Abstract
Sample preparation procedures are in most cases sample- and time-consuming and commonly require the use of a large amount of solvents. Automation in this regard can optimize the minimal-needed injection volume and the solvent consumption will be efficiently reduced. A new fully automated sample desalting and pre-concentration technique employing microextraction by packed sorbents (MEPS) cartridges is implemented and coupled to an ion cyclotron resonance Fourier-transform mass spectrometer (ICR-FT/MS). The performance of non-target mass spectrometric analysis is compared for the automated versus off-line sample preparation for several samples of aqueous natural organic matter. This approach can be generalized for any metabolite profiling or metabolome analysis of biological materials but was optimized herein using a well characterized but highly complex organic mixture: a surface water and its well-characterized natural organic matter and a marine sample having a highly salt charge and enabling to validate the presented automatic system for salty samples. The analysis of Suwannee River water showed selective C18-MEPS enrichment of chemical signatures with average H/C and O/C elemental ratios and loss of both highly polar and highly aromatic structures from the original sample. Automated on-line application to marine samples showed desalting and different chemical signatures from surface to bottom water. Relative comparison of structural footprints with the C18-concentration/desalting procedure however enabled to demonstrate that the surface water film was more concentrated in surface-active components of natural (fatty acids) and anthropogenic origin (sulfur-containing surfactants). Overall, the relative standard deviation distribution in terms of peak intensity was improved by automating the proposed on-line method.

The efficient use of MEPS technique in automated form with high resolution FT-ICR-MS gives a selective spectrum, which reveals rich see water organic content. The shown high sensitivity of detected organic matter is due to effective desalting achieved by automated MEPS coupling to FT-ICR-MS technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To date, the composition of dissolved organic matter (DOM), a major component of natural organic matter (NOM), is not well characterized at the molecular level [1]. This meaningful information is only accessible through the use of complementary high-resolution analytical technologies involving separation techniques, spectrometry and spectroscopy [2]. The key role of DOM in environmental chemistry and general carbon and element cycling [3, 4] necessitates an in-depth understanding of its structure and chemistry. DOM in both seawater and freshwater ultimately originates mainly from biological organic matter (e.g., carbohydrates, amino acids and lipids) that underwent diagenesis and humification at various levels, i.e., DOM is a product of successive biological and abiotic transformations in which most of the biotic signature has been lost [2, 5]. In general, marine DOM is found more aliphatic than terrestrial DOM with lower aromaticity and lesser phenolic hydroxyl group content [6]. For more details about humified materials, NOM and DOM readers are referred to Ref [7].
Mass spectrometry characterization of NOM, commonly relies upon various ionization like matrix-assisted laser desorption/ionization (MALDI) [8–12] or electrospray ionization (ESI) [13–21]. While the combined use of various ionization methods produces insightful complementary molecular signatures that enable advanced comprehension of the structural diversity of NOM [22], ESI is the most common choice for soft ionization and mass analysis of natural organic matter in positive and negative ion mode with a decent coverage of the NOM compositional space, forming single- and multiple-charged ions depending on experimental conditions [23].
Ultrahigh-resolution mass spectrometry Fourier-transform ion cyclotron resonance (ICR-FT/MS) at high magnetic fields is mandatory to achieve adequate resolution of the very intricate mixture composing DOM. Mass resolution in excess of 400,000 and mass accuracies with uncertainties of lower than 0.2 mg L−1 allow a complete peak resolution of any C,H,O- molecular composition in DOM [5]. The determination of molecular formulas of fulvic acid molecules have been demonstrated previously [13, 24] as well as the resolution of >6000 molecular formulas of Suwannee river fulvic acid (SRFA) [22, 25] and ultrahigh mass resolution has been used to differentiate NOM samples from different sources and various locations [26].
