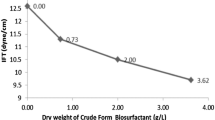
Abstract
Three hundred and thirty two bacterial colonies were isolated from soil contaminated by an oil spill. All the bacteria were cultured in a liquid medium individually, and the surface tensions of the media were compared. The bacterium whose culture medium had the lowest surface tension was identified as Pseudomonas sp. G11. A biosurfactant was produced by cultivation of the Pseudomonas sp. G11 in the LB media. For extraction of the biosurfactant, two solvent systems were used (n-hexane and a 2:1 (v/v) mixture of chloroform/MeOH), and the results were compared. Various experimental conditions (solvent composition, flow rate, etc.) were tested to optimize the analysis of the biosurfactant by asymmetrical flow field-flow fractionation (AsFlFFF). The biosurfactant was successfully separated from the culture medium by AsFlFFF when pure water was used as the carrier. From the retention data, the hydrodynamic diameter (d H) and molecular weight (M) of the biosurfactant were determined by AsFlFFF. The molecular weight was determined by using pullulans as the calibration standards. The d H and M were 49 nm and 2.3 × 105 Da when extracted with n-hexane, and 39 nm and 1.13 × 105 Da when extracted with the 2:1 mixture of chloroform/MeOH, respectively.

Separation of biosurfactant from its culture medium by flow FFF
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil spills have released over 5.6 million tons of oil into the environment since 1970 [1]. When oil spreads in the environment, low molecular weight hydrocarbons are volatilized, whereas polar components are dissolved in water. Most of the hydrocarbons remain on the surface of water and are adhered to soil particles due to their low solubility. Oil spills are often treated with synthetic surfactants to disperse the oil and to accelerate its mineralization [2]. Surfactants increase the surface area of the hydrophobic contaminants in soil or water, and thus increase their aqueous solubility and consequently accelerate their microbial degradation [3–6].
Surfactants have both hydrophilic and hydrophobic (generally hydrocarbon) moieties [7–11], and reduce the surface and the interfacial tensions in both aqueous solutions and hydrocarbon mixtures. Various synthetic surfactants are in use these days for a variety of purposes, such as dispersion, emulsification, penetration, wetting, foaming, and detergency [4–6]. Unfortunately chemical surfactants are environmentally unfriendly as they form foams on the surface of water, blocking the sunlight and oxygen. Furthermore, chemical surfactants have low biodegradability [8, 12].
Biosurfactant contains structurally diverse types of surface-active groups synthesized by microorganisms. They have some advantages over chemical surfactants: lower toxicity, higher biodegradability [13, 14], better environmental compatibility [15], higher foaming ability [7], and higher selectivity [13]. They also show specific activities even at extreme temperatures, pH levels, and salinity [13].
In this study, three hundred and thirty two bacterial colonies were isolated from soil contaminated by an oil spill. The bacterium whose culture medium had the lowest surface tension was identified as Pseudomonas sp. G11 by physiological-biochemical tests and analysis of its 16S rRNA sequence. For practical application of the biosurfactant, it needs to be purified and identified. The goal of this study is to develop a method to purify the biosurfactant produced by the Pseudomonas sp. G11.
Asymmetrical flow field-flow fractionation (AsFlFFF) is a chromatography-like separation technique that has been shown to be suitable for a wide range of macromolecules and colloidal particles (macromolecules [16–18], colloids [19], viruses [20], proteins [21], and liposomes [22]). AsFlFFF is the most versatile technique among the members of the field-flow fractionation (FFF) family, and it is applicable to molecules as small as a few nanometers up to particles of about 50-μm diameter. In this study, AsFlFFF was tested for purification and characterization of the biosurfactant produced by the Pseudomonas sp. G11.
Theory
In flow FFF, the relationship between the diffusion coefficient D and retention time t r is expressed by [23, 24].
where F out and F c are the channel and the cross-flow rates, respectively, and w is the channel thickness. Equation (1) is a simplified expression that is valid only for reasonably high retention [23]. According to Eq. (1), D can be directly determined from the retention time, which can then be used for determination of the hydrodynamic diameter, d H by the Stokes–Einstein equation:
where k is the Boltzmann constant, T the temperature, and η is the viscosity of the carrier liquid.
Generally for polymers, the relationship between D and molecular weight M is given by [25]:
from which
is obtained. A calibration plot of logD vs. log M is expected to be linear, and can be used to determine the molecular weight and its distribution of a sample.
