Abstract
A novel monolithic macroporous material was developed by cross-linking hen egg albumin (HEA) and chitosan with glutaraldehyde at subzero temperatures. A macroporous cryogel structure allowed efficient mass transport of solutes within the material. In one application, albumin was partially replaced with active enzymes (glucose oxidase and horseradish peroxidase) resulting in the production of macroporous biocatalyst preparations suitable for flow-injection analysis of glucose in the low millimolar range. In another application, the proteolytic enzymes savinase and esperase were coupled to the macroporous structure via free amino groups on the pore walls using glutaraldehyde as cross-linker/spacer agent. The low hydraulic resistance of the matrix allowed for the development of a generic, high-performance online protein digestion system utilizing the wall-bound proteases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many applications, enzymes are immobilized to solid supports and the preparations are then used repeatedly or continuously. There are a broad range of solid supports available and the choice is often critical, both from a cost standpoint and from the point of view of performance of the final functional material. Generally, the immobilization procedure should result in a highly retained biocatalyst activity and give a material with desirable mechanical properties. Frequently at the laboratory scale, beaded material is used for enzyme immobilization [1, 2]. The methods to capture the biocatalyst are generally gentle and efficient, yielding an active enzyme in the immobilized form. A crucial factor when immobilized enzymes are to be used in large-scale preparations is the cost of the support. One of the more successful concepts of enzyme capture for industrial applications is that of cross-linking the protein molecules, forming solid aggregates which can serve directly as support [3, 4]. After coupling and subsequent washing, the matrix should be ready for use. In addition, for flow-based applications, the media containing the immobilized enzyme should have sufficiently low hydraulic resistance allowing for efficient mass transport so that the efficiency coefficient of the immobilized enzyme molecules will be high. Furthermore, it is advantageous if the resin can tolerate particulate material, avoiding clogging when complex feed solutions are utilized.
With the objective of combining high efficiency matrices with the possibility to perform purification or biotransformation reactions, much attention has been given to so-called monolithic materials. Monolithic columns are considered as a new type of chromatographic media allowing for predominantly convective mass transport [5–7]. The low hydraulic resistance is the most advantageous feature of the monolithic columns compared with conventional packed-bed columns. One typical example of these materials is the monolithic convective interaction media (CIM) discs. CIM discs with immobilized glucose oxidase were used for online monitoring of glucose during cultivation of Saccharomyces cerevisiae and were successfully utilized instead of conventional packed-bed columns [8].
Recently, a new format of monolithic materials has been developed, prepared via cryogelation (i.e., gelation at subzero temperatures) [9, 10]. The macroporous cryogels with an open porous structure are produced at moderately low temperatures, such that most of the solvent (water) is frozen while the dissolved substances are concentrated in relatively small non-frozen regions, a so-called non-frozen liquid microphase [11]. The cryogel formation occurs in this liquid microphase and the crystals of frozen solvent perform as a porogen leaving behind the interconnected pores after melting. The cryogelation technique allows for preparation of macroporous hydrophilic materials with a wide range of porosity, 0.1–200 μm, depending on the type of gel precursors used [12]. The macroporous cryogel monoliths with large, 1–200 μm, interconnected pores and unique elastic and sponge-like morphology were prepared from synthetic [11–16] and natural [17–19] gel precursors mainly through free radical polymerization reaction in an aqueous medium [11, 13, 14, 16, 18] or through chemical cross-linking using the appropriate cross-linkers [15, 19] or through physical cross-linking [17]. However, the preparations of macroporous protein-based monoliths with high flow-through characteristics have not been addressed much so far. In the present work, the cryogelation technique was evaluated as a tool for the development a novel macroporous protein-based cryogel (MPC) monolith. In one scheme, active proteins were actually incorporated in the monolithic material during the cryogelation procedure. In another application, the protein-based cryogels were subsequently used as a support for the immobilization of enzymes on the pore surface of the material.
