Abstract
An electrochemical biosensor based on the immobilization of laccase on magnetic core-shell (Fe3O4–SiO2) nanoparticles was combined with artificial neural networks (ANNs) for the determination of catechol concentration in compost bioremediation of municipal solid waste. The immobilization matrix provided a good microenvironment for retaining laccase bioactivity, and the combination with ANNs offered a good chemometric tool for data analysis in respect to the dynamic, nonlinear, and uncertain characteristics of the complex composting system. Catechol concentrations in compost samples were determined by using both the laccase sensor and HPLC for calibration. The detection range varied from 7.5 × 10–7 to 4.4 × 10–4 M, and the amperometric response current reached 95% of the steady-state current within about 70 s. The performance of the ANN model was compared with the linear regression model in respect to simulation accuracy, adaptability to uncertainty, etc. All the results showed that the combination of amperometric enzyme sensor and artificial neural networks was a rapid, sensitive, and robust method in the quantitative study of the composting system.

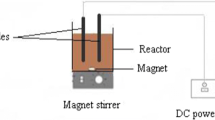
Structure of the magnetic carbon paste electrode used in the electrochemical biosensor
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds exhibit well-known adverse effects on human health and the environment. Such compounds mostly originate from various agricultural and industrial activities, including waste discharge from pulp, dyeing, petrochemical, and textile industries and the partial degradation of phenoxy contaminants in remediation processes. The toxicity of phenols generated from bioremediation, such as composting, can also bring on undesirable ecological effects and seriously damage removal efficiencies [1]. Catechol is a hazardous phenolic compound which affects the nerve center system of human beings, inhibits DNA replication, and leads to chromosomal aberration [2]. With the increasing application of composting technology in disposal of municipal solid waste, catechol is generally a direct pollutant or a by-product of the aromatic pollutant biodegradation [3]. Hence, detection of catechol from compost bioremediation of municipal solid waste is a critical issue. And with the enhancement of the demand of environmental quality, there should be a set of rapid and efficient detection techniques to match the correlative standard of environment protection.
A variety of organic compounds exist in compost systems, e.g., aromatic, aliphatic, phenolic, and quinolic derivatives with varying molecular sizes and properties. It is a complex and heterogeneous mixture with diversity, nonlinearity, and uncertain characteristics [4]. The most commonly used analytical methods for quantitative study of the composition of compost systems include spectrophotometry, gas chromatography, and high-performance liquid chromatography (HPLC) based on the absorbance spectra [5–7], but they are probably subject to interference from substrate turbidity and UV–Vis-light-absorbing substances in the compost system. The increase of background absorbance and partial overlapping of the spectral band of the interferents with that of the detectable compound leads to an improper performance of the procedures [8]. Besides, the pretreatment of the samples is cumbrous and time-consuming, and the instruments are expensive and ponderous. In an attempt to overcome the deficiencies of traditional analytical methods, the applications of enzyme sensors to specialty pollutant detection have increasingly been reported to exhibit superior sensitivity, stability, reusability, selectivity, portability, and other obvious advantages. The operation efficiency of compost systems will be much improved if biosensors are applied to detection of pollutants in the compost.
In our laboratory, an amperometric laccase sensor was developed to detect catechol in compost. Laccase is a multicopper phenol oxidase which can directly oxidize catechol and utilize dioxygen as an oxidant, reducing it to water, without H2O2 as cosubstrate or any other cofactors for its catalysis [9, 10]. In the reaction, catechol (QH2) as electron donor for the oxidized form of the enzyme, was mainly converted into quinone and/or free radical product (Q), and then was reduced on the surface of the electrode at potentials below 0 V (vs. SCE), which efficiently shuttled electrons between the laccase redox center and the electrode surface in a dynamic equilibrium. A series of reactions could produce a set of correlative response signals which were transformed into detectable electric current. The reaction equations of the redox process on the electrode surface are described as follows:
Despite the improved selectivity and tolerance of the electrochemical sensor to turbid and selfcolored samples, the detection procedures are still susceptible to the complex and heterogeneous organic components containing certain functional groups (e.g. phenolic OH, carboxyl, etc.) owing to both the redox and sorption of the interfering matrix constituents on the electrode surface. As a result, an unstable baseline and a gradually increasing response current—instead of a prompt current transition during sensor analyses—will be observed when it is applied to large quantities of compost samples. Generally the linear regression model is the most widely used model for electrochemical sensor determination, which is inherently vulnerable to local noise in the detection diagram. The quantification capability of the linear model will be limited by the dynamic, nonlinear, and uncertain characteristics of the complex composting system, and will give erroneous results if the linear range is exceeded [11, 12]. Artificial neural networks (ANNs) have been widely applied in data analysis because they can process very nonlinear and complex problems even if the data are imprecise and noisy [13]. Some researchers have successfully coupled ANNs to sensors as a chemometric tool to analyze target substance in complex systems, which showed strong nonlinear mapping and selflearning ability and enhanced the determining performance of the biosensor [14–17]. Although the detection of catechol from compost bioremediation is a single-analyte detection, nonlinear and uncertain problems also occur in the process as mentioned above, which restricts the biosensor in practical application. ANNs can efficiently identify and learn the correlated patterns between input and output values without an explicit formulation of the physical relationship of the problem [18].
