenic activity or total protein content was observed.
Quantification and correlation of birch pollen allergen product attributes. For 15 PTS and 15 SLIT products, the values for Bet v 1, Bet v 4, total protein content, and total allergenic activity were opposed in several scatter dot plots (PTS in light grey, SLIT products in dark grey). The Pearson correlation coefficient (r) and the respective 95 % confidence interval are given for each pairing. Statistically significant correlations were marked as follows: ***p < 0.001
Discussion
The characterization of allergen products is still based on IgE potency testing and not on the quantification of individual allergen components representing the active substances. ELISA is a common, simple, and easily transferrable high-throughput method, which has been used for allergen quantification for a long time despite its dependence on allergen-specific antibodies raised in animals. It has been suggested as a comparator for validation of quantitative MS. Although some ELISA systems are able to differentiate between protein isoforms [21], MS has the benefit to be a stand-alone technique allowing to differentiate between isoforms even in chemically modified allergen preparations intended for AIT [44, 45]. However, a widespread use of MS in allergen product characterization is still limited due to the high costs of instruments and their operation. At the same time, standardization programs for allergen products like CREATE and the Biological Standardisation Programme (BSP) 090 focus on selected major allergens like Bet v 1 [46–48]. Hence, complementary ELISA systems for quantification of both major and minor allergens are only available for very few allergen sources.
We developed and validated, in-house, the first sandwich ELISA for the determination of the minor birch pollen allergen Bet v 4. High specificity and precision of this method have enabled reproducible and accurate quantification of Bet v 4 in birch pollen extracts. Since the assay is highly sensitive, Bet v 4 quantification is also possible in glycerinated extracts without adapting the dilution buffer of the reference standard. Above the ULoQ at concentrations exceeding 1 ng/ml, the extract curve showed a decreased slope compared to the Bet v 4 reference (Fig. 2a). Although the OD values in this high Bet v 4 range partially surpass OD = 3, the standard deviation did not increase significantly compared to the measurement range. Hence, a measuring device-related artifact due to high OD values is unlikely. Alternatively, the phenomenon could be caused by nBet v 4 isoforms displaying epitope differences and exceeding a critical concentration. However, as only one isoform (Bet v 4.0101, GenBank X87153.1) has been described so far, the best explanation for the lower extract slope above the ULoQ is a matrix effect due to the high birch pollen extract concentrations. The ELISA and its components were found to be specific for Bet v 4 as they did not exhibit any reactivity to other birch pollen allergens (Table 1, Fig. 1). Although some of the tested birch allergen products contain nearly 500-fold more Bet v 1 than Bet v 4, the specificity of the antibodies as well as of the ELISA itself is sufficiently high to ensure valid results. Unfortunately, cross-reactivity to the minor birch allergen Bet v 8 [49] could not be tested due to a lack of availability. As it is unclear whether this minor allergen is contained in the birch pollen extract examined in immunoblot analysis (Fig. 1), the absence of an unspecific signal corresponding to the size of Bet v 8 can only be regarded as an indication for antibody specificity.