Saltwater samples such as native ocean water with a salt content in the range of 35 g L−1 and a NOM content in the range of 1 mg L−1 produce extensive yields of salt-derived cluster ions in ESI-ICR-FT/MS mass spectra, thereby obscuring signatures of NOM organic molecules because of pronounced ion suppression. Different means of marine NOM enrichment such as ultrafiltration techniques [27] or reverse osmosis following electrodialysis [28] have been implemented. In addition, solid-phase extraction (SPE) using various resins including C18 sorbents have been previously used for NOM enrichment out of the aqueous phase [29, 30]. Although common SPE cartridges can be used for this purpose, commercially available microextraction by packed sorbents (MEPS) with C18 sorbent represents an attractive choice in order to minimize the sample amount and automate the coupling between the sample treatment and the analyzer. The C18 phase did not show the best NOM extraction efficiency for marine water [30] but was the only reverse-phase material available as MEPS. Ideally, the sample requirements during preparation should correspond to the final sample volume needed for its analysis, enabling minimal sample consumption. The use of automated devices and less time-consuming methodologies which involve less human manipulation, will likely result in lesser sample wastage, more accurate reproducibility while being more economic and environmentally benign. MEPS which is also called microextraction by packed syringe, is the integration of a miniaturized SPE bed with a microvolume syringe (100–250 μL). Commercial MEPS uses an integrated barrel insert and needle (BIN) device to reduce SPE to a micro-scale suitable for small sample volumes as well as for the on-line coupling of conventional SPE techniques. Furthermore, MEPS is a portable SPE device that can operate manually or incorporated into robotics samplers [31]. Compared with liquid–liquid extraction and SPE, MEPS reduces the sample preparation time and the organic solvent consumption [32, 33].
In short, the main objective of this work is to study the feasibility of coupling MEPS to ultrahigh-resolution mass spectrometry viz. off- and on-line and the applicability for non-target analysis such as followed in metabolomics studies or in NOM characterizations. A river water sample was chosen, analyzed directly and following MEPS treatment, demonstrating the selectivity introduced by the C18-sorption procedure. Direct automated analysis of marine waters (different depths, pristine versus harbor) was studied in order to demonstrate the viability of coupling MEPS directly to ICR-FT/MS. Given the availability of competitive NOM isolation methods out of freshwater, the selectivity of MEPS fractionation is instrumental to assess MEPS performance in the case of Suwannee River water. In contrast, taking into account the elaborate nature of procedures in current use for NOM isolation out of marine, salty waters, the relative case of MEPS fractionation is an effective feature, when sufficient discrimination capacity between inorganic salts and organic matter will be achieved.
Experimental
Reagents and samples
Formic acid 98–100% and l-arginine 99% were purchased from Merck (Germany). Methanol LC-MS Chromasolv and H2O LC-MS Chromasolv were from Sigma-Aldrich (Germany). Microextraction by packed sorbents (MEPS) syringes as well as the barrel inserts and needles (BIN) were obtained from SGE Analytical Science (Melbourne, Australia).
Marine samples were collected in two different locations of Mallorca Island (Spain): a natural area, Sa Costera (39°48′37.25″N, 2°42′23.48″E), and directly in the harbor of Palma de Mallorca (39°34′03.02″N, 2°38′36.52″E). Carbon concentration in marine systems ranges from 0.5 to 2 mg/L. All surface samples were collected with a Dr. Oetker 1235 BackID-Kreativ device enabling a direct collection of the surface water and the surface film at the water/air interface through small holes over a circular surface 28 cm in diameter and a collection of the liquid (around 10 ml per event) in the peripheral channel. Other samples were collected in glass vials at 0.5 m and at 2 m depth (bottom sample 10 cm over ground).
Suwannee river samples were directly collected during the second humic acid collection campaign organized by Prof. M. Perdue 2003; its carbon concentration was in the range of 40 mg/L. More information on the sample location and characteristics can be found under http://ihss.gatech.edu/ihss2/sources.html.
All real water samples were stored in glass vials in dark at 4 °C until their use.
