Abstract
Different highly selective sorbents have been evaluated for the treatment of food and biological samples to determine chloramphenicol residues by ion mobility spectrometry (IMS). Combination of a selective solid-phase extraction (SPE) and dispersive liquid-liquid microextraction allowed a highly sensitive determination of chloramphenicol in water, milk, honey, and urine samples. The performance of selective SPE supports such as immunoaffinity chromatography (IAC) and molecular imprinted polymers (MIP) have been compared in terms of selectivity, sensitivity, trueness, precision, and reusability. Quantitative recoveries were obtained for chloramphenicol residues, ranging from 91 to 123 % for water, from 99 to 120 % for skimmed milk, and from 95 to 124 % for urine using IAC-IMS and MIP-IMS methods. Quantitative recoveries (from 88 to 104 %) were also achieved for honey samples using IAC-IMS, but low recoveries were obtained using MIP-IMS. The limit of quantification was set at 0.1 μg L−1 which is lower than the minimum required performance limit established by the EU. The proposed methodology is a simple and cost affordable alternative to chromatography methods for the highly sensitive and selective analysis of chloramphenicol residues in food and urine.

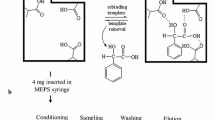
Scheme for chloramphenicol determination by selective solid-phase extraction and ion mobility spectrometry
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ion mobility spectrometry (IMS) has recently gained increased attention as a well-established analytical technique for the determination of volatile and semivolatile compounds in different matrices with a good sensitivity and high sample throughput. IMS is based on the gas-phase separation of the resulting ions formed under a weak electric field at ambient pressure [1]. Primarily, it was used for the analysis of explosives, illicit drugs, and chemical warfare agents in security control points. However, the analytical potential of IMS, particularly as regards operational speed and sensitivity, has extended its scope to the pharmaceutical, food and feed, clinical, polymer, petrochemical, and environmental fields [2].
IMS measurement of complex samples produces a mixture of reagent and analyte product ions [3], which could complicate the interpretation of IMS plasmagrams and interfere in analyte determination. Moreover, the measured number of theoretical plates of an IMS device operating at 3000 V, a gate width of 0.2 ms and 150 °C, rarely exceeded 5000. Compared to the typical number of theoretical plates obtained with other separation techniques, as 25,000 for liquid chromatography (LC), 120,000 for gas chromatography, and 300,000 for capillary electrophoresis [4], it can be easily imagine that the low separation efficiency has limited the applicability of IMS. Therefore, the incomplete resolution of peaks is a common problem in the analysis of complex samples by IMS. So, to do this kind of analysis, the use of chemometrics [5, 6] or sample pre-treatments based on diverse separation techniques such as SPE [7], solid-phase microextraction [8, 9], stir-bar sorptive extraction [10], liquid-liquid microextraction [11], single-drop microextraction [12], hollow-fiber liquid-phase microextraction [13], and paper spray [14] has been proposed. However, in most cases, the selectivity of the currently available sample treatments is not enough to remove interferences and competitive ionization problems can take place in IMS determinations.
The potential of highly selective sorbents as sample treatment in IMS has been recently demonstrated. Immunoaffinity chromatography (IAC) columns have been used for the determination of mycotoxins [15, 16] and fungicides [17] and molecularly imprinted polymers (MIPs) for the selective analysis of pesticides [18, 19], pharmaceuticals [20, 21], and nitrobenzenes [22].