In this paper, a feedforward backpropagation (BP) network model based on the connections of catechol concentrations to response currents was built, and the performance of the ANN model was studied, and the soft-determination of catechol in a complex composting system was realized. This method, coupled with the inherent high sensitivity, rapidity, robustness, and portability of the electrochemical sensor technique, enables the development of fast and inexpensive on-line monitoring systems in municipal solid waste compost bioremediation.
Experimental
Apparatus and reagents
A CHI660B electrochemistry system (Chenhua Instrument, Shanghai, China) was used for all cyclic voltammetric and amperometric measurements. An Agilent 1100 high-performance liquid chromatograph was used to determine the concentration of catechol in compost extracts. A Model PHSJ-3F laboratory pH meter (Leici Instrument, Shanghai, China), a Sigma 4K15 laboratory centrifuge (Sartorius AG, Germany), and an FD-1 vacuum freezing dryer (Boyikang Instrument, Beijing, China) were used in the assay. All the work was done at room temperature (25 °C) unless otherwise mentioned. The three-electrode system used in this work consisted of a magnetic carbon paste electrode (MCPE, diameter 8 mm) as working electrode of interest, a saturated calomel electrode (SCE) as reference electrode, and a Pt foil auxiliary electrode.
Laccase (EC 1.10.3.2, 23.3 U mg−1) was from Fluka. 3-Aminopropyltriethoxysilane (APTES), polyethylene glycol (PEG), tetraethoxysilane (TEOS), and all other chemicals were of analytical grade and used as received. All solutions were prepared with doubly distilled water. The synthesis of Fe3O4 magnetic nanoparticles and the immobilization of laccase on the surface of nanoparticles were achieved according to the procedure introduced by Zhang et al. [19].
Procedures
The preparation of MCPE and the HPLC and amperometric measurements of catechol concentration in compost extract were carried out as described in our previous work. The mixture of paraffin (400 mg) and graphite powder (500 mg) was stuffed into a polytetrafluoroethylene tube (aperture 8 mm) to fabricate an electrode. A caky magnet (diameter 8 mm and thickness 1.5 mm) was embedded at a depth of 8 mm from the electrode surface. After pretreatment, the magnetic laccase–Fe3O4 nanoparticles were firmly magnetically attached to the MCPE surface by magnetic forces. The structure of the MCPE is shown in Fig. 1.
The determination of catechol in compost extracts was carried out by HPLC and then the laccase sensor. The eluent in the HPLC method consisted of an isocratic mixture of water, acetonitrile, and acetic acid (88:10:2) at a flow rate of 0.7 mL min−1, and the concentration of catechol was detected by an ultraviolet spectrophotometer at 280 nm [20]. The cyclic voltammetry was performed between −0.6 and + 0.6 V vs. SCE at 100 mV s−1. The optimized amperometric monitoring system conditions, i.e., which afforded the highest sensitivity of the enzyme sensor, were electrolyte comprising 30 mL 67 mM phosphate buffer (pH 5.5) containing catechol and the oxidation peak potential of –0.232 V (vs. SCE).