Instrumentation
An ICR-FT/MS (Bruker Daltonics, Bremen, Germany) 12 Tesla magnet (Magnex, UK) was used for these experiments. A schematic of the instrument is given elsewhere [34]. The FTMS instrument consists of a hexapole–quadrupole–hexapole ion guide, coupled to an infinity ICR cell [35]. In the first hexapole, ions can be accumulated for variable time (for enrichment) before they are guided to the quadrupole, which can serve as a mass filter (when it is used in the combined DC/AC electric field mode). However, no ion isolation experiments were done in this study. Thus, the quadrupole acts here as a simple linear ion guide with a pressure of 3 × 10−6 mbar to linearize the ions and reduce the radial dispersion of ions along their way to the second hexapole. In the second hexapole, a relatively high pressure of argon gas (10−2 mbar) is maintained.
The formed product ions together with the remaining precursor ions are subsequently accelerated toward the ICR cell through a series of ion accelerating and decelerating lenses to overcome the magnetic fringing field. Once trapped inside the ICR cell, a dipolar radial excitation pulse can be triggered from the excitation plates of the ICR cell to excite ions radially. This causes an increase in the cyclotron motion of all ions for detection. A time domain transient is obtained with a size of 4 M words and is Fourier-transformed to obtain a frequency spectrum, which is then converted by Apex Control program (Bruker Daltonics, Bremen, Germany) to a mass spectrum. All ion excitations were performed in broad band mode (frequency sweep radial ion excitation). Simulations of ion trajectories inside ICR cells in both thermal motion and also during radial excitation are well-known [36–38].
Ions were accumulated in the first hexapole for 1 s and in the second hexapole (collision cell) for 100 ms prior to ICR ion detection. The base pressure in the ICR vacuum chamber was 5 × 10−10 mbar. Electrospray ionization source (Apollo II, Bruker Daltonics, Bremen, Germany) was used in the negative ionization mode to ionize the studied analytes in pure methanol. In the studied off-line mode, the samples were directly injected into the electrospray ionization source by using a micro-liter pump with a liquid flow rate of 2 μL min−1. Nitrogen was used for both sheath gas as well as curtain gas. Ions are formed in the source by applying a voltage difference of 4 kV and accelerating the formed ions by 500 eV inside a glass capillary prior to ion injection into two successive ion funnels. A source heater temperature of 200 °C was maintained to ensure rapid solvent evaporation in the ionized droplets. The instrument was previously calibrated by the use of arginine-negative cluster ions starting from a methanolic arginine solution of 10 mg L−1. Data acquisition and handling were performed by using the Data Analysis Software (Bruker Daltonics, Bremen, Germany). In the on-line mode, an autosampler (Gilson, USA) was used with specific operation steps that are provided below.
Automatic on-line method features
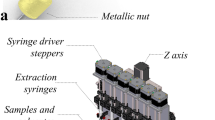
The sample treatment procedure was performed by using MEPS. Each bin of MEPS contains ~4 mg of C18 packing, whose particle size is 45 μm and its pore size is 60 Å. Only one single bin was coupled to a Gilson 223 sample changer (USA) by means of a simple tubing fitting consisting of two screws and their ferules as well as two pieces of 0.5 mm i.d. × 3 cm length Teflon tube.
The program to perform the sample preparation was as follows. Initially, the autosampler aspirates and subsequently delivers 310 µL methanol in order to clean and condition the C18 cartridge. Afterwards, the autosampler first aspirates and then delivers 410 µL H2O containing 1% formic acid for further cleaning the sorbent and to prepare it for the successive pre-concentration step. The following step consists of aspirating 2.210 mL of acidified sample (2% vol formic acid) at a low flow rate (0.1 mL min−1) to facilitate the interaction between the analytes and the sorbent. In this case, only analytes with strong hydrophobic properties are retained on the C18 stationary phase inside MEPS. Then, the sample is delivered through the MEPS to the waste at the same flow rate. In the following step, 1.210 mL of acidified water (1% vol formic acid) is aspirated and later delivered at a flow rate of 0.2 mL min−1 in order to remove salts from the sorbent.