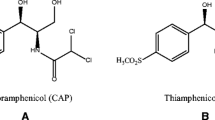
The main objective of this study has been the critical comparison of the capabilities of different highly selective sorbents, such as IAC and MIPs columns, as sample pre-treatments in IMS. The determination of chloramphenicol in water, skimmed milk, honey, and urine samples was employed as example model to evaluate the proposal. Chloramphenicol is a broad-spectrum antibiotic very effective against both Gram-positive and Gram-negative bacteria. It was widely used to treat human and animal infections. However, it is not authorized in the EU since 1994 for use in food-producing animals due to its association with the induction of aplastic anemia. Nevertheless, chloramphenicol is still being used in the EU for human medicine and in treatments of non-food-producing animals [23]. Considering the evidences of in vivo carcinogenicity and genotoxicity of chloramphenicol, it is not appropriate to establish an acceptable daily intake, and maximum residue limits have neither been assigned for this antibiotic [24]. Screening methods are able to determine chloramphenicol residues at the minimum required performance limit (MRPL), established at 0.3 μg kg−1 and include diverse techniques like microbial inhibition tests, immunoassay, electrochemistry, and chromatography. Confirmatory methods, typically based on chromatographic methods coupled to mass spectrometry detectors, provide limits of quantification (LOQs) in the range of 0.01 to 0.3 μg kg−1 [23]. However, the main drawbacks of the aforementioned methodologies are the long analysis time required and the instrumentation cost. Thus, a logical analytical scheme for the detection of chloramphenicol residues in foods would be based on the application of rapid screening methods, with appropriate sensitivity and selectivity, followed by the confirmation of positive samples by liquid chromatography-tandem mass spectrometry.
Material and methods
Material, reagents, and samples
Cyanogen bromide (CNBr) activated Sepharose-4B from GE Healthcare Biosciences (Uppsala, Sweden) was used as solid support. Polypropylene SPE tubes (3 mL) with polyethylene frits (20 μm porosity) were obtained from Scharlab (Barcelona, Spain). SupelMIP chloramphenicol molecular imprinted polymer was obtained from Supelco (Bellefonte, PA, USA).
Chloramphenicol (2,2-dichloro-N-[1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide) pure standard was purchased from Sigma-Aldrich (St. Louis, MO, USA) and stock solutions were prepared in 2-propanol and kept at 4 °C in amber glass vials. Chloroform, methanol, 2-propanol, and buffer constituents were also obtained from Scharlab.
Skimmed milk and honey samples were obtained from the Spanish market and urine samples were obtained from different healthy volunteers, after appropriate information and oral consent. All the samples were stored at 4 °C in the fridge until analysis. A lyophilized, partially defatted, raw bovine milk reference material (MI1413-2/CM), acquired from TestVeritas S.R.L. (Padova, Italy) with a certified chloramphenicol concentration of 1.56 μg kg−1 (n = 11) and a satisfactory range from 0.24 to 2.88 μg kg−1, was analyzed to assess the trueness of the methodology.
IAC procedure
The anti-chloramphenicol monoclonal antibody was produced in our laboratory following procedures for hybridoma generation [25]. Antibody was purified by ammonium sulfate precipitation and protein G affinity chromatography, being later fully characterized by enzyme-linked immunosorbent assay (ELISA). IAC columns were home-made produced by covalent immobilization of the antibody on Sepharose gel following previous studies [26]. IAC columns were produced using 5 mg antibody per gram of Sepharose gel, which is equivalent to 0.5 mg monoclonal antibody per column.
The IAC column was equilibrated with 5 mL phosphate buffer saline (PBS) plus 5 mL deionized water under gravity flow (approximately 1 mL min−1). Samples (from 1 to 20 mL) were loaded through the column, washed with 2 mL deionized water, smoothly dried by passing air through the IAC by means of an empty syringe, and eluted with 1 mL 2-propanol. Honey samples (from 1 to 20 g) were dissolved using 2 mL deionized water per gram of honey prior to their extraction and analysis in order to improve fluidity through the column.
A volume of the elution solution was extracted by dispersive liquid-liquid microextraction (DLLME) in order to pre-concentrate chloramphenicol, but also to change the solvent for increasing the sensitivity of the IMS measurement. Thus, taking into consideration the residual water amount of the elution fraction (30 % v/v), 150 μL of elution solution was mixed with 450 μL water and 50 μL chloroform as extraction solvent, in order to maintain the same water/2-propanol/chloroform ratio than that used in the aforementioned DLLME procedure. A cloudy solution was formed that was stable for a long time. Then, the mixture was centrifuged for 2 min at 5000 rpm and 10 μL of the chloroform extract (sedimented in the bottom of the conical tube) was directly injected and analyzed by IMS. The concentration of chloramphenicol in samples was calculated by interpolating the IMS signal a calibration curve prepared in the same conditions than samples using the DLLME procedure.
