Abstract
Five double-target multiplex plasmids to be used as calibrants for GMO quantification were constructed. They were composed of two modified targets associated in tandem in the same plasmid : (1) a part of the soybean lectin gene and (2) a part of the transgenic construction of the GTS40-3-2 event. Modifications were performed in such a way that each target could be amplified with the same primers as those for the original target from which they were derived but such that each was specifically detected with an appropriate probe. Sequence modifications were done to keep the parameters of the new target as similar as possible to those of its original sequence. The plasmids were designed to be used either in separate reactions or in multiplex reactions. Evidence is given that with each of the five different plasmids used in separate wells as a calibrant for a different copy number, a calibration curve can be built. When the targets were amplified together (in multiplex) and at different concentrations inside the same well, the calibration curves showed that there was a competition effect between the targets and this limits the range of copy numbers for calibration over a maximum of 2 orders of magnitude. Another possible application of multiplex plasmids is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiplex PCRs consist in the amplification of several targets together in the same reaction well. Depending on the number of amplified targets, the reaction can be a duplex (two targets) [1], triplex (three targets) [2], quadruplex (four targets) [3], pentaplex (five targets) [4], hexaplex (six targets) [5], heptaplex (seven targets) [6], octaplex (eight targets) [7] or nonaplex (nine targets) [8–10] reaction.
Multiplex products can be distinguished on agarose gel [1–4, 6–8, 10] thanks to size differences, on microarrays [11, 12] thanks to different hybridization properties or by capillary gel electrophoresis [5, 9, 13] (differences in size and dye colour). Distinction of targets on agarose gel involves amplifying targets of different sizes. The more targets that are amplified in the same well, the wider is the range of fragment size that must be considered to allow good distinction on a gel. This situation can lead to a decrease of sensitivity when processed products are tested.
Results of multiplex PCR can also be obtained faster by using real-time PCR. This can be done using different chemistries such as (1) SYBR Green, where amplicons are distinguished by their melting temperature (T m) after a distinction protocol [14], or (2) hydrolysis probes (such as TaqMan probes), where amplicons are distinguished by the reporter dyes bound to the probes [15]. In the case of real-time PCR, there are generally not more than five amplified targets in the same reaction. This is due to the limited number of channels available on real-time PCR devices but also to the number of dyes available and that are clearly distinguishable from each other.
Another important difference to be made between different types of multiplex reactions is the fact that several targets may be amplified with (1) different primers, (2) common primers [16] or (3) bipartite primers [17].
When a multiplex reaction is used for GMO quantification, two targets are simultaneously amplified: a part of an endogenous gene (generally a single copy gene specific for the plant species considered) and a transgenic target [18].
The primer concentration has to be adjusted to obtain accurate threshold cycles (Ct) for both targets. A limited primer concentration for the endogenous target permits one to limit its amplification. This avoids consumption of the reagents by the most abundant target and decreases the competition effect acting on the detection level of the transgenic target.
Different types of external calibrants can be used for GMO quantification. Standards can be made of DNA extracted from certified reference materials (CRMs) consisting of flours containing different GMO contents [19]. With this technique, the GMO percentage is generally calculated as a mass fraction. The production of CRMs is an extremely complex, time-consuming and expensive process [20]. To comply with European regulations, specific CRMs must be produced for each approved GM line. Commission Recommendation 2004/787/EC [21] introduced the concept of haploid genome equivalents to express the results of quantitative analyses as GM-DNA copy numbers in relation to target taxon-specific DNA copy numbers. In this way, the use of linearized plasmid DNA as a standard provides a cheaper and more flexible alternative to conventional reference material [20]. Some papers indicate that GMO quantification could differ if genomic DNA or plasmid DNA is used for the GMO percentage determination [22, 23]. An interlaboratory trial was conducted to compare plasmid and genomic DNA for quantitative PCR calibrations and showed that plasmid DNA constitutes a suitable alternative to genomic DNA as a calibrant [24]. This was also shown by other independent studies [25–27]. Validated plasmid calibrants [28] are also commercially available (http://www.diagenode.com) and constitute tools widely used for GMO quantification.
For the first time, plasmids containing a single marker insert were built (single-target plasmids). Two targets are needed for GMO quantification: a species-specific target (endogenous target) and a transgenic target (construct- or event-specific). With single-target plasmids, the use of two plasmids to establish the calibration curve might be a source of problems because the concentrations of the plasmids must be determined very precisely and no variation in concentration caused by dilution is tolerated. The problem can be solved by integrating the two fragments [29] or more [30, 31] into the same plasmid. The endogenous and the transgenic target are present in a 1:1 ratio. Such a tandem-marker plasmid represents an ideal construction because it permits one to carry out the relative quantification regardless of accurate evaluation of the plasmid concentration. Presently, standards have exactly the same sequence as the targets to be detected.
In this paper we propose a new type of calibrant: multiplex calibrants. They consist of tandem-marker plasmids containing modified sequences of the original targets. Inserts were amplified with the same primers as those used on the original targets but a modification of the internal sequence was created to distinguish the calibrants from the original sequence.