To elute the analytes, 260 μL methanol are aspirated at a flow rate of 0.1 mL min−1. Subsequently, 206 µL of the aspired methanolic solution are delivered to the electrospray through a long (50 cm) PEEK tubing at a low flow rate (0.1 mL min−1) to ensure that no washing solvent remains inside the PEEK tube which leads directly to the electrospray source. Afterwards, a 4 µL fraction of the previously aspired solution is delivered to the ionization source at 0.01 mL min−1 (for electrospray conditioning). The remaining methanol volume (50 μL), which was previously aspired and has passed through the sorbent, is delivered to the ESI at a flow rate of 2 µL min−1 for mass spectrometric acquisition (200 scans in 20 min).
Finally, a cleaning step was performed in order to clean both the ionization source and the PEEK tubing. Firstly, 0.5 mL were aspirated from the solvent reservoir (50:50 methanol–water solution) at 0.3 mL min−1 and later delivered at the same flow rate to the ionization source (through MEPS). Then, 500 μL of methanol were aspirated at a flow rate of 0.3 mL min−1. Therefore, the whole aspirated volume was delivered according to the following sequence. First 400 μL are delivered at a flow rate of 0.3 mL min−1, subsequently 50 μL at a flow rate of 0.02 and the last 50 μL at 0.1 mL min−1. These different flow rates were performed in order to provide the best cleaning of both the tubing and the ionization chamber to avoid carry over and interferences due to the matrix as well as previous analyses.
Results and discussion
Given the disproportionate ratio of inorganic salts and organic matter in typical seawater in the range of >20,000:1, a treatment procedure composed of both clean-up and pre-concentration is mandatory in order to provide concentrated and desalted marine DOM samples.
The coupling between the sample treatment unit and the analytical instrument can be performed discontinuously with two possible configurations viz. (a) off-line, in which the operator acts as interface collecting the sample aliquots and introducing them into the analyzer, and (b) at-line, in which a robotic interface is programmed to perform the transfer step. On the other hand, the treated sample can be introduced into the analyzer on-line by means of an automated and continuous transfer [39]. The descriptions of the studied couplings (off- and on-line) as well as their applications are provided below.
Off-line C18 MEPS treatment of Suwannee River water
The first experiments were performed with Suwannee River water as an example of a very complex organic mixture. Classical XAD8-resin (fulvic and humic acids) and reverse osmosis (RO)-extracts of this river water are part of the International Humic Substances Society reference materials and are well studied materials. These fulvic, humic, and RO-fractions are complex organic matrixes of large chemical diversity. Thus a ESI(−)-ICR-FT/MS comparison of Suwannee river water to the concentrated fraction obtained after C18-MEPS extraction would give a good indication of preferential chemical selectivity in the column retention.
The concentration setup was chosen to increase the method sensitivity for the semiquintitative comparison of the two modalities. For all common masses, the peak intensity was, on average, 65–75% in the MEPS-concentrated fraction relative to the diluted real water (Fig. 1); the ratio of the average peak height of the more than 15,000 unique signals (S/N 2) of the two modalities was 91% showing that some signals were even relatively higher in the concentrated fraction as a possible effect of differences in suppression effects (not of contamination). Initially, an aliquot of the Suwannee river water sample was diluted 1 to 10 with H2O. Subsequently, it was manually pre-concentrated by using off-line C18 MEPS method. Firstly, the MEPS-sorbent was conditioned with 1 mL methanol and afterwards with 1 mL water before using it for the first time. The first step of the pre-concentration procedure consist on cleaning and conditioning the C18 sorbent with 100 μL MeOH and 250 μL of acidified H2O (1% vol formic acid). The following step is the pre-concentration of the sample. In order to have all carboxylic acids in neutral form to improve the interaction with the sorbent, a sample aliquot (1 mL) was acidified by using 2% vol of formic acid. The C18-packed sorbent is cleaned-up by using 1 mL acidified H2O (1% vol Formic acid) in order to remove the salt content to avoid ion suppression effect, mainly due to Na+. This step will especially be important for the marine samples because of their high salt content. Subsequently, the retained analytes were eluted with solvents. For this step, different solvents were tested: methanol, methanol:water mixtures, acidified methanol and methanol-containing ammonium formate. It was found that the best eluent is methanol based on the relative abundance and the number of signals per nominal mass being highest in ICR-FT/MS. The optimum elution volume found for the MEPS columns was 50 μL. This volume was selected in order to reach a compromise between the acquired number of scans needed in ICR-FT/MS and the minimum volume possible to achieve the highest pre-concentration factor. The elution was injected in the FT-ICR/MS by using a micro-liter pump at a flow rate of 2 μL min−1. In addition, to minimize carryover, MEPS was washed with 100 μL methanol and 250 μL H2O between runs. These optimizations were the first steps to determine automatization parameters when coupling the sample preparation device to ICR-FT/MS.