Experimental
Reagents
Five pullulan standards (Shodex P-92, Showa Denko, Tokyo, Japan) were used for the calibration of the AsFlFFF system. The pullulan standards were dissolved in doubly distilled and degassed water, and then filtered through a 0.45-μm syringe filter (Whatman, Maidstone, UK) before being injected into the AsFlFFF system. The pullulan concentration was about 10 mg mL−1, which corresponds to the injection mass of about 200 μg. The nominal molecular weights and the polydispersities of the pullulan standards are summarized in Table 1. The Pseudomonas sp. G11 was cultivated in the Luria–Bertani (LB) media (Difco Laboratories, USA). The culture media contains 0.5% sodium chloride, 0.5% yeast extract, and 1% tryptone (casein peptone).
Preparation of biosurfactant for AsFlFFF analysis
Three hundred and thirty two bacterial colonies showing oil-degradability were isolated from soil contaminated by an oil spill in the city of Daejeon, Korea. Among them, the bacterium whose culture medium had the lowest surface tension was chosen and identified as Pseudomonas sp. G11 by physiological-biochemical tests and analysis of its 16S rRNA sequence [26].
The preparation process for analysis of the biosurfactant is shown in Fig. 1. The Pseudomonas sp. G11 was cultivated in LB media at 30 °C for 24 h in a bottle on a rotary shaker (EYELA, Japan). The whole culture broth was then centrifuged at 6,000 rpm for 20 min to remove the cells, and then 1N HCl was added to adjust the pH to 2.0. The supernatant was collected (about 1 L) and solvent extraction was performed to isolate the biosurfactant from the culture media. Two different solvent systems (n-hexane and a 2:1 (v/v) mixture of chloroform/MeOH) were employed. During extraction, the solution was stirred at 200 rpm for 24 h at 4 °C. The organic phase was taken, the solvent was evaporated at 50 °C (EYELA, Japan), and the residue was diluted in 0.2 M phosphate buffer (pH 7.0). The crude biosurfactant was freeze-dried (Freezone 4.5, LABCONCO, USA). For AsFlFFF analysis, the freeze-dried biosurfactant was dissolved in the carrier liquid.
Instruments
The AsFlFFF channel was assembled in a similar manner as described previously [23, 24] with a 190-μm-thick Mylar spacer and a regenerated cellulose membrane (Millipore, Bedford, MA, USA) having the cut-off molecular weight of 10,000 Da. The channel geometry was trapezoidal with the tip-to-tip length of 28.2 cm and breadths at the inlet and the outlet of 2.1 cm and 0.47 cm, respectively. The channel thickness determined from the retention time of the Ferritin (Sigma Aldrich, St. Louis, Missouri, USA) was 168.3 μm [27]. A degasser (DEGASYS, DG-2410, UniFlows, Tokyo, Japan) was placed before the pump. A piston pump (Young-Lin SP930D, Anyang, Korea) and a syringe pump (Model 100, KD Scientific, USA) were used to drive the channel and the injection flow, respectively. The channel and the cross-flow rates were measured using liquid flowmeters (Optiflow 1000, Agilent Technologies, Palo Alto, USA). Two on-line detectors were used, which were a Young-Lin M720 UV detector (Anyang, Korea) set at 254 nm, followed by a Young-Lin RI 750F refractive index detector. The detector signal was collected by using the software supplied by Postnova (Salt Lake City, Utah, USA). For calculation of the hydrodynamic diameter (d H), the retention time corresponding to the first moment of the fractogram was converted to d H by using Eqs. (1) and (2). For calculation of the molecular weight (M), the same retention time was converted to M by using the calibration plot (see Eq. (4)) prepared with a series of pullulan standards. The carrier liquid for AsFlFFF was pure water, which was heated and filtered through a 0.45-μm cellulose membrane filter (Millipore, hydrophilic). For all AsFlFFF experiments, the sample was introduced into the channel using a 20-μL loop injector (Rheodyne, Cotati, CA, USA).
Results and discussion
Selection of carrier liquid for AsFlFFF analysis of biosurfactant
Figure 2 shows AsFlFFF fractograms of the biosurfactant extracted with the 2:1 (v/v) mixture of chloroform/MeOH. The fractogram of the blank is also shown, where the carrier itself was injected. In normal operations of AsFlFFF, water containing small amounts of salt and/or surfactant, instead of pure water, is used as the carrier in order to prevent the interaction between the sample and the channel membrane, to keep the system clean, and to protect the system against bacteria multiplication [28]. In water with 0.02% sodium azide (NaN3) and 0.05% sodium dodecyl sulfate (SDS), all components were eluted at the void time without retention. In water with 0.02% sodium azide, some degree of retention is observed, but not enough to be separated from the void peak. Finally in pure water, a well-retained band was observed which was well separated from the void peak. In all experiments in Fig. 2, the same sample solution was injected, whose concentration was above the critical micelle concentration (CMC).