We show their application in relative GMO quantification with some limits due to competition effects when they are used in multiplex.
Experimental
Soybean samples
The samples used consisted of transgenic CRM developed by the Institute for Reference Materials and Measurements (IRMM; Geel, Belgium). They were made of powdered dried soybean (IRMM 410) containing 0.1, 0.5, 1, 2 or 5% Roundup Ready soybean expressed in percentage of mass. Commercial Roundup Ready soybean kernels were also used after they had been ground with a ZM100 grinder (Retsch, Haan, Germany).
Commercial multiple-target plasmid DNA
The plasmid reference molecule constructed by Kuribara et al. [30] and validated in an interlaboratory trial by Shindo et al. [28] was used for comparison of results. The plasmid contains a DNA sequence of the construct of event GTS 40-3-2 (Roundup Ready soybean), parts of the sequences of Cauliflower mosaic virus promoter p35S, the Agrobacterium tumefaciens nopaline synthase terminator Tnos and the soybean lectin gene. The plasmid is a linearized DNA, digested with a restriction enzyme and diluted in salmon sperm DNA.
Plasmid solutions containing 20,125, 1,500, 20,000 and 250,000 copies (absolute amounts in 2.5 μl) were provided as one set [GM soybean (RRS) detection plasmid set] by Diagenode (Liège, Belgium).
DNA isolation on CRMs was performed with a Wizard magnetic DNA purification system for food kit (Promega, Madison, USA) on a test portion of 50 mg. DNA was eluted in 150 µl of elution buffer. Extraction was performed with a King Fisher robot (Thermo Life Sciences, Basingstoke, UK). Plasmid DNA was isolated from bacterial cultures with a High Pure plasmid isolation kit from Roche Diagnostics (Mannheim, Germany). Plasmids were linearized with BamHI enzyme (Roche Diagnostics, Mannheim, Germany) and the products of this linearization (10 U of BamHI were used per microgram of plasmid DNA in 1 h at 37 °C) were purified with the last steps of a phenol-chloroform extraction [32]. The quality and quantity of DNA extracted from the samples were determined by using a UV/vis 918 spectrophotometer (GBC, Australia) and measuring the absorbance at 260 nm (A 260) and 280 nm (A 280). The DNA purity was determined by using the A 260/A 280 ratio.
Primers and probes were synthesized by Eurogentec (Seraing, Belgium). The probes were labelled with the reporter dye FAM at the 5′ end and with the quencher dye TAMRA at the 3′ end. Specific probes and primers common to several constructs are presented in Fig. 1. The specific primers used to create the modified sequences contained the sequence of each common primer but extended at their 3′ end with either the original Roundup Ready soybean or lectin sequence or their several modified counterparts as presented in Figs. 1 and 2a.
Construction of single-target and double-target multiplex plasmids (illustrated for the construction of DMC1). a Design of the modified sequences illustrated for Lecvar1 and RRvar1. b Association of the two modified sequences. Two PCRs were performed in parallel: one PCR with primers 1 and 2 to generate a product of amplification corresponding to the sequence of the lectin calibrator with an additional tail of ten nucleotides; the PCR with primers 3 and 4 gives a product of amplification corresponding to the Roundup Ready soybean calibrator lengthened with a ten nucleotide tail complementary to one of the lectin calibrators. A part of each PCR product was put together and amplified with primers 1 and 4 situated at the ends of the expected double fragment. c Cloning and checking of the double-target multiplex calibrants (DMCs). Fragments associated give a PCR product of 174 bp. Double fragments were cloned into pCR2.1 with a TOPO TA cloning kit (Invitrogen)
Primers used to link the modified fragments (Fig. 2b) had a polyA or polyT tail integrating the sequence of a BclI restriction site (T↓GATCA). Primer 2 in Fig. 2b had the following sequence: 5'AAAA TGATCAGGCATAGAAGGTGAAGTTGAAGGA-3. Primer 3 in Fig. 2b had the following sequence: 5′-TGATCA TTTTGCCATGTTGTTAATTTGTGCCAT-3′.
Construction of double-target multiplex plasmids
The technique of association of the modified fragments consists in amplifying each fragment with a nontailed and a tailed primer (Fig. 2b). The 3′ and 5′ ends of both tailed sequences were complementary to each other and contained a BclI restriction site to enable possible control of the correct integration of the desired fragments by means of restriction enzymes.
Combinations of the two fragments in each of the five versions were tested with primers Lectin-F and RRS-R located at the ends of the double insert (Fig. 2c) and the appropriate Lecvar and RRvar probes (Fig. 1).
The results show the successful integration of both fragments. Integration of the correct sequences was confirmed by pyrosequencing.
Real-time PCR
Real-time PCRs (35 µl) were performed using an ABI5700 instrument (Applied Biosystems, Foster City, CA, USA) with the quantitative PCR MasterMix (Eurogentec, Seraing, Belgium). For 17.5 µl of MasterMix, 2.5 µl of each primer (5 mM), 2.2 µl of probe (9 mM), 5.3 µl of doubly distilled water and 5 µl of DNA were added.