Initially, the sample volume chosen was 1 mL of the diluted sample (1 to 10 in methanol). Nevertheless, no significant difference is observed when 100 μL of non-diluted sample is directly pre-concentrated in MEPS. Therefore, 100 μL were used for further studies. Due to the qualitative nature of our study, the recovery from C18 was not measured.
The real water resulted in 15,400 signals (S/N 2) and the MEPS was concentrated similarly 15,300. Calculation of the elementary composition led to 2,080 formula for the real water and 1,840 for the MEPS concentrate from which an equal number of proportion in the number of CHO, CHOS, CHON, and CHONS molecules could be found (78%, 10%, 8%, 3%, respectively). In Fig. 1a, the van Krevelen diagram (atomic H/C ratio versus O/C of the obtained formula) resulting from the off-line MEPS analysis of Suwannee river is depicted. In addition, in Fig. 1b the obtained van Krevelen diagram for the direct analysis (after centrifugation) of the same sample is shown. In light of these spectra, C18 microextraction shows a high structural chemical selectivity. The extraction eliminates the highly polar, oxygen-rich and tannin-like compounds because of their higher polar properties; the strongly aromatic compounds probably interacted strongly to the MEPS and then this part is also almost missing in the diagram. Furthermore, C18 microextraction fractionated the proportions of fatty acids relative to other non-fatty acids components. More aliphatic compounds present a higher interaction with MEPS due to their higher apolarity. As can be seen in Fig. 2, we can find a complex spectrum as there are up to ten peaks per nominal mass. Most differences were found in lowest and high m/z range; the middle m/z range around m/z 400 is less affected and show similar intensity patterns in original water relative to the C18-MEPS extraction (see nominal mass 405 Fig. 2).
Comparative spectrum in the m/z 405 nominal mass where signals are very similar in intensity patterns a diluted real water Suwannee River; b MEPS extraction. At lower m/z, lower mass defects signals are intensity reduced for the MEPS extracts; at higher m/z lower mass defect signals are reduced for the original water
On-line C18 MEPS setup
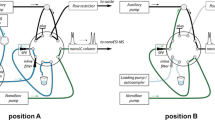
Figure 3 gives a schematic representation of the auto sampler used for the automation of the whole process. In Fig. 3 is depicted both the connection to the electrospray ionization source and the MEPS coupling. The auto sampler can aspirate liquid samples on the basis of non-compressible liquid located inside a tube, which connects the syringe pump with MEPS. The tube must be initially purged with methanol–water mixture (50–50% vol) before running any experiment. The MEPS cartridge and needle (BIN) is located between the probe and a 5-mL volume loop. As can be seen in this figure, the commercial available bin was connected to the autosampler by using a Teflon tubing fitting consisting of two small tubes of 0.5 mm i.d × 3 cm length, two screws and their two ferules. The autosampler injection port was used as a transfer line to the FT-ICR/MS. The volume inside the autosampler probe was calculated according to the dimensions provided by the manufacturer, which result in 210 μL, this volume was checked experimentally. Therefore, the real volume that passes through the bin is the subtraction of the programmed amount minus 210 μL.