Experimental
Chemicals
Freeze-dried hen egg albumin (HEA) powder was a kind gift from Källbergs Industri AB (Töreboda, Sweden). The proteolytic enzymes savinase (16 KNPU mL−1) and esperase (6 KNPU mL−1) were kind gifts from Novozymes Biopharma AB (Lund, Sweden). Chitosan from crab shells (min. 82% deacetylated) was from Protan Laboratories (Redmond, WA, USA). Glutaraldehyde (25% aqueous solution, grade II), glucose oxidase (G-6891, 1,200 U mL−1), horseradish peroxidase (P-8125, 113 U mg−1) and Staphylococcal enterotoxin B from S. aureus (S-4881, 5 mg mL−1) were purchased from Sigma (St. Louis, MO, USA). Water and other chemicals were of analytical quality.
Preparation of MPC monoliths
Aqueous solutions of hen egg albumin (15–25% w/v) were mixed in different ratios with chitosan (2% w/v in 0.5% acetic acid) under stirring. The pH of the solution was adjusted to 4.0 with 5 M HCl. After addition of glutaraldehyde as cross-linker, the reaction mixture was vigorously stirred for 15 s, poured into plastic columns (inner ∅ 13 mm), and then immediately frozen at −18 °C for 15 h. The resulting MPCs were thawed at 4 °C and washed thoroughly under running distilled water accompanied by intermittent squeezing of the spongy material. As the last step in the preparation of an MPC, a reduction of the formed Schiff bases was carried out using a 20 mM solution of NaCNBH3 in 0.1 M sodium phosphate buffer (Na-PB), pH 7.0 containing 0.15 M NaCl. After Schiff base reduction the cryogel plug was placed (i.e., slightly squeezed) into an adaptor-equipped glass column with an inner ∅ of 10 mm.
Enzyme immobilization
As a model system for enzymes catalyzing the conversion of low molecular weight substrates, glucose oxidase (GOD) and horseradish peroxidase (HRP), reconstituted in distilled water (15 and 113 U mL−1 final activity, respectively) were added to the albumin/chitosan solution before addition of glutaraldehyde. The enzyme-containing cylindrical cryogel plug (13 × 10 mm) was placed in a glass column (inner ∅ 10 mm) and integrated into a flow-injection analysis (FIA) system. The gel was washed for 2 h by pumping 0.1 M Na-PB, pH 7.0 through the column.
For enzymes acting on macromolecular substrates, a different immobilization strategy was necessary. On bare cryogels, produced as described above, the proteolytic enzymes savinase and esperase were immobilized, utilizing primary amino groups on the pore wall surface. Again, glutaraldehyde was used as cross-linker/spacer and the matrix was incubated in 1% (v/v) in 100 mM Na-PB, pH 6.0 containing 0.15 M NaCl for 3 h at 4 °C. After washing (with the same buffer), a solution containing 0.5 KNPU mL−1 (200-mL total volume) of each protease activity in 100 mM Na-PB, pH 6.0 was applied at a flow rate of 2 mL min−1 for 5 h under recirculation at 4 °C. The cryogel was then extensively washed with Na-PB for 36 h to remove any trace of unbound enzymes. As a last step in the preparation of the protease-MPC, another reduction of formed Schiff bases was performed as described earlier. After equilibration in ammonium bicarbonate buffer (50 mM, pH 8.0) the cryomaterial was ready for protein degradation studies. To improve the digestion capacity of the matrix, the column was operated at 40 °C.
Characterization of the MPC
Morphology
The cryogel samples for imaging with scanning electron microscopy (SEM) were fixed in 2.5% glutaraldehyde in 0.12 M Na-PB, pH 7.2, overnight. The samples were then successively dehydrated in ethanol and critical-point dried. The dried samples were coated with gold/palladium (40:60) and examined using a JEOL JSM-5600 LV scanning electron microscope.