The allergen content in an extract-based product is influenced by various factors like the year and season of the harvest as well as the maturity grade of the source material [50, 51]. In addition, the composition of allergen isoforms was shown to differ in pollen from different origins [15] and differences in industrial processing of the raw material might influence the content and stability of proteins in allergen extracts. Especially polcalcins like Bet v 4, which depend on binding of Ca2+ ions to maintain both stability [30] and allergenicity [16, 52], might be affected by differences in manufacturing processes. Nevertheless, Bet v 4 could be detected at levels above the LLoQ in all birch allergen products analyzed in this study. This result is in contrast to published data which indicated that some commercially available birch pollen extracts did not contain Bet v 4 [2]. The reason for this apparent discrepancy might be a difference in sensitivity of the applied methods (western blotting versus ELISA). However, our data also shows remarkable differences between the products with respect to their Bet v 4 content, irrespective of their intended use in either birch pollen allergy diagnosis or therapy (Fig. 4). Great variability was also found in total allergenic activity, Bet v 1, and total protein content. This observation is in line with other studies showing a similarly great variability in allergen content in natural allergen extracts of various sources [4, 53, 54]. Nevertheless, our data confirm that the factors currently used for defining the quality of allergen extracts from birch pollen (total protein content, total allergenic activity, and Bet v 1 content) are clearly interdependent. Interestingly, none of the investigated factors correlated with the minor allergen Bet v 4. This finding is important with respect to the goal of allergen product standardization. Despite the identification of Bet v 4 as a pan-allergen, there is no data available from controlled clinical trials clarifying whether or not Bet v 4 is important for the success of AIT in birch pollen-allergic patients sensitized to this allergen. However, our data indicate that, due to a lack of correlation between Bet v 4 and the parameters routinely assessed in batch control, it will be difficult to establish Bet v 4 content as an additional specification in the control of birch pollen extracts. As more data on content and variability of Bet v 4 in birch pollen extracts is required, the ELISA presented in this study will be used further on to monitor Bet v 4 in birch allergen products and thus contribute to the understanding of the importance of minor allergens for allergic patients and AIT.
Abbreviations
- AIT:
-
Allergen immunotherapy
- Bet v:
-
Birch allergen (Betula verrucosa, nomenclature according to the International Union of Immunological Societies)
- ELISA:
-
Enzyme-linked immunosorbent assay
- mAb:
-
Monoclonal antibody
- MS:
-
Mass spectrometry
- nBet v:
-
Native birch allergen
- PTS:
-
Prick test solution
- rBet v:
-
Recombinant birch allergen
- SLIT:
-
Sublingual immunotherapy
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
References
Akkerdaas JH, Wensing M, Knulst AC, Krebitz M, Breiteneder H, de Vries S, Penninks AH, Aalberse RC, Hefle SL, van Ree R (2003) How accurate and safe is the diagnosis of hazelnut allergy by means of commercial skin prick test reagents? Int Arch Allergy Immunol 132(2):132–140
Focke M, Marth K, Valenta R (2009) Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Investig 39(5):429–436
Focke M, Marth K, Flicker S, Valenta R (2008) Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy 38(8):1400–1408
Kaul S, Luttkopf D, Kastner B, Vogel L, Holtz G, Vieths S, Hoffmann A (2007) Mediator release assays based on human or murine immunoglobulin E in allergen standardization. Clin Exp Allergy 37(1):141–150
van Ree R (1997) Monoclonal versus monospecific polyclonal antibodies and purified major allergens: their application in allergen standardization. Arb.Paul Ehrlich Inst.Bundesamt Sera Impfstoffe Frankf A M. 91:174–176