Reaction mixes were distributed in a 96-well reaction plate (Eurogentec). The wells were covered with optical caps (Eurogentec). The plates were centrifuged (500 rpm, 10 s) to eliminate possible air bubbles at the bottom of the wells.
The PCR conditions were as follows: 2 min at 50 °C, 10 min to 95 °C and 50 cycles of 15 s at 95 °C and 1 min at 60 °C. The baseline (normalized fluorescence signal before exponential PCR amplification occurs) was adjusted and a threshold level of fluorescence was fixed in the middle of the linear region of the amplification curve when represented on a semilogarithmic graph (y-axis with log fluorescence level as a function of cycles). This permitted us to compare the different Ct values of the samples.
Qualitative PCR
PCRs for cloning (20 µl) were performed using a GeneAmp9600 instrument (Applied Biosystems, Foster City, CA, USA). The reaction mix was composed of 2 µl of tenfold-concentrated buffer, 2 µl of 2.5 mM MgCl2, 2 µl of 0.2 mM dNTPs, 2 µl of each primer (5 mM), 0.75 U (0.1 µl) of platinum Taq DNA polymerase, 6.9 µl of doubly distilled water and 3 µl of three times diluted DNA. Buffer, MgCl2, dNTPs, Taq polymerase and primers were purchased from Invitrogen (Merelbeke, Belgium).
The PCR conditions were as follows: 4 min at 95 °C and 35 cycles of 15 s at 94 °C, 15 s at 60 °C and 15 s at 72 °C. A final elongation was done for 5 min at 72 °C. Once the run had ended, the tubes were kept at 4 °C.
Cloning into pCR2.1
The amplified fragments were ligated into pCR2.1 plasmid vector (Invitrogen, Merelbeke, Belgium) by using a TOPO TA cloning kit (Invitrogen, Merelbeke, Belgium). The PCR results were visualized on 2.5% agarose gel and the concentration was estimated by means of a visual comparison with a quantitative molecular weight marker (SmartLadder, Eurogentec, Seraing, Belgium).
The fragments were cloned into the 3.9-kb pCR2.1-TOPO vector following the instructions for the TOPO TA cloning kit.
Subcloning into pUC18
pUC18 (10 µg) vector was cut with 100 U of HindIII and XbaI (Roche Diagnostics, Germany) to prepare the vector for integration of the fragment of interest.
pCR2.1 plasmids with the integrated targets were cut with the same two restriction enzymes (10 µg of plasmid with 100 U of HindIII and XbaI) to retrieve a 285-bp fragment to be inserted in pUC18.
Purification and extraction of bands on low-melting gel were done following the recommendations for the QIAquick gel extraction kit (QIAGEN, Germany). The amounts of DNA were estimated on gel by means of visual comparison with a quantitative molecular weight marker (SmartLadder, Eurogentec, Liège, Belgium) and volumes corresponding to 10 ng of the fragments were used for ligation into the pUC18 vector.
The molar ratio of the vector and the fragment for the ligation was 1:4 (sticky ends). Ligation was done in a final volume of 60 µl with 2 U of T4 DNA ligase (Roche Diagnostics, Germany) following the recommendations of the manufacturer (catalogue no. 716359, Roche Diagnostics)
Subsequent transformation to competent Escherichia coli TOP10 cells (Invitrogen) and plasmid DNA extraction were done as described already.
Plasmid copy number determination
The amount in terms of copy number of plasmid stock solution was determined as follows. The quantity of plasmid DNA was determined by UV spectrophotometry taking into consideration that 1 U of absorbance at 260 nm corresponds to a concentration of 50 µg/ml. The molar weight of one plasmid molecule is derived from the combination of its size in base pairs and the mean molar weight of one base pair set at 635 Da [32]. Knowing the number of moles available, one can deduce the copy number of plasmids by means of the Avogadro number.
Dilutions of plasmids
Dilutions were performed in water using low-retention tubes (Eppendorf, Hamburg, Germany) and tips (Molecular BioProducts, San Diego, CA, USA). The DNA quantities were first adjusted to 109 copies per 5 µl. Successive dilutions were realized to reach the following copy numbers in 5 µl of solution : 250,000 ± 19,000, 25,000 ± 2,000, 5,000 ± 400, 1,000 ± 90 and 100 ± 9 or 3,000,000 ± 200,000, 300,000 ± 23,000, 30,000 ± 2,500, 3,000 ± 260 and 300 ± 28. The uncertainty of measurement given for these figures only takes into consideration the pipetting errors during dilution. The uncertainty on the concentration of the starting stock solution was not considered because it is of less importance for the interpretation of the results. Dilutions were done stepwise without exceeding tenfold dilution factors.