Flow characteristics
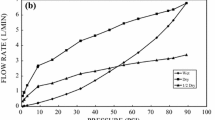
The flow properties of the monolithic cryogels were investigated and compared with those of a conventional beaded packing material (Sepharose CL-4B). Two columns (of equal size and volume, 10 × 25 mm) were used and the mobile phase (water) was pumped at linear flow rates in the range of 76–760 cm h−1 (1–10 mL min−1). Water was passed through the column for 1 min at each flow rate. The flow control and pressure drop over the columns were registered using a Biologic DuoFlow Chromatography System from BioRad (Hercules, CA, USA).
Enzyme assays
One cryogel containing both GOD and HRP was gently squeezed into a glass column (10 × 25 mm) equipped with dual-end adaptors and the column was then mounted in a FIA system (flow rate 2 mL min−1, sample loop volume 250 μL). Upon injection of the appropriate substrates (i.e., 4-aminoantipyrine and phenol: 2 mM and 25 mM, respectively) together with an increasing concentration of glucose (1–5 mM), the colored product quinomine (Eqs. 1 and 2) was detected at 592 nm using a NovaSpec II flow-spectrophotometer from Pharmacia (Uppsala, Sweden). A calibration curve of absorbance vs. concentration of glucose was constructed.
The activity of the protease-coated MPC was investigated by injecting pulses (1 mL, 0.144 mg mL−1) of Staphylococcal enterotoxin B (SEB), a 28-kDa exotoxin derived from Staphylococcus aureus. SEB was chosen in this study owing to its inherent resistance to protease degradation [20]. However, the enzymes savinase and esperase from Bacillus lentus used in combination, have in earlier studies (unpublished data) completely digested SEB without any pretreatment (i.e., no denaturation using urea or guanidine, which is otherwise commonly used/needed). By varying the residence time of the analyte in the cryogel column, digestion profiles for the proteolytic products could be obtained. The degradation studies were performed using a fast protein liquid chromatography (FPLC) system from BioRad. Mass spectrometric analysis of the column eluate, collected as top fractions (0.5 mL) at different flow rates (0.3–3 mL min−1) was performed utilizing a QSTAR® pulsar-i-QTOF mass spectrometer (PE Sciex, Toronto, Canada) equipped with a nanospray ionization source. The eluate containing digested SEB was mixed 2:1 (v/v) with acetonitrile containing 0.1% formic acid prior to MS analysis and the nanospray interface was set to positive ion mode with a source voltage of +850 V. The quadrupole system was adjusted to scan between m/z 500 and 2,000 in TOF-MS mode, whereas in product ion mode (i.e., MS/MS, collision-induced dissociation (CID)) a range of m/z 50–2,000 was chosen.
As a rough estimate of the MPC digestion capacity, the peak intensities of SEB peptides from the digestion map (i.e., conclusively identified with tandem MS and the bioinformatic web tool Mascot (http://www.matrixscience.com)) were accumulated for 20 s and plotted vs. residence time of the protein in the column. Additionally, peak envelopes related to intact or moderately truncated forms of SEB present in the eluate were concomitantly processed and plotted in the same manner.
Results and discussion
Preparation of MPC monoliths
MPC monoliths were prepared by cross-linking a mixture composed of two biopolymers, hen egg albumin and chitosan, with glutaraldehyde. The formed MPC could be defined as a porous hydrophilic material with an interpenetrating polymeric network (a so-called IPN gel). It is worthwhile to note that cryogelation of hen egg albumin (15–25% w/v) in the absence of chitosan was unsuccessful, resulting in mechanically fragile (weak) cryogels. Hence, different ratios of egg albumin and chitosan were tested to optimize the mechanical strength and flow-through properties of the MPC monoliths (Table 1).