AsFlFFF fractograms of the biosurfactant extracted with a 2:1 mixture of chloroform/MeOH in various carriers. Experimental conditions: F in = 2.5 mL min−1, F c/F out = 2.20, and focusing time = 70 s. The carrier liquid is 1 blank, 2 water with 0.02% NaN3 + 0.05% SDS, 3 water with 0.02% NaN3, and 4 pure water
Figure 3 shows the AsFlFFF fractograms of the culture medium and its components obtained by using pure water as the carrier liquid. The culture medium and all its single components are not retained and eluted at the void time. This result ensures that the retained band shown in Fig. 2 (with pure water as the carrier) is of the biosurfactant. Pure water was then chosen as the carrier liquid in all AsFlFFF analysis of the biosurfactant in this study.
AsFlFFF fractograms of the culture media and its components. Experimental conditions are the same as those in Fig. 2 except F c/F out = 0.46 and the carrier liquid was pure water
AsFlFFF calibration
Determination of molecular weight (M) in AsFlFFF requires a calibration. Five pullulan standards were used for calibration, and their nominal molecular weights are listed in Table 1. Figure 4 shows fractograms of the pullulan standards (left) and the calibration curves (right) obtained at F c/F out = 0.41. The calibration plot shows an excellent linearity with R 2 values of 0.999. This calibration plot can be used to determine molecular weights of unknown samples, as explained in the “Theory” section. The molecular weights obtained here are not absolute, as they are determined from the calibration plot prepared with pullulan, which is not of the same chemical structure as the sample. When the calibration plot shown in Fig. 4 was used to determine the molecular weights of the pullulans, the measured M values are within the relative error of 4% or less (see Table 2).
AsFlFFF fractograms of pullulan standard (left) and the calibration curve (right). Experimental conditions are the same as those in Fig. 2 except F c/F out = 0.41 and the carrier liquid was pure water
The calibration plot changes when the experimental conditions change. In this study, two different flow rate conditions were used for analysis of the biosurfactant, F c/F out=0.41 and 2.36. Table 2 also shows the measured molecular weights of the pullulans using the calibration plot prepared at F c/F out = 2.36, which again showed an excellent linearity and the measured M values within the relative error of about 5 % or less. The R 2 values, intercept, and the slope of the calibration plot obtained at two different conditions are also shown in Table 2.
Characterization of biosurfactant
Figure 5 shows AsFlFFF fractograms of the biosurfactants extracted with fn-hexane (A) and a 2:1 (v/v) mixture of chloroform/MeOH (B). The hydrodynamic diameters (d H) and molecular weights (M) were determined for the fractograms shown in Fig. 6 using the pullulan-based calibration, and the results are summarized in Table 3. Accurate determination of d H and M requires the use of the calibration standards having the same chemical composition as the sample. In this work, the pullulan standards were used because the standards having the same chemical composition as the surfactant were not available. Thus the d H and M data shown in Fig. 6 and in Table 3 are not accurate: rather, they are relative. No particular trends in either d H or M against storage time were observed in Fig. 6. This finding is in accordance with a preliminary finding that the surface tension of the biosurfactant was maintained at about 25 dyne/cm for about 11 days, after which the surface tension slowly started to increase [26].
AsFlFFF fractograms of biosurfactant extracted with n-hexane (a) and a 2:1 (v/v) mixture of chloroform/MeOH (b). Experimental conditions are the same as those in Fig. 2 except F c/F out=0.46 and 2.53 for a and b, respectively. The carrier liquid was pure water
As shown in Fig. 6, d H and M are not constant over time, rather they fluctuate. All AsFlFFF analyses were repeated at least twice in this study (usually in a row), and the short-term reproducibility of AsFlFFF in determination of d H and M was within the relative error of less than about 5%. The exact cause of the fluctuation in measured d H and M is not clear at this point. The molecular conformation of the biosurfactant may change with the extracting solvent. An accurate answer to this requires further study. On average, d H and M of the biosurfactant were 48.7 nm and 2.27 × 105 Da when extracted with n-hexane, and 39.1 nm and 1.13 × 105 Da when extracted with a 2:1 (v/v) mixture of chloroform/MeOH, respectively. Accurate explanation for these differences and also for the fluctuation in the d H and M data requires further detailed study. In general, the peak areas of the biosurfactant extracted with a 2:1 (v/v) mixture of chloroform/MeOH were approximately three times larger than those of the biosurfactant extracted with n-hexane. This indicates the mixed solvent system is more efficient than n-hexane for extraction of the biosurfactant, which agrees with a previous study [29].