Pyrosequencing
Biotinylated RRS-R and Lectin-R primers were ordered from Eurogentec. PCRs were performed with the biotinylated reverse primers and the common forward primers in a total volume of 50 µl containing 5 µl (10 ng) of genomic DNA, 5 µl tenfold-concentrated buffer, 4 µl of 2.5 mM MgCl2, 4.5 µl of 0.2 mM dNTPs, 2 µl of each primer (5 mM), 1.25 U (0.25 µl) of platinum Taq DNA polymerase and 27.25 µl of doubly distilled water. Buffer, MgCl2, dNTPs, Taq polymerase and primers were purchased from Invitrogen (Carlsbad, CA, USA)
The PCR conditions were as follows: 4 min at 95 °C and 50 cycles of 15 s at 94 °C, 15 s at 60 °C and 15 s at 72 °C. Once the run had ended, the tubes were stored at −20 °C.
Immobilization of 10 µl of PCR products onto streptavidin-coated beads, separation of strands by denaturation in NaOH, neutralization of the immobilized strands and annealing of sequencing primers were done with the vacuum Prep Tool (AB Biotage, Sweden) by following the sample preparation guidelines for the PSQ96 system (http://www.pyrosequencing.com). Pyrosequencing was performed by using a PSQ96 analyser (AB Biotage, Sweden) according to the manufacturer's instructions.
Results and discussion
Construction of single-target multiplex calibrants
The original sequences of interest correspond to the p35S-CTP junction of the transgenic construct of the GTS 40-3-2 event in soybean and to a part of the soybean lectin gene for the endogenous target. These two regions are routinely used for GMO quantification purposes.
Primers and probes used were validated [33] and produced amplicons of approximately 80 bp.
The newly designed targets have to address some requirements. They have to be amplified with an efficiency similar to that of the original target and must enable separate discrimination of each target through use of specific probes either in real-time PCR or on microarrays
To obtain new targets with characteristics as similar as possible to the original ones, they have to be amplified with the same primers. Distinction between the various newly designed targets was possible thanks to a variation of their internal sequence. To yield amplicons with similar T m, the sequence of the probe was reshaped just by changing the order of the bases in-between the primers in such a way that the new sequence was specific to the new target but kept the same T m and GC percentage as the target from which it was derived (Table 1).
Sequences of all the modified targets were synthesized de novo. Because the sequence of the p35S-CTP target had a size of 83 bp, five sets of two oligonucleotides (of 57 and 59 bp) having complementary 3′ end sequences corresponding to the modified sequence of their respective probe region were prepared (Fig. 2a). During PCR, the primers hybridize and the missing 3′ ends are synthesized, giving the newly expected sequences. The so-created targets can be amplified with the same primers as the original targets. Each amplicon was then cloned in a plasmid.
Five different sequences showing variations of the internal sequence for the Roundup Ready soybean target were built (Fig. 1a). Similarly, five variants were built for the lectin target (Fig. 1b).
The newly created sequences are identical to the original sequence in terms of their length, GC percentage and T m following the equation \( {T_{\rm{m}}} = 81.5 + 16.6 \times \left[ {{\hbox{lo}}{{\hbox{g}}_{{10}}}\left( {{\hbox{salt}}\,{\hbox{concentration}}\,{\hbox{in}}\,{\hbox{moles}}\,{\hbox{per}}\,{\hbox{litre}}} \right)} \right] + 0.41 \times \left( {\% {\hbox{GC}}} \right) - \left( {{{675} \mathord{\left/{\vphantom {{675} {{\hbox{length}}\,{\hbox{in}}\,{\hbox{base}}\,{\hbox{numbers}}}}} \right.} {{\hbox{length}}\,{\hbox{in}}\,{\hbox{base}}\,{\hbox{numbers}}}}} \right) \). Their respective T m values calculated by Primer Express (Applied Biosystems) presented some differences because in this software program the position of the different bases in the probe area is taken into account (Table 1).
Once cloning had been done in pCR2.1, the presence of inserts was checked by PCR using RRS-F and RRS-R for Roundup Ready soybean amplicons and Lectin-F and Lectin-R for lectin plasmids (Fig. 2a).
Absence of cross-hybridization between probes and modified sequences was tested by real-time PCR (data not shown). The tests concluded that the probes fit well to their respective target and there was no interaction with the original sequence nor with the other modified inserts.
These plasmids built with modified sequences as shown in Fig. 1 constitute two sets of single-target multiplex plasmids (here also called single-target multiplex calibrants).
Such single-target multiplex plasmids may be of interest as internal PCR controls to show, for instance, that in prespotted plates [34] the absence of specific amplification of a defined target is not due to defective reagents or complete inhibition. However, for quantification purposes with GMOs single-target multiplex plasmids are not appropriate.