MIP procedure
A commercially available MIP specific for chloramphenicol, with a bed weight of 25 mg, was employed as SPE support. MIP was equilibrated with 5 mL 2-propanol plus 5 mL deionized water under gravity flow. Samples (from 1 to 20 mL) was loaded through the column, washed with 2 mL 2-propanol 20 % (v/v) in deionized water, completely dried using vacuum, and eluted with 1 mL 2-propanol. Honey samples (from 1 to 20 g) were likewise dissolved using 2 mL deionized water per gram of honey to increase sample fluidity. The MIP elution fraction was also extracted by DLLME, using 100 μL elution solution plus 500 μL water and 50 μL chloroform, to maintain the optimized solvent ratios. The concentration of chloramphenicol in samples was calculated by interpolating the IMS signal in a calibration curve prepared using the DLLME procedure.
IMS determination
An IONSCAN-LS from Smiths Detection (Morristown, NJ, USA) equipped with a 63Ni foil radioactive ionization source was used for the IMS determination of chloramphenicol. IM station software (version 5.389) was used for plasmagram acquisition and data processing. Plasmagrams were acquired in negative ion mode using 4-nitrobenzonitrile, with a reduced mobility (K 0) of 1.652 cm2 V−1 s−1, as internal calibrant and hexachloroethane, with a K 0 of 2.261 cm2 V−1 s−1, as ionization reactant. The number of segments per analysis was 120, with 20 co-added scans per segment and every plasmagram containing 479 data points. The shutter grid width was 0.2 ms, the value optimized by the manufacturer, and plasmagrams were collected with a scan period of 30 ms. A counterflow of dry nitrogen, set at 350 mL min−1, was introduced as drift gas at the end of the drift region. The electric field strength in the drift region was 287 V cm−1, with a total drift voltage of 2008 V and a drift tube length of 7 cm.
Sample introduction was performed by thermal desorption from a polytetrafluoroethylene (PTFE) membrane, using 10 μL injection volume. Desorption, inlet, and drift tube temperatures were adjusted to 255, 260, and 111 °C, respectively. The sample tray, containing the PTFE membrane, was inserted in the heated zone using a 1-s post-dispense delay and the sample was held in this position for 60 s. Thus, the total analysis time for IMS acquisition was 1 min.
Reference UHPLC-MS procedure
Ultra high-performance liquid chromatography (UHPLC) coupled to a triple quadrupole mass spectrometer was selected as the reference methodology in order to obtain maximum sensitivity and selectivity in the analysis of chloramphenicol residues. UHPLC was performed on a Waters Acquity UPLC system from Waters (Milford, MA, USA), equipped with a binary solvent delivery system, an autosampler, and a BEH C18 (1.7 μm, 2.1 × 50 mm) column. An injection volume of 5 μL of the IAC extracts was directly analyzed by UHPLC-MS. The mobile phase consisted of (A) 0.1 % (v/v) formic acid in water and (B) methanol. The gradient started at 5 % of B was linearly increased to 95 % in 4 min, and then maintained 1 min at a flow rate of 0.4 μL min−1. The obtained retention time under the aforementioned conditions of chloramphenicol was 3.44 min. Tandem mass acquisitions were performed in a Waters Acquity triple quadrupole mass spectrometry detector, equipped with a Z-spray electrospray ionization source, with 3.5 kV capillary voltage, 120 °C source temperature, and 350 °C desolvation temperature. The employed parameters were as follows: ESI+, parent ion 321 m/z and daughter ions 257 and 152 m/z, using 25 V cone energy, and 15 and 25 eV collision energy, respectively.
Results and discussion
IMS plasmagrams of chloramphenicol
The ion mobility plasmagram of a chloramphenicol standard solution in 2-propanol is depicted in Fig. 1. The peaks present in the early portion of the plasmagram between 8 and 14 ms, which also occur in the blank injections, are product ions from IMS reactant and calibrant compounds. In negative ion mode, a chloride source (hexachloroethane) was used as reactant, and after the initial formation of negatively charged chloride, additional collisions lead to stable secondary ions, the most common of which is Cl−(H2O) n . It can react with oxygen of air, used as drift gas, which provided stable secondary ions such as O2 −(H2O) n . In summary, the hexachloroethane reactant and the 4-nitrobenzonitrile calibrant yields alternate reactant ions such as Cl−, NO3 −, or NO2 −, resulting in a complex signal, between 8 and 14 ms difficult to be analyzed [27].