Chitosan is a hydrophilic, biocompatible, and biodegradable cationic polysaccharide with low toxicity, which has been used in several biomedical applications [21, 22]. In acetic acid, chitosan is positively charged from protonated amino groups owing to a pK value of 6.3 [23]. At high pH (>7) the amino groups are deprotonated and thus, chitosan undergoes reactions typical of amines, of which N-acylation and Schiff base formation reactions are the most important [21]. Spongy and elastic MPC structures were produced only within a rather narrow range of component concentrations as shown in Table 1. In the further study, MPC monoliths produced with a ratio HEA/chitosan of 19 (w/w) and a glutaraldehyde concentration of 0.0054% (v/w) were used.
One of the features of performing gelation at subzero temperatures is the effect of concentration of the reagents in the unfrozen liquid microphase, where the chemical reaction can proceed despite the bulk of the reaction mass resembling solid ice. High concentration of the reagents (hen egg albumin, chitosan and glutaraldehyde) in the liquid microphase accelerates a chemical reaction at subzero temperature even when conditions lie far away from the optimal ones in a homogeneous solution at ambient temperature (Schiff base formation requires slightly alkaline conditions, and the pH of the protein/chitosan solution for cryogel formation was 4.0). The formed MPC monoliths were of bright yellow color owing to the formation of Schiff bases. Reduction of the Schiff bases (which are unstable at pH < 6) was carried out using sodium cyanoborohydride.
The prepared MPCs were structurally stable at temperatures up to 60 °C. An attempt to re-swell the gel after complete drying from an aqueous solution was however unsuccessful. The MPC became brittle and weak under these conditions and the material fell apart in flakes even under very small mechanical strains. As could be expected, during the drying procedure, the porous structure would collapse and this would be accompanied by a probable partial denaturation of the protein molecules, resulting in an irreversible protein aggregation. Hence, for long-term storage of the MPC in wet state, the presence of a bacteriostatic agent such as sodium azide would certainly be required for preservation.
The proper control over two main parameters, namely the chemical reaction (which proceeds in unfrozen liquid microphase) and formation of ice crystals (which perform as porogen), allowed for reproducible production of the MPCs. It was shown earlier that macroporous polyacrylamide-based cryogel monoliths (pAAm monoliths) were prepared reproducibly under the same experimental conditions [11] and the porous properties of pAAm monoliths were effectively regulated by changing many parameters such as monomer concentration [14], content of initiating system in the reaction mixture, cross-linker, and solvent used [24]. The preparation of the macroporous cryogel monoliths from natural polymers (as protein dispersions) is complex per se compared with the cryostructuration of synthetic precursors. However, while the cryostructuration conditions for the preparation of MPC were optimized (i.e., optimal composition of the reaction mixture, content of the cross-linker (glutaraldehyde), and freezing temperature), MPC with similar behavior (flow-through properties and biocatalytic activity) were prepared. It is noteworthy that reagents used for the preparation of MPC are robust and available and were used without any additional treatment (purification).
Porosity properties of the MPC
SEM studies of the MPC monoliths revealed an interconnected super-macroporous network with pore diameters ranging from around 40 to 120 μm (Fig. 1a,b). Further investigations of the SEM images showed that the pore walls within each pore had a pronounced microporous system (0.05- to 0.1-μm pore size), allowing molecular inter-pore diffusion (Fig. 1c).
To determine the flow characteristics in terms of backpressure of the protein cryogel in comparison to a typically used bead-based matrix (Sepharose), chromatographic columns of the same size and volume were packed with the different resins. The super-macroporous structure of the MPC monoliths yielded, as was desired, considerably lower flow resistance compared with the beaded material (Fig. 2). For the MPC column, even at linear flow rates up to 800 cm h−1, little or no compression was observed. By contrast, for the packed bed of Sepharose significant compression was exhibited even at linear flow rates around 300 cm h−1 (4 mL min−1). At a flow rate of 500 cm h−1 severe compression of the packed bed occurred, indicating that the chromatographic properties were heavily compromised.