Construction and testing of double-target multiplex calibrants
Five plasmid standards having a different internal region were constructed for the endogenous target (the lectin target) and for the transgenic target (35 S-CTP-EPSPS junction) (see Fig. 2) because a calibration curve should be based on five points [35]. Use of ten plasmids (five for the endogenous target and five for the transgenic target) would have complicated the manipulations and led to the risk of errors. Moreover, for GMO quantification, single plasmids are not good calibrants because plasmids with the endogenous and transgenic targets are separately quantified and errors at this step may have a great impact on quantification. Correct plasmid calibrants should integrate endogenous and transgenic targets in tandem so targets are present in exactly the same number of copies.
Associations of modified transgenic and endogenous targets were done as explained in “Experimental” to construct five different double-target multiplex calibrants (DMCs): DMC1 (Lecvar1 and RRvar1), DMC2 (Lecvar2 and RRvar2), DMC3 (Lecvar3 and RRvar3), DMC4 (Lecvar4 and RRvar4) and DMC5 (Lecvar5 and RRvar5). The newly constructed plasmids were each derived from a single colony of transformant. In all of them, the presence of the double inserts was checked by real-time PCR using the four possible combinations of primer pairs and probe (Table 2). The results for Ct in this table are consistent with a tandem presence of the targets.
Double inserts were transferred from pCR2.1 plasmids to pUC18 to respond to the requirements of the p-ENGL library and for deposition in the official plasmid and complementary DNA collection (LMBP) of the Belgian Coordinated Collection of Micro-Organisms (BCCM), centralized at Ghent University. Integration of the correct sequences was confirmed by pyrosequencing.
The modified lectin fragments were first tested independently with Lectin-F and Lectin-R primers and the appropriate Lecvar probe. This was also done with RRS-F and RRS-R and the appropriate RRvar probe for modified Roundup Ready soybean fragments (Fig. 2c)
For the DMCs, an earlier signal (smaller Ct) was generally obtained for lectin than for Roundup Ready soybean fragments (Table 2). This difference between Ct for the two targets was not due to a problem of construction of the double calibrants as was also observed with the original targets on transgenic soybean grains. DNA extracted from GTS40-3-2 kernels was tested with the original lectin and Roundup Ready soybean targets. Twenty-two repetitions were performed. This led to the following mean Ct values: 24.90 (standard deviation 0.22) for lectin and 25.84 (standard deviation 0.27) for the Roundup Ready soybean targets.
Use of DMCs for quantification
Quantification tests using the DMCs were carried out in two different ways. First, the experiments were done with DMCs present in pCR2.1 plasmids. The quantities used for construction of calibration curves were 3,000,000, 300,000, 30,000, 3,000 and 300 copies of plasmids. Second, experiments were done with the double inserts transferred into pUC18 plasmids. In this case, the quantities used for the calibration curves were 250,000, 25,000, 5,000, 1,000 and 100 copies of plasmids. A range from 3,000,000 to 300 copies has the advantage of showing more pronounced effects but is less representative than the range between 250,000 and 100 copies. This range fits better to the copy numbers encountered in routine analysis. Values below 100 copies were not used here because they generate an additional variability owing to the larger standard variations generally observed for these low copy numbers.
Use of a single DMC in separate amplifications for calibration purposes
An example of calibration curves based on the dilution of one DMC (here DMC2) and quantification of CRMs is presented in Fig. 3. These calibration curves are suitable for Roundup Ready soybean quantification (Table 3). Here evidence is given that a calibration curve can be built with amplicons detected by a probe that is different from the one for the target to be measured, and the use of two such calibration curves results in correct relative quantifications.
In our approach, we favoured a method where several calibrants at different copy numbers could be amplified in the same well.
Use of a different DMC for each calibration point amplified separately
In this experiment, each DMC was quantified and diluted to obtain the following copy numbers in 5 µl (volume used in a PCR) of DNA: 250,000 copies of DMC1, 25,000 copies of DMC2, 5,000 copies of DMC3, 1,000 copies of DMC4 and 100 copies of DMC5.
The DMCs were separately amplified and calibration curves (for lectin and Roundup Ready soybean) were used to quantify five CRMs at 5, 2, 1, 0.5 and 0.1% of Roundup Ready soybean.
The estimated values from calibration curves obtained with Lecvar and RRvar primers and probes are presented in Table 4. They were compared with quantitative determinations obtained with the validated multiple-target plasmids developed by a Japanese Research group [28, 30]. The relative standard deviations (RSDs) meet the performance requirements recommended (RSD ≤ 25%) by the European Network of GMO Laboratories (ENGL) [36]. GMO percentages, RSDs and bias in similar ranges were published earlier in connection with other quantitative (plasmid and genomic) GMO detection systems [25–27, 37].
Like in previous studies, a lower precision and a greater bias were observed for low levels of DNA content compared with higher ones [25, 28, 37, 38] but per GM content the data obtained were not worse with a different DMC for each calibration point than in other published systems which were considered as acceptable.
These results prove that the calibration curves can be built for each calibration point with a different probe that also differs from the one for the target to be measured and that the combination of two such calibration curves can give acceptable [36] relative quantifications in terms of precision and trueness.