Plasmagrams of chloramphenicol presented a main peak at 16.90 ± 0.05 ms drift time (K 0 of 1.221 ± 0.005 cm2 V−1 s−1) and a second smallest peak at 17.55 ± 0.02 ms (K 0 of 1.079 ± 0.002 cm2 V−1 s−1). Accordingly, the reported chloramphenicol K 0 value is consistent with previously published values (1.22 ± 0.05 cm2 V−1 s−1) [28].
An alarm was generated in the IMS software to alert for the presence of chloramphenicol in the samples, using the following peak descriptors: (i) K 0 value of 1.221 cm2 V−1 s−1, (ii) a variability value of 75 μs of the peak drift time, to compensate for small changes of the expected K 0 value, (iii) a full width value at the half-maximum height (FWHM) of the peak of 200 μs, (iv) the width of the peak must be no more than 1.5 times the programmed FWHM, and (v) a signal higher than the signal threshold value (10 arbitrary units). The total analysis time of chloramphenicol determinations by IMS was set at 30 s.
Analytical features of chloramphenicol determination by IMS
The height of the peak at 16.90 ms, obtained from the average plasmagram of a 3-s window around chloramphenicol desorption time, was employed as analytical response. Determination coefficient and linear range of the procedure were checked using calibration curves of chloramphenicol dissolved in 2-propanol and chloroform from 10 to 200 μg L−1 using seven concentration levels. The obtained slopes were statistically comparable, independently of the solvent used, and the main analytical figures of merit are shown in Table 1. The instrumental limits of detection (iLOD) and quantification (iLOQ) were calculated as three and ten times, respectively, the standard deviation of the intercept divided by the slope of the calibration line, obtaining values of 10 and 30 μg L−1, respectively. However, the obtained sensitivity was not enough to reach the MRPL of chloramphenicol in food (0.3 μg L−1) [23]. Accordingly, a pre-concentration of sample was carried out in order to improve the sensitivity of the method.
Development of IAC-IMS procedure
Chloramphenicol IAC columns were produced by using high specific monoclonal antibodies coupled to CNBr-activated Sepharose gel. The affinity of anti-chloramphenicol antibodies was characterized by enzyme-linked immunosorbent assay using a conjugate immobilized indirect assay with an average IC50 value of 3.2 μg L−1. IAC columns were produced using 5 mg antibody per gram of Sepharose gel as proposed in previous studies [26]. Thus, the antibody was covalently immobilized on 100 mg of Sepharose gel with a coupling efficiency close to 100 %.
The most appropriate washing solution was established to provide an adequate sample clean-up and no analyte elution from the column. Several mixtures of 2-propanol in water, from 0 to 100 % (v/v), were evaluated to check the release of chloramphenicol from the IAC column. Therefore, IAC columns were loaded with 100 ng chloramphenicol (1 mL of 100 μg L−1 standard), washed with 1 + 1 mL of 2-propanol from 0 to 100 % (v/v) in water, and eluted with 1 + 1 mL 2-propanol. The obtained results are shown in Fig. 2, where it can be seen that chloramphenicol began to be detected in the washing solution at 2-propanol concentration equal or higher than 5 % (v/v). Accordingly, pure water was selected as washing solution in order to avoid analyte losses during the washing step. Pure 2-propanol was selected as the most appropriate elution solvent taking into consideration the chloramphenicol quantitative elution in the first 1 mL volume and the compatibility with IMS detection.
Once SPE procedure was defined, chloramphenicol IAC columns were characterized in terms of analyte binding capacity and reusability of the immunosorbent. Columns were loaded with 10 μg of chloramphenicol (2 mL of 5 mg L−1 standard), washed with 2 mL water, and eluted with 1 mL 2-propanol, with an obtained binding capacity of 1.01 ± 0.15 μg chloramphenicol. The column reusability was evaluated by repeated recovery studies using spiked samples. Recovery values were calculated for ten repeated cycles using an IAC column loaded with 1 mL of water spiked at 50 μg L−1 chloramphenicol in independent studies. Quantitative recoveries, between 85 and 122 % with RSD values lower than 10 %, were obtained in all cases; therefore, the same column can be reused at least ten times.