The aspiration flow rate was studied. Values higher than 0.3 mL min−1 results in a worse correlation between the programmed volume and the real volume aspired since the obtained r 2 was 0.9992 for 0.3 mL min−1 and r 2 = 0.981 for 0.5 mL min−1. This difference is probably due to the overpressure that the bin generates inside the manifold. The delivering was also studied over the range 0.2-1 mL min−1. This step can be performed at higher flow rates than the aspiration step without any pressure problems. In fact, better volume standard deviation (0.89%) was obtained at higher flow rate and then 1 mL min−1 was chosen.
The spectra acquisition was performed at low flow rate (2 μL min−1) in order to accommodate a long acquisition time (20 min) with an appropriate number of scans (200) for better reproducible results. In order to obtain only constructive scans, the acquisition started just a few seconds after the eluent entered the ionization chamber. Besides, the program was made to finish the acquisition when all the eluent entered in the chamber.
Using the above-described methodology, each single analysis takes 1 h and 8 min. This time could be improved but at the expense of reproducibility. Then, it was selected for further studies.
On-line C18 MEPS treatment of marine samples
The on-line setup from the extraction of highly salt-containing sample to their flow injection analysis with ICR-FT/MS was successful and led to typical spectra without NaCl cluster.
The ultrahigh resolving power and mass accuracy of ICR-FT/MS allow to determine the molecular-level differences between marine surface film samples from more natural versus anthropogenic impacted area and from different depths. In Fig. 4, the van Krevelen diagrams from the four different samples three taken at different depths (A, surface; B, 0.5 m; C, 2 m) and one from the harbor (D) are shown. As can be seen, the van Krevelen pattern show the typical marine CRAM trace [40] with more saturated structures than the previously analyzed Suwannee river surface water sample (these having more phenolic type of structures with higher double bound equivalences, DBE). One first differentiation with increasing depth is the increase in signals of lower m/z assignable to series of variously saturated fatty acids probably from biological origin. The deep sample C was taken in mixed zone at 2 m depth where biological activity is higher, leading to double as much signals as in 0.5 m depth or at the surface. In addition, 27% of total signals of the surface sample A was sulfur-containing compared to 15.5% and 14.5% in sample B or C, respectively, showing a relative concentration of sulfur compounds in the surface film. These may be of natural origin or ubiquitous ionic/anionic surfactants used as detergents and found in many environmental compartments. For visual comparison, a van Krevelen diagram is shown for more than 220 surfactants from a compound database (http://www.terrabase-inc.com). These results show the relative increase in surface-active components in the surface film of pristine marine water. A detailed characterization of these compounds was not intended herein and will be presented elsewhere with results from a Mediterranean cruise (B. Koch, Alfred von Wegener Institut Bremerhaven, Germany, February 2009, personal communication). The same tendency in higher amount of surfactant-like compounds is shown in the surface water collected in the harbor (Fig. 4d). Many signals show the higher organic “contamination” of that sample (especially with relative higher number of condensed structures with higher double bond equivalences). Here, more than 20% of the signals contained sulfur and the typical detergent fingerprint from Fig. 5 can also be observed.
van Krevelen diagrams of the CHO and CHOS detergents of the ionic and non-ionic surfactant database from http://www.terrabase-inc.com/ (CHO open diamonds, CHOS full diamonds)
Comparison between the studied configurations
Figure 6 shows the obtained results from a reproducibility test performed on MEPS implementation (a) off-line (by using the micro-liter syringe to perform the elution step from MEPS and the simultaneous injection in the ICR-FT/MS) and (b) on-line (the fully automated and modified GILSON system). The marine sample was measured nine times in both modes under the same experimental conditions. Each histogram shown in Fig. 6 represents a specific pattern for the percent measured deviation of signal intensity (of each mass) from the mean. The percent deviation is calculated as STD/mean × 100, where STD is the standard deviation from the unweighted mean. As can be seen, the histogram for the on-line measurements has a noticeable statistical shift of the percent deviation from the mean to the extreme left side. This indicates that most measured masses have statistical percent deviation from the mean lower than 33% in the on-line mode. The off-line mode shows a noticeable broadening of the percent deviation from the mean, whereby the maximum mounts (measured masses) have a very high percent deviation of 65%. This is due to bias introduced by the low volume handling manually. The weighted average in the case of on-line measurements (25.3%) is obviously lower than for the case of off-line measurements (46.6%).