Amplification of DMCs in the presence of genomic DNA
The DMC targets were designed in such a way that they can be amplified together and distinguished separately. They can also be distinguished from the original lectin and Roundup Ready soybean sequences. So a DMC could constitute an interesting inhibition control because the target has the same size, T m and GC content as the original target and can be amplified with the same primers and comparable efficiencies. PCR efficiencies were calculated using the slope of calibration curves based on serial dilutions of genomic and plasmid DNA [24]. PCR efficiencies determined on the same materials were comparable for lectin and Roundup Ready soybean targets. The efficiencies of amplification for genomic and plasmid DNA were above 90% and therefore acceptable following the ENGL criterion [36]. The PCR efficiencies for genomic and plasmid DNA were also comparable to those in other studies [24, 25].
In the case of quantification, we have to determine if samples and calibrants can be amplified in the same well. To test the influence of plasmid DNA during amplification on genomic DNA, genomic DNA was amplified with different quantities of plasmid DNA (1% Roundup Ready soybean). DMCs were tested with their appropriate Lecvar and RRvar probes and genomic DNA was tested with the original Lec and RR probes.
The Ct values of genomic DNA in the presence of increased quantities of plasmid DNA showed an influence on detection of lectin and Roundup Ready soybean targets (Table 5). This influence was less pronounced on the lectin target than on the Roundup Ready soybean target. This was due to the fact that there are 100 times more copies of the lectin target than of the Roundup Ready soybean target in the sample tested (CRM containing 1% Roundup Ready soybean target). The Ct values increased in the presence of greater amounts of plasmid DNA and this resulted even in nondetection for the original genomic Roundup Ready soybean target. In other experiments with greater amounts of plasmid DNA (range from 3,000,000 to 300 copies, data not shown) the influence on the lectin target was also well marked.
In the same way, the presence of genomic DNA can influence detection of plasmid DNA present at a low copy level.
This experiment shows us that calibrants and sample DNA to be tested have to be amplified separately unless the plasmids are used in small amounts as internal positive controls to detect possible inhibition.
Amplification of several DMCs in the same reaction mix
The aim of the following experiment was to test if several DMCs, each at a different level, can be amplified in the same reaction mix.
Amplification of five DMCs at different concentrations in the same reaction mix
All calibrants were amplified together to test if amplification of five calibrants was possible by PCR in a single test tube. Each calibrant was present in a certain number of copies (250,000 copies of DMC1, 25,000 copies of DMC2, 5,000 copies of DMC3, 1,000 copies of DMC4 and 100 copies of DMC5). The primers used for lectin and Roundup Ready soybean targets were the same for each calibrant and distinction was possible thanks to the appropriate Lecvar and RRvar probes.
The results in Table 6 show there was an influence of higher concentrations of plasmids, leading to nondetection of lower concentrations. Competition occurred between targets. Amplification of all DMCs in a single PCR tube is therefore not possible for the range of plasmid concentrations considered.
Influence of DMC concentration
To check the influence of the higher quantities of plasmids on lower quantities, several binary mixes were realized. At a fixed copy number of DMC1 (copy number from 300 to 3,000,000) increased amounts of plasmid DMC3 were added. A summary of the result is presented in Table 7.
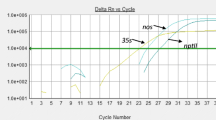
From this experiment, we can conclude that precise quantification of a defined target is hampered by a competitor molecule present in an amount exceeding that of the target by 100 times (we have to note the fact that plasmids DMC1 and DMC3 are amplified with the same primers and this increases competition between plasmids). A 10 times excess seems to have no influence. When we looked at the amplification curves for DMC1, we observed that even in the case where the Ct values remain identical, the level of the plateau phase decreased as a function of the quantities of DMC3 added (Fig. 4a–c). The effect was particularly noticeable for the lowest quantities of plasmid DMC1. Once we reached 300,000 copies of DMC1, at least an equal quantity of DMC3 had to be added to have an influence on the amplification curves (Fig. 4d). The same observation applied to experiments with 3,000,000 copies of DMC1 (Fig. 4e).
Influence of higher plasmid DNA amounts in a mix on the shape of the amplification curves of plasmids introduced in lower amounts (amplifications were realized with the RRS primers and RRvar1 probe): a 300 copies of DMC1, b 3,000 copies of DMC1, c 30,000 copies of DMC1, d 300,000 copies of DMC1 and e 3,000,000 copies of DMC1
These data imply that it is not possible to have a good calibration curve if the five calibrants are amplified together in the same reaction mix. It is better to perform several PCRs to obtain the calibration curve.
Amplification of two or three DMCs at different concentrations in the same reaction mix
Tests were done to divide the calibrants used for the calibration curve into two PCR mixes. To create a calibration curve with five calibrants, one reaction mix contained two calibrants and the second reaction mix contain three calibrants. Both experiments were done with the central concentration (5,000 copies) added either to the two highest or to the two lowest concentrations.