(2007) CHMP: guideline on allergen products: production and quality issues, EMEA/CHMP/BWP/304831/2007
(2010) Allergen products, general monographs. In: European pharmacopoeia, 6.6 und 7.3, Strasbourg, France: Council of Europe, pp 6903–6906 und 5541–5544
Arilla MC, Ibarrola I, Garcia R, de La Hoz B, Martinez A, Asturias JA (2004) Quantification of the major allergen from cypress (Cupressus arizonica) pollen, Cup a 1, by monoclonal antibody-based ELISA. Int Arch Allergy Immunol 134(1):10–16
Asturias JA, Arilla MC, Aguirre M, Gomez-Bayon N, Martinez A, Palacios R, Sanchez-Gascon F, Martinez J (1999) Quantification of profilins by a monoclonal antibody-based sandwich ELISA. J Immunol Methods 229(1–2):61–71
Arilla MC, Eraso E, Ibarrola I, Algorta J, Martinez A, Asturias JA (2002) Monoclonal antibody-based method for measuring olive pollen major allergen Ole e 1. Ann Allergy Asthma Immunol 89(1):83–89
Duffort O, Calabozo B, Gonzalez R, Carpizo JA, Barber D, Polo F (2004) Monoclonal antibody-based ELISA to quantify the major allergen of Cynodon dactylon (Bermuda grass) pollen, Cyn d 1. Int Arch Allergy Immunol 135(4):277–283
Jimeno L, Duffort O, Serrano C, Barber D, Polo F (2004) Monoclonal antibody-based ELISA to quantify the major allergen of Artemisia vulgaris pollen, Art v 1. Allergy 59(9):995–1001
Ramirez J, Carpizo JA, Ipsen H, Carreira J, Lombardero M (1997) Quantification in mass units of Bet v 1, the main allergen of Betula verrucosa pollen, by a monoclonal antibody based-ELISA. Clin Exp Allergy 27(8):926–931
Seppala U, Dauly C, Robinson S, Hornshaw M, Larsen JN, Ipsen H (2011) Absolute quantification of allergens from complex mixtures: a new sensitive tool for standardization of allergen extracts for specific immunotherapy. J Proteome Res 10(4):2113–2122
Erler A, Hawranek T, Kruckemeier L, Asam C, Egger M, Ferreira F, Briza P (2011) Proteomic profiling of birch (Betula verrucosa) pollen extracts from different origins. Proteomics 11(8):1486–1498
Engel E, Richter K, Obermeyer G, Briza P, Kungl AJ, Simon B, Auer M, Ebner C, Rheinberger HJ, Breitenbach M, Ferreira F (1997) Immunological and biological properties of Bet v 4, a novel birch pollen allergen with two EF-hand calcium-binding domains. J Biol Chem 272(45):28630–28637
Twardosz A, Hayek B, Seiberler S, Vangelista L, Elfman L, Gronlund H, Kraft D, Valenta R (1997) Molecular characterization, expression in Escherichia coli, and epitope analysis of a two EF-hand calcium-binding birch pollen allergen, Bet v 4. Biochem Biophys Res Commun 239(1):197–204
Moverare R, Westritschnig K, Svensson M, Hayek B, Bende M, Pauli G, Sorva R, Haahtela T, Valenta R, Elfman L (2002) Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol 128(4):325–335
Sekerkova A, Polackova M (2011) Detection of Bet v1, Bet v2 and Bet v4 specific IgE antibodies in the sera of children and adult patients allergic to birch pollen: evaluation of different IgE reactivity profiles depending on age and local sensitization. Int Arch Allergy Immunol 154(4):278–285
Rossi RE, Monasterolo G, Monasterolo S (2003) Detection of specific IgE antibodies in the sera of patients allergic to birch pollen using recombinant allergens Bet v 1, Bet v 2, Bet v 4: evaluation of different IgE reactivity profiles. Allergy 58(9):929–932
Foetisch K, Dahl L, Jansen B, Becker WM, Lidholm J, van Ree R, Broll H, Kaul S, Vieths S, Holzhauser T (2011) Development and in-house validation of allergen-specific ELISA tests for the quantification of Dau c 1.01, Dau c 1.02 and Dau c 4 in carrot extracts (Daucus carota). Anal Bioanal Chem 399(2):935–943
Wopfner N, Gruber P, Wallner M, Briza P, Ebner C, Mari A, Richter K, Vogel L, Ferreira F (2008) Molecular and immunological characterization of novel weed pollen pan-allergens. Allergy 63(7):872–881
Ledesma A, Villalba M, Batanero E, Rodriguez R (1998) Molecular cloning and expression of active Ole e 3, a major allergen from olive-tree pollen and member of a novel family of Ca2+-binding proteins (polcalcins) involved in allergy. Eur J Biochem 258(2):454–459