Although the on-line methodology is more time-consuming (38 min more per sample than the off-line), it is fully automated and then no operator is needed when the autosampler is running. Furthermore, the showed above-repetitivity studies point out the suitability of the on-line method over the off-line to ensure high-quality results.
Conclusion
The on-line coupling and full automation of MEPS to ICR-FT/MS was successfully established and exemplified with highly complex NOM materials and extreme salty samples, and is thus ideally suited for non targeted approaches such as metabolomics studies. Here, especially in the context of NOM analysis, the sample amount was not necessarily the issue but their salinity that made them perfect for the validation of the approach. We showed the main differences in the chemical diversity of dissolved organic carbon within a water profile and particularly were able to point out the constitution of the surface layer of marine water being particularly rich in surface-active anthropogenic molecules, especially in the harbor sample. The proposed on-line methodology offers many advantages over off-line such as conventional C18 and ICR-FT/MS procedures. MEPS allows not only to minimize the amount of organic solvents but also to reduce the amount of sample relative to the minimal needed for injection. Furthermore, the developed method provides good-quality results in terms of repeatability and it is fully automated. In addition, the commercial availability of MEPS is important in this field since it simplifies the transference of this methodology to other research laboratories such as when automation of high sample amounts is needed in metabolomics studies.
Abbreviations
- MEPS:
-
Microextraction by packed sorbents
- ICR-FT/MS:
-
Ion cyclotron resonance Fourier-transform mass spectrometry
- BIN:
-
Barrel insert and needle
- DOM:
-
Dissolved organic matter
- NOM:
-
Natural organic matter
References
Hedges JI, Eglinton G, Hatcher PG, Kirchman DL, Arnosti C, Derenne S, Evershed RP, Kogel-Knabner I, de Leeuw JW, Littke R, Michaelis W, Rullkotter J (2000) Org Geochem 31:945–958
Hertkorn N, Ruecker C, Meringer M, Gugisch R, Frommberger M, Perdue EM, Witt M, Schmitt-Kopplin P (2007) Anal Bioanal Chem 389:1311–1327
Gaspar A, Harir M, Lucio M, Hertkorn N, Schmitt-Kopplin P (2008) Rapid Commun Mass Spectrom 22:3119–3129
Sleighter RL, Hatcher PG (2008) Mar Chem 110:140–152
Hertkorn N, Frommberger M, Witt M, Koch B, Schmitt-Kopplin P, Perdue EM (2008) Anal Chem 80:8908–19
Koch BP, Witt MR, Engbrodt R, Dittmar T, Kattner G (2005) Geochim Cosmochim Acta 69:3299–3308
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley, New York
Fievre A, Solouki T, Marshall AG, Cooper WT (1997) Energy Fuels 11:554–560
Remmler M, Georgi A, Kopinke FD (1995) Eur J Mass Spectrom 1:403–407
Shinozuka T, Shibata M, Yamaguchi T (2004) J Mass Spectrom Soc Jpn 52:29–32
Mugo SM, Bottaro CS (2004) Rapid Commun Mass Spectrom 18:2375–2382
Mugo SM, Bottaro CS (2007) Rapid Commun Mass Spectrom 21:219–228