The amount of water in the elution fraction from IAC columns affected the chloramphenicol IMS signal intensity, compromising sensitivity of the methodology (see Electronic Supplementary Material (ESM) Fig. S1). Thus, the use of a DLLME was proposed in order to (i) remove water and (ii) pre-concentrate the MIP extract. Therefore, after the IAC-SPE procedure, 150 μL of the eluted fraction was extracted by DLLME using 450 μL deionized water and 50 μL chloroform in order to maintain the optimized ratio of solvents (see ESM Fig. S2). In the same way, a calibration line was prepared using 70 % (v/v) 2-propanol and extracted by DLLME. Table 1 shows the main analytical figures of merit of the IAC-IMS procedure regarding linearity, linear range, precision, iLOD, and iLOQ values. The total time for an IAC-IMS analysis of a sample was set in less than 10 min, and several samples (6–8) can be easily run in parallel.
Trueness of the IAC-IMS method was established by the evaluation of recoveries on water, skimmed milk, urine, and honey spiked with chloramphenicol at four concentration levels (0.1, 0.5, 5.0, and 50.0 μg L−1). Different sample amounts were loaded on the IAC column depending on the spiked level, in order to include it inside the IMS linear range, and recoveries were determined (see Table 2). The obtained values ranged from 91 to 96 % for water, from 99 to 110 % for skimmed milk, from 88 to 104 % for honey, and from 95 to 116 % for urine. Thus, method quantitation limit (MQL) was set at 0.06 μg L−1 for all evaluated matrices. Figure 3 shows the IMS plasmagrams of the four abovementioned matrices spiked at 50 μg L−1 chloramphenicol. Chloramphenicol residues were accurately calculated for all samples evaluated till concentrations of 0.1 μg L−1; thus, IAC methodology can be satisfactorily employed as screening tool for regulatory purposes.
Development of MIP-IMS procedure
A MIP procedure was developed employing commercial MIP supports specific for chloramphenicol. First of all, the adequate washing and elution solution was established as aforementioned for IAC supports. MIP column was loaded with 100 ng chloramphenicol, washed with different 2-propanol/water mixtures (from 0 to 100 % (v/v)), eluted with pure 2-propanol, and then analyzed by IMS. Figure 2 shows the obtained results, where it can be seen that a 20 % (v/v) 2-propanol in water could be employed as washing solution without elution of chloramphenicol. Pure 2-propanol was employed as elution solvent and the SPE support was taken to dryness, so no residual water was found in this case in the elution fraction by ATR-FTIR analysis. DLLME of the MIP-SPE extract was performed using 100 μL of the eluted fraction, 500 μL deionized water, and 50 μL chloroform to maintain the adequate ratio of solvents (see ESM). Table 1 shows the main analytical figures of merit of the MIP-IMS procedure carried out on chloramphenicol standards prepared in 2-propanol. The total analysis time was scarcely higher (12 min) than that employed for IAC-IMS, including the vacuum drying step. Likewise, several samples can be extracted in parallel in order to increase the sample throughput.
MIP columns were characterized similarly to IAC supports, in terms of chloramphenicol binding capacity (higher than 10 μg) and reusability (until ten times). The MIPs presented a higher binding capacity than IAC supports as it is conventionally generalized, due to its higher number of active sites [29]. Recoveries were performed in water, skimmed milk, urine, and honey samples spiked with 0.1, 0.5, 5.0, and 50.0 μg L−1 chloramphenicol. Table 2 shows the obtained recoveries for the different matrices. Quantitative recoveries were obtained for water (from 91 to 96 %), skimmed milk (from 90 to 120 %), and urine (from 101 to 124 %). The MQL values for these matrices were set at 0.09 μg L−1. Concerning honey samples, recoveries were lower than 50 % due to the presence of matrix compounds in the SPE extract, which strongly interfere the IMS analysis, as it can be seen in Fig. 3. In any case, modifications of the clean-up procedure of the MIP-IMS procedure would be recommended to avoid interferences from non-specifically bounded compounds which interfere in the chloramphenicol determination of highly complex matrix, without dramatically affecting the global performance of the method in terms of low recoveries and sample throughput.