Suphioglu C, Ferreira F, Knox RB (1997) Molecular cloning and immunological characterisation of Cyn d 7, a novel calcium-binding allergen from Bermuda grass pollen. FEBS Lett 402(2–3):167–172
Barber D, de La Torre F, Feo F, Florido F, Guardia P, Moreno C, Quiralte J, Lombardero M, Villalba M, Salcedo G, Rodriguez R (2008) Understanding patient sensitization profiles in complex pollen areas: a molecular epidemiological study. Allergy 63(11):1550–1558
Mari A (2001) Multiple pollen sensitization: a molecular approach to the diagnosis. Int Arch Allergy Immunol 125(1):57–65
Hauser M, Roulias A, Ferreira F, Egger M (2010) Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol 6(1):1
Tinghino R, Twardosz A, Barletta B, Puggioni EM, Iacovacci P, Butteroni C, Afferni C, Mari A, Hayek B, Di FG, Focke M, Westritschnig K, Valenta R, Pini C (2002) Molecular, structural, and immunologic relationships between different families of recombinant calcium-binding pollen allergens. J Allergy Clin Immunol 109(2):314–320
Burrows B, Bloom JW, Traver GA, Cline MG (1987) The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med 317(21):1309–1314
Neudecker P, Nerkamp J, Eisenmann A, Nourse A, Lauber T, Schweimer K, Lehmann K, Schwarzinger S, Ferreira F, Rosch P (2004) Solution structure, dynamics, and hydrodynamics of the calcium-bound cross-reactive birch pollen allergen Bet v 4 reveal a canonical monomeric two EF-hand assembly with a regulatory function. J Mol Biol 336(5):1141–1157
Karamloo F, Wangorsch A, Kasahara H, Davin LB, Haustein D, Lewis NG, Vieths S (2001) Phenylcoumaran benzylic ether and isoflavonoid reductases are a new class of cross-reactive allergens in birch pollen, fruits and vegetables. Eur J Biochem 268(20):5310–5320
Cadot P, Nelles L, Srahna M, Dilissen E, Ceuppens JL (2006) Cloning and expression of the cyclophilin Bet v 7, and analysis of immunological cross-reactivity among the cyclophilin A family. Mol Immunol 43(3):226–235
Reese G, Ballmer-Weber BK, Wangorsch A, Randow S, Vieths S (2007) Allergenicity and antigenicity of wild-type and mutant, monomeric, and dimeric carrot major allergen Dau c 1: destruction of conformation, not oligomerization, is the roadmap to save allergen vaccines. J Allergy Clin Immunol 119(4):944–951
Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166(2):368–379
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal.Biochem. 72(0003–2697 (Linking)):248–254
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2005) ICH-Guideline Q2 (R1) Validation of analytical procedures: text and methodology. http://www.ich.org
European Pharmacopoeia (2008) Statistical Analysis of Results of Biological Assays and Tests. Strasbourg, France, Council of Europe. Ph. Eur. 8th edn, pp 607–635
Food and Drug Administration, Center for Drug Evaluation and Research (2001) Guidance for industry: bioanalytical method validation
Wild D (2005) The immunoassay handbook. Elsevier Ltd, Oxford
Nič M, Jirát J, Košata B, Jenkins A, McNaught A (eds) (2009) IUPAC compendium of chemical terminology. IUPAC, Research Triangle Park, NC
European Medicines Agency (2011) Guideline on bioanalytical method validation
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6(2):65–70
R Core Team (2014) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria
Reuter A, Luttkopf D, Vieths S (2009) New frontiers in allergen standardization. Clin Exp Allergy 39(3):307–309
Carnes J, Himly M, Gallego M, Iraola V, Robinson DS, Fernandez-Caldas E, Briza P (2009) Detection of allergen composition and in vivo immunogenicity of depigmented allergoids of Betula alba. Clin Exp Allergy 39(3):426–434
Neske F, Schorner C, Buchheit KH, Costanzo A, Hanschmann KM, Himly M, Holzhauser T, Ferreira F, Kaul S, Vieths S, van RR (2009) BSP090—the follow-up to CREATE. Arb.Paul Ehrlich Inst.Bundesamt Sera Impfstoffe Frankf A M. 96(0936–8671 (Linking)):12–19