MPC as biocatalyst scaffold
As albumin presents a constitutive component of the MPC monolith, the replacement of a fraction of this protein with a functional biocatalyst seems to be a convenient method of enzyme immobilization. Indeed, the enzymes GOD and HRP retained approximately 80% of enzyme activity when integrated in the MPC structure, implying that the proteins were gently confined within the pore walls of the macroporous gel. The monolith was placed in a glass column which was mounted in a FIA system. Upon injection of the appropriate substrates for HRP together with an increasing concentration of glucose, the colored product quinomine could be detected (Fig. 3). The linear glucose calibration curve obtained strongly indicates that low molecular weight compounds can diffuse through the pore walls and are quantitatively processed by the trapped enzymes. In addition, the elasticity of the MPC allows for gentle squeezing and easy column packing. When the monolith expands in the column it occupies the whole volume; hence there is no risk of having a bypass flow between the column walls and the cryogel.
Flow injection analysis of glucose using MPC monolith prepared by partial replacement of albumin with glucose oxidase and horseradish peroxidase. The GOD/HRP MPC monolith was placed in an FIA system. A 2 mL min−1 flow rate of 0.1 M Na-PB, pH 7.0 was applied. When injecting the substrates 4-aminoantipyrine and phenol (2 mM and 25 mM, respectively) concomitantly with an increasing concentration of glucose, the absorbance of the colored product quinomine could be detected at 592 nm using a flow-spectrophotometer
For biocatalysts requiring a higher degree of steric availability for their substrate interaction, a different immobilization strategy was used. By simultaneous co-immobilization of the proteolytic enzymes savinase and esperase, utilizing available amino groups on the pore wall surface, an MPC with excellent proteolytic properties was achieved. The activity of the protease-coated MPC was investigated by injecting pulses of staphylococcal enterotoxin B (SEB) through an FPLC system. As a rough estimate of the MPC digestion capacity, the intensities of SEB peptides occurring in the mass spectrum were accumulated and plotted vs. the residence time of the protein in the column. Additionally, peak envelopes related to intact or moderately truncated forms of SEB in the eluate were concomitantly processed and plotted in the same manner (Fig. 4).
Proteolytic degradation of SEB in the MPC monolith column (10 × 25 mm) modified with immobilized savinase and esperase. The column eluate after discrete injections of SEB (1 mL, 0.144 mg mL−1) was collected in fractions at the investigated flow rates. The peak intensities (accumulated for 20 s in m/z range 500–2,000 in TOF-MS mode) from the occurring SEB peptides (filled circles) were calculated from MS-spectra and used as a rough estimate of the MPC digestion capacity. Concurrently, peak intensities related to intact or moderately truncated SEB molecules were identified and plotted (open circles) in the same manner. The MS analysis of the collected fractions from the column eluate also demonstrated that SEB was essentially degraded to peptides at residence times above 2 min in the cryogel
The efficient mass transport within MPC pore and the high proteolytic activity allowed for relatively fast continuous degradation of proteins even as stable as SEB. Mass spectrometric analysis of the collected fractions from the column eluate demonstrated that SEB was essentially degraded to peptides at residence times above 2 min in the gel. The surface-bound biocatalysts maintained >70% of their initial activity over 7 days at a continuous exposure to temperatures of around 40 °C.
Conclusion
When a mixture of hen egg albumin and chitosan was cross-linked with glutaraldehyde, an elastic sponge-like material, a macroporous protein cryogel monolith, was produced with a narrow range of component composition. Partial substitution of albumin with a functional protein allowed for the production of material with a high degree of built-in enzyme activity, excellent mass transfer characteristics, and beneficial hydrodynamic properties. The immobilized (integrated) enzymes were used to assay glucose in a FIA setup, yielding quantitative detection of the analyte at flow rates of at least 2 mL min−1.
When the biocatalyst was immobilized by covalent coupling to the pore walls of the MPC, instead of being captured within the micropore structure, the enzyme maintained its activity for 5–7 days at temperatures around 40 °C and far larger analytes could be processed. In this case, an online proteolytic degradation of the exotoxic compound staphylococcal enterotoxin B was successfully performed.