Application in compost extracts
The amperometric determination of the catechol concentration was applied in compost bioremediation. The composting process has been introduced previously. The components of compost were soil, straw, scraps, and bran, and the water ratio was 51%. The soil was collected from 100-cm depth underground from an unfrequented hillside of Yuelu Mountain (Changsha, China), from which large organic scraps were removed. Aerobic compost was then managed for 40 days under the conditions of 30 °C temperature and 0.033 m3 h−1 ventilation. A 10-g compost sample was placed in a flask and 200 mL water was added. The suspension was agitated on a mechanical vibrator at 200 rpm for 2 h. The supernatant was centrifuged at 10,000 rpm for 5 min, and then filtered to get the filtrate as the compost extract. The dosage of catechol into each compost extract was controlled [21].
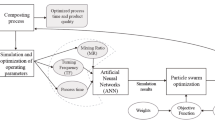
Artificial neural networks
Matlab 7.0 (Mathworks, Natick, MA), and in particular its Neural Network Toolbox, was employed to develop the ANN models. The measured data of compost samples with catechol and corresponding amperometric responses of laccase sensor were analyzed using a feedforward BP network model. The data were divided into three subsets, one for training, one for testing, and the other for model validation. A series of 29 compost extract samples was used to train the network. Other ten extract samples were taken to be the external test set to estimate the modeling performances. And another eight extract samples validated the ANN model application. The training set is used for adjusting the connection weights, while the testing set is used for the determination of network geometry and model parameters. Finally, the validation set is used for testing the optimality and generalization ability of the model developed [22, 23]. For the precision of the ANN modeling research, the extract samples containing a maximum or a minimum catechol concentration were included in the training set, avoiding the need for extrapolation when checking the model with the external test.
Results and discussion
Characterization of artificial neural network
In the experiment, under the optimum conditions, the continuous curve of current versus time was obtained corresponding to the catechol concentration. Instead of direct response current as the only independent variable in linear regression, the time profile of the amperometric signal, which was relative to response current, current stabilizing time, and some other current change characteristics, was adopted in the ANN modeling. Besides response current, current stabilizing time could also act as an identification factor of the analytical environment in the electrochemical sensor determination. The change of the stabilizing time exhibited some available information according to different concentrations and analytes. The time profile of the amperometric signal could represent all characters and relations between the sensor response and the analyte concentration in the complex system, which formed an intact and scientific structure to perform a nonlinear modeling for catechol determination. Figure 2 shows the ANN architecture. It was built with input neurons taken from data points of each amperometric curve within 120 s before the detection current reached steady state. The concentration of catechol is the target for ANN modeling. Since a relevant report [24] stated that ANNs with single hidden layer mapping structure could resolve the nonlinear problem in electrochemical signal analysis, networks with more than one hidden layer were not considered.
The normalization of original data was recognized to improve the network performance for confidentiality and generalization of the neural network model [25]. In this work, the input values of both the training and the test subsets were normalized by
where \(x\prime \) is the normalized value for input variable, x is the original value, and x min and x max are the minimal and maximal original values of primitive data, respectively. After simulation of the networks, the estimated results were reconverted by inverse function of Eq. (3) to be compared with the target values.
Network optimization
The network performance was quantified by calculating the root mean square of errors (RMSE) between the expected and predicted catechol concentrations:
where y i and t i are respectively the predicted and the expected concentration values by the ANN, and n is the number of the test samples which equals to 10 in this work.
Feedforward backpropagation with random initial weights and biases was used to train the networks to avoid selecting fixed conditions which might favor one particular network design. Variability will occur due to random initial values in the networks with exactly the same program [26] ; hence, each ANN program was run more than seven times to get the average RMSEs for the external test set to result in a true measure of performance.
At first, the hidden neuron numbers and combinations of tan-sigmoidal (Tansig), log-sigmoidal (Logsig), pure-lineal (Purelin), and sat-lineal (Satlins) transfer functions were evaluated. The calculated RMSEs were plotted against different hidden neuron numbers and combinations of transfer functions in hidden and output layers synchronously. The lowest RMSE value was obtained with 15 hidden neurons and Satlins–Purelin as transfer function as shown in Fig. 3.