In a mix containing three calibrants at different concentrations, an increase of Ct was always observed for the lowest concentration. So if the central point of the calibration was put in the reaction mix with the highest concentrations, Ct for this point suffered from the effect of competition. If the central point was put with the two lowest concentrations, it was the lowest concentration (100 copies) that suffered from competition.
When we used these calibration curves to quantify CRMs, we observed that points which suffer from competition were outliers and generated a bias in the calibration curve.
In the case of mix 3 + 2 (Fig. 5b), we observed that the central point was an outlier. Owing to its central position, an effect was essentially reported on the coefficient of determination (R 2) and the estimation of the GMO percentage was similar to the values in Table 4. In the case of mix 2 + 3 (Fig. 5c), the values for 100 copies generated a bias leading to large overestimation of percentages. Better results were obtained when the point was suppressed. These results confirmed the observation in “Influence of DMC concentration” that a DMC cannot be amplified in the presence of more than 10 times of a competitor without being subject to competition.
Calibration curves obtained with plasmids amplified separately or in different mixes. a DMC amplified separately (DMC1, 250,000 copies; DMC2, 25,000 copies; DMC3, 5,000 copies; DMC4, 1,000 copies; DMC5, 100 copies). b DMC amplified in two mixes (mix 1: DMC1, 250,000 copies; DMC2, 25,000 copies; DMC3, 5,000 copies; mix 2, DMC4, 1,000 copies; DMC5: 100 copies). The lowest concentration in mix 1 (DMC3, 5,000 copies, central point of the calibration curve) is subject to competition and appears like an outlier in the calibration curve. c DMC amplified in two mixes (mix 1: DMC1, 250,000 copies; DMC2, 2,000 copies; mix 2: DMC3, 5,000 copies; DMC4, 1,000 copies; DMC5, 100 copies). The lowest concentration in mix 2 (DMC5, 100 copies) is subject to competition. DMC5 appears like an outlier in the calibration curve and generates a bias in the calibration curve
Conclusions
One of the aims that was targeted when constructing the DMC plasmids was to build a system that could be used for quantification of GMOs with biochips. That is why different probes for calibrants were required. The experiments outlined in this study clearly show that one of the prerequisites for such a purpose was met. It is perfectly possible to build up a calibration curve with DNA calibrants that differ in their internal sequence from that of the original sequence which will have to be measured with the help of the calibration. To our knowledge this is the first time that proof of concept for this has been shown. However, owing to competition effects, it is clear that the calibrants cannot be amplified together with the sample DNA, at least for the purpose of GMO quantification because the range of copy numbers to consider is very large. As such, this is not a problem, but another limit that was pointed out is the fact that it is also impossible to amplify several calibrants together, once again owing to competition effects linked to the large range of copy numbers to consider for the calibrants. Even when a multiplex reaction with three calibrants is considered, the range of copy numbers is still too wide and results in a significant competition for the calibrant with the lowest copy number. Plasmids as designed here are a very good model to check the limits of multiplex reactions in a quantitative perspective when the same primers are used. A 10 times excess of competitor has no effect, while a 100 times excess of competitor significantly modifies the Ct value. This does not preclude the use of such plasmids with a modified internal sequence as internal PCR controls for qualitative purposes (e.g. in plates such as those designed by the Institute for Health and Consumer Protection of the Joint Research Centre [34]).
References
Permingeat HR, Reggiardo MI, Vallejos RH (2002) J Agric Food Chem 50:4431–4436
James D, Schmidt AM, Wall E, Green M, Masri S (2003) J Agric Food Chem 51:5829–5834
Singh CK, Ojha A, Kachru DN (2007) J AOAC Int 90:1517–1525
Matsuoka T, Kuribara H, Akiyama H, Miura H, Goda Y, Kusakabe Y, Isshiki K, Toyoda M, Hino A (2001) Shokuhin Eiseigaku Zasshi 42:24–32
García-Cañas V, González R, Cifuentes A (2004) Electrophoresis 25:2219–2226
Germini A, Zanetti A, Salati C, Rossi S, Forre C, Schmid S, Marchelli R, Fogher C (2004) J Agric Food Chem 52:3275–3280
Shrestha HK, Hwu KK, Wang SJ, Liu LF, Chang MC (2008) J Agric Food Chem 56:8962–8968