Critical comparison IACs vs MIPs
The potential of IAC and MIP columns was evaluated through the comparison of both procedures in terms of precision, recoveries, robustness, MQL, binding capacity and reusability, acquisition costs, and selectivity, among others.
As it can be seen in Table 3, precision, robustness, and MQL values provided by IAC and MIP treatments were comparable. On the other hand, recoveries close to 100 % were obtained by IAC-IMS in all the matrices evaluated. However, honey samples presented important interferences that preclude chloramphenicol determination after MIP-IMS.
The high selectivity brought by the IAC cartridge was superior to the selectivity provided by the MIP cartridge for all the matrices evaluated (see Fig. 3). Compared with the signal obtained after IAC treatment, additional signals can be observed in the plasmagram of chloramphenicol spiked at the level of 50 μg L−1 in urine, skimmed milk, and honey samples after clean-up with MIPs, probably due to unspecific interactions between interferents and polymer. Regarding binding capacity, IAC cartridges showed a ten times lower value than that of the MIP, being comparable the reusability of both types of cartridges.
MIPs have demonstrated to be very stable at high temperatures, higher than 120 °C, and treatments with organic solvents, strong acids and bases, being the IACs unstable with organic solvents, pH extremes, and at high temperatures. Although, the possible leaching of the MIP polymer during use is an important aspect to consider during method development. The commercial availability of MIP columns for a wide range of analytes is higher than that of IAC sorbents which should be custom-made developed for the analysis of new analytes. Moreover, development of antibodies may take several months, in front of the production period of MIPs that is from days to weeks. Regarding acquisition costs, MIPs have a relative low cost as compared to monoclonal antibodies employed in the production of IACs, which requires several phases like hapten design and synthesis, immunization of animals, production and clonation of hybridoma, and large scale production of antibodies [30].
In summary, although IACs are relatively expensive, the higher selectivity in front of MIPs and the quantitative recoveries obtained for all the evaluated matrices make IAC the preferred technique to improve the selectivity and sensitivity of IMS. So, IAC-IMS was selected for the analysis of chloramphenicol in food and clinical samples.
Validation of the proposed procedure
Analytical figures of merit of the proposed IAC-IMS methodology are shown in Table 1 and it was discussed in “Development of IAC-IMS procedure” section. Additionally, the extraction efficiency of IAC sorbents and trueness of the whole method was also evaluated by the analysis of a milk reference material with a certified chloramphenicol content of 0.24–2.88 μg L−1. The lyophilized milk sample was reconstituted in 9.30 g of distilled water, following the manufacturer recommended procedure. After the reconstitution, it was stored at 4 °C overnight and used the day after. Before using the reference sample, it was heated in water bath at 40 °C up to the complete dissolution of all the clots. Therefore, it was cooled at room temperature and gently shaken. The aforementioned solution was analyzed using the IAC-IMS procedure in two independent replicates. The chloramphenicol concentration found using the proposed method was 1.12 ± 0.07 μg L−1, which is included in the range of concentration certified by the supplier, indicating the agreement of the proposed procedure with the certified reference value.
Trueness of the IAC-IMS method was also assessed by method comparison using UHPLC-MS/MS as reference methodology for the analysis of chloramphenicol. A total of 32 samples, including 12 milk, 12 honey, and 8 urine samples, were extracted by using the proposed IAC method and analyzed by both, IMS and UHPLC-MS/MS methodologies. Chloramphenicol was not detected in any of them; thus, four milk, three honey, and five urine blank-samples were blind-spiked at concentrations from 0.1 to 2.0 μg L−1 chloramphenicol. Concentration values found by the proposed IMS procedure were compared with those obtained by the reference UHPLC-MS/MS method and they were in good accordance as it can be seen in Table 4, with bias values between −17.6 and 18.2 %. Deming regression, which considers the standard deviation of both methods, was employed to evaluate the agreement between chloramphenicol concentrations obtained by the reference UHPLC-MS/MS and proposed IMS methods. Comparison of results afforded a regression with a slope of 0.94 (95 % confidence interval was from 0.88 to 1.01) and an intercept of 0.015 (95 % confidence interval was from −0.033 to 0.064), indicating that results obtained by both procedures were statistically comparable.