Obtained RMSEs in catechol concentration prediction for different transfer function combinations and neuron numbers in the hidden layer with input neuron number of 12 and Levenberg–Marquardt backpropagation (trainlm) as optimization algorithm. Numbers 1–5 represent the transfer function combinations Tansig–Purelin, Logsig–Satlins, Satlins–Purelin, Logsig–Purelin, and Tansig–Satlins, respectively
The effects of different optimization algorithms on the model performance were then evaluated as shown in Fig. 4. The ANN models with trainbfg, trainbr, traingd, and traincgb as optimization algorithms, respectively, could not meet the performance goal when the minimum gradient was reached in the training process. The ANN models by traingdm and traingdx got lower RMSE values sometimes, but were not steady with the increasing of training time. So trainlm was selected as the optimal algorithm to obtain the lowest RMSE value, steady result, and short training time. Considering that the complexity of the model was increased, and the training time was prolonged remarkably with no obvious decrease of RMSE when the input neuron number was increased in our experiment, the input neuron number of 12 was selected with adequate accuracy of simulation. The final optimization results of the ANN model are shown in Table 1.
Performance of the best ANN
In order to obtain accurate catechol concentrations from ANN models, triplicate calculation results of the optimized network were selected and averaged with RMSEs most close to 8.6549 µM for catechol concentration. The concentrations of catechol from compost extract in the experiment varied between 7.5 × 10–7 and 4.4 × 10–4 M. As shown in Fig. 5, it is obvious that the predictions by the optimized ANN model are in good agreement with measured results with low error levels. The linear regression shows a nice fit of data in the training set with remarkably high correlation coefficients of nearly 1, and in the test set, the linear regression yielded high correlation coefficients (R 2 > 0.999). These results confirm the excellent prediction ability of the ANN model used in this study.
Comparison of prediction results between regression model and ANN model in composting system
Through simple mathematical analysis of the data detected by the biosensor, a linear regression was obtained with a linear range of 7.5 × 10–7–2.75 × 10–4 M. The equation of the linear regression model is:
where x is catechol concentration (µM) and y is the response current value (µA). The correlation coefficient (R 2) of the linear regression is 0.9945.
Practically, when biosensor with linear data analysis is applied to determine the real samples, the concentration of analyte often exceeds the linear detection range of biosensor, which will affect the accuracy of determination. Therefore, for the sake of obtaining a more applicable and convenient detection method, the combination of ANN with biosensor technology turns out to be a good analytical tool. The ANN, owing to its strong prediction ability for situations not included in the training process, can enlarge the determining limit, and then make the determination and the result analysis quick [24]. In this study, the linear range of the linear model for catechol determination was only 7.5 × 10–7–2.75 × 10–4 M, while the catechol concentrations directly analyzed by the ANN model varied between 7.5 × 10–7 and 4.4 × 10–4 M, which far exceeded the determining range of other catechol sensors, too [27–28]. The ANN and linear regression models established here were applied to the eight external compost extract samples. Figure 6 shows the correlation between experimental and predicted values of the regression and ANN models. The prediction results by the ANN model were more precise than the linear regression, and the prediction result by linear regression was far from accurate at high levels of catechol beyond the linear range, while the fitting degree of experimental and predicted value using the ANN model was satisfactory. So ANN was superior to the linear regression especially for the determination of high levels of catechol in the compost system. Furthermore, due to the black-box approach that depends only on the observed values, ANN can easily resolve the complicated properties in the compost system. The results showed that the correlation coefficient, adaptability to uncertainty, etc., obtained after combining the biosensor with ANNs were superior to direct determination of the catechol concentration by the biosensor in the compost system. Obviously, combined with the ANN model, the direct detection range for catechol in the compost system of the biosensor was widened, which would enhance the performance of the detection system in further applications in real compost extract sample determination.
Conclusions
In view of the harm that catechol can cause to human health and the environment, it is practically significant to detect catechol in environmental samples. Composting systems possess complex, dynamic, nonlinear, and uncertain properties which cause difficulty in realizing accurate pollutant detection by common methods. The ANN model is a so-called black box as it does not need any model structure specification, and holds the strong learning capability and adaptability. A BP–ANN model was established for data analysis in the sensor detection system as a chemometric tool to determine the catechol concentration in compost systems more effectively. All the results showed that the combination of amperometric enzyme sensor and artificial neural networks was a rapid, sensitive, and robust method in the quantitative study of composting systems. The direct detection range for catechol of the biosensor was extended to 7.5 × 10–7–4.4 × 10–4 M, which was superior to the direct determination by the biosensor with linear data analysis. In future work, this biosensor combined with ANN model could be a good analytical tool for further application in monitoring real contaminated compost and other complex environments which are relevant to human life and health.