Vieths S, Kaul S, Hanschmann KM, Schorner C, Barber D, Ledesma A, Chapman M, Hrabina M, Moingeon P, Fiebig H, Reese G, Weber B, van RR, Buchheit KH, Costanzo A (2012) Establishment of recombinant major allergens Bet v 1 and Phl p 5a as Ph. Eur. reference standards and validation of ELISA methods for their measurement. Results from feasibility studies. Pharmeur.Bio Sci.Notes 2012(2075–2164 (Linking)):118–134
van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, Villalba M, Durham SR, Becker WM, Aalbers M, Andre C, Barber D, Cistero BA, Custovic A, Didierlaurent A, Dolman C, Dorpema JW, Di FG, Eberhardt F, Fernandez CE, Fernandez RM, Fiebig H, Focke M, Fotisch K, Gadermaier G, Das RG, Gonzalez ME, Himly M, Kinaciyan T, Knulst AC, Kroon AM, Lepp U, Marco FM, Mari A, Moingeon P, Monsalve R, Neubauer A, Notten S, Ooievaar-de HP, Pauli G, Pini C, Purohit A, Quiralte J, Rak S, Raulf-Heimsoth M, San Miguel Moncin MM, Simpson B, Tsay A, Vailes L, Wallner M, Weber B (2008) The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy 63(3):310–326
Mahler V, Fischer S, Heiss S, Duchene M, Kraft D, Valenta R (2001) cDna cloning and characterization of a cross-reactive birch pollen allergen: identification as a pectin esterase. Int Arch Allergy Immunol 124(1–3):64–66
Esch RE (1997) Allergen source materials and quality control of allergenic extracts. Methods 13(1):2–13
Castro AJ, de Dios AJ, Cuevas J, Romero PJ, Alche V, Rodriguez-Garcia MI (2003) Pollen from different olive tree cultivars contains varying amounts of the major allergen Ole e 1. Int Arch Allergy Immunol 131(3):164–173
Hayek B, Vangelista L, Pastore A, Sperr WR, Valent P, Vrtala S, Niederberger V, Twardosz A, Kraft D, Valenta R (1998) Molecular and immunologic characterization of a highly cross-reactive two EF-hand calcium-binding alder pollen allergen, Aln g 4: structural basis for calcium-modulated IgE recognition. J Immunol 161(12):7031–7039
Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, Valenta R, Vrtala S (2012) Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol 159(3):253–262
Eichler I, Gotz M, Jarisch R, Eichler HG, Moss R (1988) Reproducibility of skin prick testing with allergen extracts from different manufacturers. Allergy 43(6):458–463
Acknowledgments
This study was realized thanks to the support of ALK-Abelló Arzneimittel GmbH, Allergopharma GmbH & Co. KG, Bencard Allergie GmbH, HAL Allergy GmbH, Leti Pharma GmbH, Stallergenes GmbH (listed in alphabetical order), and their representatives, giving consent for the use of their birch pollen extracts designated for batch release control at the PEI. Many thanks go to Prof. Dr. Paul Rösch, University of Bayreuth, Germany, for providing the recombinant Bet v 4.
Conflict of interest
In the past 5 years, Stefan Vieths has received a speaker’s honorarium from Phadia (now Thermo Fisher Scientific), Uppsala; an honorarium for organizing and chairing an educational seminar on food allergens from Fresenius Academy, Dortmund; honoraria for acting as expert reviewer for the Allergen Online Database of the Food Allergy Resource and Research Program, Lincoln NE; and consultancy fees by the Institute for Product Quality, Berlin.
Thomas Holzhauser had consultant arrangements with Institut für Produktqualität and Monsanto Company. He received fees from Die Akademie Fresenius for speaking and organizing education. He received reimbursement for lectures from the European Academy of Allergology and Clinical Immunology, World Allergy Organization, Deutscher Allergie- und Asthmabund e.V., International Life Sciences Institute/Health and Environmental Sciences Institute, International Association for Food Protection, AOAC International, Chinesisch-Deutsches Zentrum für Wissenschaftsförderung, Phadia AB, and SAG Süderelbe Projektgesellschaft AG & Co. KG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver Dehus and Julia Zimmer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dehus, O., Zimmer, J., Döring, S. et al. Development and in-house validation of an allergen-specific ELISA for quantification of Bet v 4 in diagnostic and therapeutic birch allergen products. Anal Bioanal Chem 407, 1673–1683 (2015). https://doi.org/10.1007/s00216-014-8418-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8418-z