Kujawinski EB, Freitas MA, Zang X, Hatcher PG, Green-Church KB, Jones RB (2002) Org Geochem 33:171–180 PII S0146-6380(0101)00149-00148
Stenson AC, Landing WM, Marshall AG, Cooper WT (2002) Anal Chem 74:4397–4409
Solouki T, Freitas MA, Alomary A (1999) Anal Chem 71:4719–4726
Brown TL, Rice JA (2000) Anal Chem 72:384–390
Alomary A, Solouki T, Patterson HH, Cronan CS (2000) Environ Sci Technol 34:2830–2838
Ikeda K, Arimura R, Echigo S, Shimizu Y, Minear RA, Matsui S (2000) Water Sci Technol 42:383–390
Plancque G, Amekraz B, Moulin V, Toulhoat P, Moulin C (2001) Rapid Commun Mass Spectrom 15:827–835
Persson L, Alsberg T, Kiss G, Odham G (2000) Rapid Commun Mass Spectrom 14:286–292
Kujawinski EB, Hatcher PG, Freitas MA (2002) Anal Chem 74:413–419
Hertkorn N, Frommberger M, Witt M, Koch B, Schmitt-Kopplin P, Perdue EM (2008) Anal Chem 80:8908–8919
Gaspar A, Kunenkov EV, Lock R, Desor M, Perminova I, Schmitt-Kopplin P (2009) Rapid Commun Mass Spectrom 23:683–688
Llewelyn JM, Landing WM, Marshall AG, Cooper WT (2002) Anal Chem 74:600–606
Stenson AC, Marshall AG, Cooper WT (2003) Anal Chem 75:1275–1284
Sleighter RL, Hatcher PG (2007) J Mass Spectrom 42:559–574
Benner R, Biddanda B, Black B, McCarthy M (1997) Mar Chem 57:243–263
Koprivnjak JF, Pfromm PH, Ingall E, Vetter TA, Schmitt-Kopplin P, Hertkorn N, Frommberger M, Knicker H, Perdue EM (2009) Geochim Cosmochim Acta 73:4215–4231
Kim S, Simpson AJ, Kujawinski EB, Freitas MA, Hatcher PG (2003) Org Geochem 34:1325–1335
Dittmar T, Koch B, Hertkorn N, Kattner G (2008) Limnol Oceanogr-Methods 6:230–235
Wynne P, Hibbert R, DiFeo D, Dawes P (2008) Column 4:12–17
El-Beqqali A, Abdel-Rehim M (2007) J Sep Sci 30:2501–2505
Morales-Cid G, Cárdenas S, Simonet BM, Valcárcel M (2009) Electrophoresis 30:1684–1691
Kanawati B, Harir M, Schmitt-Kopplin P (2009) Int J Mass Spectrom (In press)
Caravatti P, Allemann M (1991) Org Mass Spectrom 26:514–518
Kanawati B, Wanczek KP (2007) Rev Sci Instrum 78:074102
Kanawati B, Wanczek KP (2008) Int J Mass Spectrom 269:12–23
Kanawati B, Wanczek KP (2008) Int J Mass Spectrom 274:30–47
Santos B, Simonet BM, Rios A, Valcarcel M (2006) Trac-Trends Anal Chem 25:968–976
Hertkorn N, Benner R, Frommberger M, Schmitt-Kopplin P, Witt M, Kaiser K, Kettrup A, Hedges JI (2006) Geochim Cosmochim Acta 70:2990–3010
Acknowledgment
G. Morales-Cid wishes to thank the Spain’s Ministry of Innovation and Science for the award of a FPI fellowship (Grant BES-2005-10603) to work with the German colleagues and the additional economic support within the mobility program. Dr. Marianna Lucio is thanked for the help in sampling the harbor sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morales-Cid, G., Gebefugi, I., Kanawati, B. et al. Automated microextraction sample preparation coupled on-line to FT-ICR-MS: application to desalting and concentration of river and marine dissolved organic matter. Anal Bioanal Chem 395, 797–807 (2009). https://doi.org/10.1007/s00216-009-3025-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3025-0