References
Lante A, Crapisi A, Krastanov A, Spettoli P (2000) Process Biochem 36:51–58
Topping DC, Bernard LG, O’Donoghue JL, English JC (2007) Food Chem Toxicol 45:70–78
Canofeni S, Sario SD, Mela J, Pilloton R (1994) Anal Lett 27:1659–1662
Campitelli P, Ceppi S (2008) Geoderma 144:325–333
Faure D, Bouillant ML, Jacoud C, Bally R (1996) Phytochemistry 42:357–359
Kim KR, Kim H (2000) J Chromatogr A 866:87–96
Corcia AD, Bellioni A, Madbouly MD, Marchese S (1996) J Chromatogr A 733:383–393
Miró M, Frenzel W (2003) Analyst 128:1291–1297
Yaropolov AI, Skorobogat’ko OV, Vartanov SS, Varfolomeyev SD (1994) Appl Biochem Biotech 49:257–280
Xu F (1999) Laccase: molecular properties, enzymology, and preparation of laccase. In: Flickinger MC, Drew SW (eds) Encylopedia of bioprocess technology, fermentation biocatalysis and bioseparation. Wiley, New York, pp 1545–1554
Bessant C, Saini S (1999) Anal Chem 71:2806–2813
Hajmeer MN, Basheer IA (2003) Int J Food Microbiol 82:233–243
Lek S, Guégan JF (1999) Ecol Model 120:65–73
Gutés A, Céspedes F, Alegret S, del Valle M (2005) Biosens Bioelectron 20:1668–1673
Gutés A, Céspedes F, Alegret S, del Valle M (2005) Talanta 66:1187–1196
Cortina M, Gutés A, Alegret S, del Valle M (2005) Talanta 66:1197–1206
Ni Y, Qiu P, Kokot S (2005) Anal Chim Acta 537:321–330
Sahoo GB, Ray C, Mehnert E, Keefer DA (2006) Sci Total Environ 367:234–251
Zhang Y, Zeng GM, Tang L, Huang DL, Jiang XY, Chen YN (2007) Biosens Bioelectron 22:2121–2126
Chapuis-Lardy L, Contour-Ansel D, Bernhard-Reversat F (2002) Plant Sci 163:217–222
Zeng GM, Tang L, Shen GL, Huang GH, Niu CG (2004) Int J Environ Anal Chem 84:761–774
Onkal-Engin G, Demir I, Engin SN (2005) Environ Modell Softw 20:843–850
Siripatrawan U, Linz JE, Harte BR (2006) Sensor Actuat B 119:64–69
Gutés A, Ibáñez AB, Céspedes F, Alegret S, del Valle M (2005) Anal Bioanal Chem 382:471–476
Günaydin HM, Doğan SZ (2004) Int J Proj Manag 22:595–602
Bachmann TT, Leca B, Vilatte F, Marty JL, Fournier D, Schmid RD (2000) Biosens Bioelectron 15:193–201
Roy JJ, Abraham TE, Abhijith KS, Kumar PVS, Thakur MS (2005) Biosens Bioelectron 21:206–211
Jarosz-Wilkołazka A, Ruzgas T, Gorton L (2005) Talanta 66:1219–1224
Acknowledgements
The study was financially supported by the National Natural Science Foundation of China (No.50608029), the National 863 High Technologies Research Foundation of China (No.2004AA649370), the National Basic Research Program (973 Program) (No. 2005CB724203), the Natural Foundation for Distinguished Young Scholars (No.50425927, No.50225926) and Program for Changjiang Scholars and Innovative Research Team in University (IRT0719).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, L., Zeng, G., Liu, J. et al. Catechol determination in compost bioremediation using a laccase sensor and artificial neural networks. Anal Bioanal Chem 391, 679–685 (2008). https://doi.org/10.1007/s00216-008-2049-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2049-1