The simplicity and robustness of the developed immobilization procedures along with the mild conditions used, allow for high activity yields of immobilized enzymes. As only cheap and easily available chemicals are used for immobilization, the method is expected to be an attractive alternative to the existing immobilization techniques used in the biotechnological industry, not only in analytical applications as shown here, but also in preparative applications. This is a topic of ongoing studies to be reported in the future.
References
Taylor RF (1985) Anal Chim Acta 172:241–248
Mateo C, Fernandez-Lorente G, Cortes E, Garcia JL, Fernandez-Lafuente R, Guisan JM (2001) Biotechnol Bioeng 76:269–276
Brown GB (1976) Mosbach K (ed) Methods Enz 44:263–280
Sheldon RA, Schoevaart R, Van Langen LM (2003) Spec Chemic Mag 23:40–42
Xie S, Svec F, Frechet JM (1997) J Chromatogr A 775:65–72
Masanori M, Hiroshi K, Norio I, Hiroyoshi M, Kazuki N, Hiroshi J, Ken H, Tohru I, Nobuo T (2002) J Chromatogr A 961:53–63
Paull B, Roux C, Dawson M, Doble P (2004) J Forensic Sci 49:1181–1186
Strancar A, Koselj P, Schwinn H, Josic D (1996) Anal Chem 68:3483–3488
Lozinsky VI, Plieva FM, Galaev IYu, Mattiasson B (2001) Bioseparation 10:163–188
Kumar A, Plieva FM, Galaev IYu, Mattiasson B (2003) J Immunol Methods 283:185–194
Plieva FM, Andersson J, Galaev IYu, Mattiasson B (2004) J Sep Sci 27:828–836
Plieva FM, Galaev IYu, Mattiasson B (2007) J Sep Sci 30:1657–1671
Persson P, Baybak O, Plieva F, Galaev IYu, Mattiasson B, Nilsson NB, Axelsson A (2004) Biotechnol Bioeng 88:224–236
Plieva FM, Karlsson M, Aguilar MR, Gomez D, Mikhalovsky S, Galaev IYu (2005) Soft Matter 1:303–309
Plieva FM, Karlsson M, Aguilar MR, Gomez D, Mikhalovsky S, Galaev IYu, Mattiasson B (2006) J Appl Polymer Sci 100:1057–1066
Savina IN, Cnudde V, D’Hollander S, Van Hoorebeke L, Mattiasson B, Galaev IYu, Du Prez F (2007) Soft Matter 3:1176–1184
Bloch K, Lozinsky VI, Galaev IYu, Yavriyanz K, Vorobeychik M, Azarov D, Damashkaln LG, Mattiasson B, Vardi P (2005) J Biomed Mater Res 75A:802–809
Plieva F, Oknianska A, Degerman E, Galaev IYu, Mattiasson B (2006) J Biomater Sci Polym Edn 17:1075–1092
Le Noir M, Plieva F, Hey T, Guiesse B, Mattiasson B (2007) J Chromatogr A 1154:158–164
Tweten RK, Iandolo JJ (1983) J Bacteriol 153:297–303
Kumar G, Bristow JF, Smith PJ, Payne GF (2000) Adv Chitin Sci 4(EUCHIS’99):345–348
Khor E, Lim LY (2003) Biomaterials 24:2339–2349
Shamov MV, Bratskaya SYu, Avramenko VA (2002) J Colloid Interfac 249:316–321
Plieva FM, Huiting X, Galaev IYu, Bergenståhl B, Mattiasson B (2006) J Mater Chem 16:4065–4073
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedström, M., Plieva, F., Galaev, I.Y. et al. Monolithic macroporous albumin/chitosan cryogel structure: a new matrix for enzyme immobilization. Anal Bioanal Chem 390, 907–912 (2008). https://doi.org/10.1007/s00216-007-1745-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1745-6