Conclusion
IMS determination provides a rapid and high sensitive determination of organic compounds in simple matrices, but the analysis of complex ones cannot be made directly and a highly selective sample treatment is required. In this study, IAC and MIP have been evaluated and compared for the analysis of traces of chloramphenicol in food and urine samples by IMS. Excellent results, in terms of sensitivity, reusability of columns, and sample throughput, were obtained for both of them. However, MIP treatment provided honey extracts much more dirties than those obtained using IAC, thus presenting important interferences. Consequently, the off-line coupling of selective IAC supports and DLLME with IMS analysis offers an attractive strategy to obtain sensitive and selective analysis of chloramphenicol residues, which can be useful in food safety monitoring studies with a MQL value of 0.06 μg L−1, which is lower than the MRPL established by the EU.
In summary, IAC-IMS can be considered as an interesting and serious alternative to available screening methods based on immunoassays, reducing the number of false positive samples and providing quantitative data. On the other hand, the IAC-IMS method is simple, cost affordable, and does not required skilled operators to obtain accurate and precise results compared to alternative methods based on UHPLC-MS/MS. Moreover, a minimal consumption of organic solvents was required and it only used 1 mL 2-propanol and 50 μL chloroform per sample. Thus, it can be considered an environmentally friendly methodology that follows the principles of green analytical chemistry and it provides results statistically comparable to chromatographic reference methods. Nevertheless, the proposed IAC-IMS methodology provides a high specificity but only for a target analyte and in the case of multiresidue determination the acquisition and optimization of additional selective sorbents is required.
References
Eiceman GA. Ion-mobility spectrometry as a fast monitor of chemical composition. TrAC Trend Anal Chem. 2002;21:259–75.
Armenta S, Alcala M, Blanco M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal Chim Acta. 2011;703:114–23.
Harrington PD, Reese ES, Rauch PJ, Hu LJ, Davis DM. Interactive self-modeling mixture analysis of ion mobility spectra. Appl Spectrosc. 1997;51:808–16.
Asbury GR, Hill HH. Evaluation of ultrahigh resolution ion mobility spectrometry as an analytical separation device in chromatographic terms. J Microcolumn. 2000;12:172–8.
Zamora D, Blanco M. Improving the efficiency of ion mobility spectrometry analyses by using multivariate calibration. Anal Chim Acta. 2012;726:50–6.
Armenta S, Blanco M. Pros and cons of benzodiazepines screening in human saliva by ion mobility spectrometry. Anal Bioanal Chem. 2011;401:1935–48.
Soleimani M, Azam M, Azimi M, Borhani K. SPE-IMS as a new analysis technique for identification and quantification of metalaxyl residue in cucumber. Ital J Food Sci. 2012;24:3–8.
Holopainen S, Luukkonen V, Nousiainen M, Sillanpää M. Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta. 2013;114:176–82.
Kalhor H, Ameli A, Alizadeh N. Electrochemically controlled solid-phase micro-extraction of proline using a nanostructured film of polypyrrole, and its determination by ion mobility spectrometry. Microchim Acta. 2013;180:783–9.
Lokhnauth JK, Snow NH. Stir-bar sorptive extraction and thermal desorption-ion mobility spectrometry for the determination of trinitrotoluene and l,3,5-trinitro-l,3,5-triazine in water samples. J Chromatogr A. 2006;1105:33–8.
Armenta S, Garrigues S, de la Guardia M, Brassier J, Alcalà M, Blanco M. Analysis of ecstasy in oral fluid by ion mobility spectrometry and infrared spectroscopy after liquid–liquid extraction. J Chromatogr A. 2015;1384:1–8.
Márquez-Sillero I, Aguilera-Herrador E, Cárdenas S, Valcárcel M. Determination of 2,4,6-tricholoroanisole in water and wine samples by ionic liquid-based single-drop microextraction and ion mobility spectrometry. Anal Chim Acta. 2011;702:199–204.
Saraji M, Jafari MT, Sherafatmand H. Hollow fiber-based liquid–liquid–liquid microextraction combined with electrospray ionization-ion mobility spectrometry for the determination of pentazocine in biological samples. J Chromatogr A. 2010;1217:5173–8.
Holopainen S, Nousiainen M, Sillanpää MET, Anttalainen O. Sample-extraction methods for ion-mobility spectrometry in water analysis. TrAC Trend Anal Chem. 2012;37:124–34.