Hernandez M, Esteve T, Pla M (2005) J Agric Food Chem 53:7003–7009
Heide BR, Heir E, Holck A (2008) Eur Food Res Technol 227:527–535
Onishi M, Matsuoka T, Kodama T, Kashiwaba K, Futo S, Akiyama H, Maitani T, Furui S, Oguchi T, Hino A (2005) J Agric Food Chem 53:9713–9721
Hamels S, Glouden T, Gillard K, Mazzara M, Debode F, Foti N, Sneyers M, Teresa E, Pla M, Berben G, Moens W, Bertheau Y, Audeon C, van Den Eede G, Remacle J (2009) Eur Food Res Technol 228:1438–2377
Xu J, Zhu SF, Miao HZ, Huang WS, Qiu MY, Huang Y, Fu XP, Li Y (2007) J Agric Food Chem 55:5575–5579
Nadal A, Coll A, La Paz JL, Esteve T, Pla M (2006) Electrophoresis 27:3879–3888
Hernandez M, Rodriguez-Lazaro D, Esteve T, Prat S, Pla M (2003) Anal Biochem 323:164–170
Huang P, Pan TZ (2004) J Agric Food Chem 52:3264–3268
Leimanis S, Hernández M, Fernández S, Boyer F, Burns M, Bruderer S, Glouden T, Harris N, Kaeppeli O, Philipp P, Pla M, Puigdomènech P, Vaitilingom M, Bertheau Y, Remacle J (2006) Plant Mol Biol 61:123–139
Rudi K, Rud I, Holck A (2003) Nucleic Acids Res 31(11):e62
Foti N, Onori R, Donnarumma E, De Santis B, Miraglia M (2006) Eur Food Res Technol 222:209–216
Weighardt F, Barbati C, Paoletti C, Querci M, Kay S, De Beuckeleer M, Van den Eede G (2004) J AOAC Int 87:1342–1355
Trapmann S, Catalani P, Conneely P, Corbisier P, Gancberg D, Hannes E, Guern L, Kramer GN (2002) The certification of reference materials of dry-mixed soya powder with different mass fractions of Roundup Ready soya. Certified reference materials IRMM-410-S. European Commission, DG-JRC, IRMM. http://irmm.jrc.be/rm/cert-reports/IRMM-410s_report.pdf
European Commission (2004) Recommendation 2004/787/EC. Off J Eur Union L 348:18–24
Charels D, Broeders S, Corbisier P, Trapmann S, Schimmels H, Linsinger T, Emons H (2007) J Agric Food Chem 55:3258–3267
Charels D, Broeders S, Corbisier P, Trapmann S, Schimmels H, Linsinger T, Emons H (2007) J Agric Food Chem 55:3268–3274
Burns M, Corbisier P, Wiseman G, Valdivia H, McDonald P, Bowler P, Ohara K, Schimmel H, Charels D, Damant A, Harris N (2006) Eur Food Res Technol 224:249–258
Taverniers I, Van Bockstaele E, De Loose M (2004) Anal Bioanal Chem 378:1198–1207
Block A, Schwarz (2003) Eur Food Res Technol 216:421–427
Mattarucchi E, Weighardt F, Barbati C, Querci M, Van den Eede G (2005) Eur Food Res Technol 221:511–519
Shindo Y, Kuribara H, Matsuoka T, Futo S, Sawada C, Shono J, Akiyama H, Goda Y, Toyoda M, Hino A (2002) J AOAC Int 85:1119–1126
Yang L, Pan A, Zhang K, Yin C, Qian B, Chen J, Huang C, Zhang D (2005) Transgenic Res 14:817–831
Kuribara H, Shindo Y, Matsuoka T, Takubo K, Futo S, Aoki N, Hirao T, Akiyama H, Goda Y, Toyoda M, Hino A (2002) J AOAC Int 85:1077–1089
Yang L, Guo J, Pan A, Zhang H, Zhang K, Wang Z, Zhang D (2007) J Agric Food Chem 55:15–24
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Association Française de Normalisation (2005) ISO 21570. Foodstuffs - methods of analysis for the detection of genetically modified organisms and derived products - quantitative nucleic acid based methods. Association Française de Normalisation, Paris
Querci M, Kleter G, Malingreau JP, Broll H, Van den Eede G (2008) Scientific and technical contribution to the development of an overall health strategy in the area of GMOs. JRC-IHCP reference reports. Report EUR 23542 EN. http://ec.europa.eu/dgs/jrc/downloads/jrc_reference_report_2008_11_healthstrategy_gmos.pdf
Love JL, Scholes P, Gilpin B, Savill M, Lin S, Samuel L (2006) J Microbiol Method 67:349–356
European Network of GMO Laboratories (2008) Definition of minimum performance requirements for analytical methods of GMO testing. EU DG-JRC ENGL method performance requirements. EU DG-JRC, Ispra. http://gmo-crl.jrc.ec.europa.eu/guidancedocs.htm
Huang C-C, Pan T-Z (2005) J Agric Food Chem 53:3833–3839
Rønning SB, Vaitilingom M, Berdal KG, Holst-Jensen A (2003) Eur Food Res Technol 216:347–354
Acknowledgements
This research was done within a Belgian research project (S-6140) financed by DG4 and DG6 of the former Belgian Federal Ministry of Agriculture. We are grateful to Aurélie Hosselet (HECE Fleurus) for her technical help and Nicole Wellens (Isogen Life Sciences, De Meern, the Netherlands) for advice with pyrosequencing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Debode, F., Marien, A., Janssen, E. et al. Design of multiplex calibrant plasmids, their use in GMO detection and the limit of their applicability for quantitative purposes owing to competition effects. Anal Bioanal Chem 396, 2151–2164 (2010). https://doi.org/10.1007/s00216-009-3396-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3396-2