Conclusions
Results show that AsFlFFF is potentially useful for analysis of biosurfactants. The method provides a separation (and thus purification) of the biosurfactant from the culture media, and also information on hydrodynamic diameter and molecular weight. It is noted that AsFlFFF analysis of a biosurfactant requires careful examination on various experimental conditions including the carrier composition to prevent the interaction between the sample and the AsFlFFF channel membrane, which often causes distortion of the elution profile. Thus a care must be taken when AsFlFFF is used with a concentration-sensitive detector only (such as UV/VIS or RI). A combination of AsFlFFF with a molecular-weight-sensitive detector (such as a light scattering detector or differential viscometer) may yield more accurate data in molecular weight, size, and the molecular conformation. One of the merits of AsFlFFF is that it is an elution technique, allowing collection of slices (fractions) of the elution profile. More study is planned for spectroscopic analysis of the fractions, and for further optimization of AsFlFFF.
References
International Tanker Owners Pollution Federation (ITOPF) 2004 <http://www.itopf.com/stats.html> (accessed 20 Jan 2005)
Iqbal S, Khalid ZM, Malik (1995) Lett Appl Micorbiol 21:176–179
Kingston PF (2002) Spill Sci/Technol Bull 7:53–61
Deshpande S, Shiau BJ, Wade D, Sabatini DA, Harwell JH (1999) Water Res 33:351–360
Doong RA, Lei WG (2003) J Hazardous Materials 96:15–27
Lang S, Philp JC (1998) Antonie van Leeuwenhoek 74:59–70
Haferburg D, Hommel R, Claus R, Kleber HP (1986) Adv Biochem Engin/Biotech 33:53–93
Parkinson M (1985) Biotech Advs 3:65–68
Wagner DB, Furnier GR, Saghai-Maroof MA, Williams SM, Dancik BP, Allard RW (1987) Proc Natl Acad Sci USA 84:2097–2100
Zajic JE, Panchal CJ (1976) CRC Crit Rev Microbiol 5:39–66
Zajic JE, Seffens W (1984) CRC Crit Rev Biotechnol 1:87–107
Mulligan CN, Yong RN, Gibbs BF (2001) Eng Geol 60:371–380
Fiechter A (1992) Trends Biotechnol 10:208–217
Zajic JE, Guignard H, Gerson DF (1977) Biotechnol Bioeng 19:1303–1320
Ishigami Y, Gama Y, Hagahora H, Yamaguchi M, Nakahara H, Kamata T (1987) Chem Lett 5:763–766
Adolphi U, Kulicke WM (1997) Polymer 38:1513–1519
Giddings JC, Benincasa MA (1992) J Liq Chromatogr 15:1729–1747
Kirkland JJ, Dilks CH Jr, Rementer SW (1992) Anal Chem 64:1295–1303
Thielking H, Roessner D, Kulicke WM (1995) Anal Chem 67:3229–3233
Thielking H, Kulicke WM (1998) J Microcol Sep 10:51–56
Litzen A, Wahlund KG (1989) J Chromatogr A 1989 476:413–421
Moon MH, Giddings JC (1993) J Pharm Biomed Anal 11:911–920
Wahlund KG, Giddings JC (1987) Anal Chem 59:1332–1339
Litzen A, Wahlund KG (1991) Anal Chem 63:1001–1007
Beckett R, Jue Z, Giddings JC (1987) Environ Sci Technol 21:289–295
Shim SH (2006) MSc thesis. Hannam University, Korea
Litzen A (1993) Anal Chem 65:461–470
Moon MH (1995) Bull Kor Chem Soc 16:613–619
Kuyukina MS, Ivshuna IB, Philp JC, Christofi N, Dunbar SA, Ritchkova MI (2001) J Microbiol Methods 46:149–156
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (R05-2004-000-12516-0). Some of authors were financially supported by the 2nd stage BK21 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, S.K., Shim, S.H., Park, K.R. et al. Purification and characterization of a biosurfactant produced by Pseudomonas sp. G11 by asymmetrical flow field-flow fractionation (AsFlFFF). Anal Bioanal Chem 386, 2027–2033 (2006). https://doi.org/10.1007/s00216-006-0823-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0823-5