Khalesi M, Sheikh-Zeinoddin M, Tabrizchi M. Determination of ochratoxin A in licorice root using inverse ion mobility spectrometry. Talanta. 2011;83:988–93.
Sheibani A, Tabrizchi M, Ghaziaskar HS. Determination of aflatoxins B1 and B2 using ion mobility spectrometry. Talanta. 2008;75:233–8.
Armenta S, de la Guardia M, Abad-Fuentes A, Abad-Somovilla A, Esteve-Turrillas FA. Off-line coupling of multidimensional immunoaffinity chromatography and ion mobility spectrometry: a promising partnership. J Chromatogr A. 2015;1426:110–7.
Jafari MT, Rezaei B, Zaker B. Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem. 2009;81:3585–91.
Rezaei B, Jafari MT, Khademi R. Selective separation and determination of primidone in pharmaceutical and human serum samples using molecular imprinted polymer-electrospray ionization ion mobility spectrometry (MIP-ESI-IMS). Talanta. 2009;79:669–75.
Jafari MT, Badihi Z, Jazan E. A new approach to determine salicylic acid in human urine and blood plasma based on negative electrospray ion mobility spectrometry after selective separation using a molecular imprinted polymer. Talanta. 2012;99:520–6.
Jafari MT, Kamfirozi M, Jazan E, Ghoreishi SM. Selective extraction and analysis of pioglitazone in cow plasma using a molecularly imprinted polymer combined with ESI ion mobility spectrometry. J Sep Sci. 2014;37:573–9.
Lu W, Li H, Meng Z, Liang X, Xue M, Wang Q, et al. Detection of nitrobenzene compounds in surface water by ion mobility spectrometry coupled with molecularly imprinted polymers. J Hazard Mat. 2014;280:588–94.
European Food Safety Authority. Scientific opinion on chloramphenicol in food and feed. EFSA J. 2014;12–3907:1–145.
Food and Agriculture Organization of the United Nations/World Health Organization. Summary report of the sixty-second meeting of JECFA. FAO Food Nutrit Papers. 2004;41–6:1–12.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Esteve-Turrillas FA, Mercader JV, Agulló C, Abad-Somovilla A, Abad-Fuentes A. Development of immunoaffinity columns for pyraclostrobin extraction from fruit juices and analysis by liquid chromatography with UV detection. J Chromatogr A. 2011;1218:4902–9.
West C, Baron G, Minet J. Detection of gunpowder stabilizers with ion mobility spectrometry. J Forensic Sci Int. 2007;166:91–101.
Jafari MT, Khayamian T, Shaer V, Zarei N. Determination of veterinary drug residues in chicken meat using corona discharge ion mobility spectrometry. Anal Chim Acta. 2007;581:147–53.
Picó Y. Food contaminants and residue analysis. Elsevier ISBN: 978-0-444-53019-6; 2008.
Esteve-Turrillas FA, Abad-Somovilla A, Quiñones-Reyes G, Agulló C, Mercader JV, Abad-Fuentes A. Monoclonal antibody-based immunoassays for cyprodinil residue analysis in QuEChERS-based fruit extracts. Food Chem. 2015;187:530–6.
Acknowledgments
Authors gratefully acknowledge the financial support of the Ministerio de Economía y Competitividad (AGL2012-39965-C02-01-02/ALI, CTQ-2012-38635, and CTQ-2014-52841) and Generalitat Valenciana (PROMETEO-II 2014-077).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the appropriate ethics committee and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare no competing financial or nonfinancial interest.
Additional information
These results were presented as a poster communication at the “XXV Reunión Nacional de Espectroscopía—VIII Congreso Ibérico de Espectroscopía” (XXV RNE-VIII CIE) held in Alicante (Spain) in July 2016 and it won the Poster Prize for excellent presentation of particularly significant innovative analytical research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 346 kb)
Rights and permissions
About this article
Cite this article
Armenta, S., de la Guardia, M., Abad-Fuentes, A. et al. Highly selective solid-phase extraction sorbents for chloramphenicol determination in food and urine by ion mobility spectrometry. Anal Bioanal Chem 408, 8559–8567 (2016). https://doi.org/10.1007/s00216-016-9995-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9995-9