Abstract
A non-ionic surfactant, polyoxyethylene 10 lauryl ether (POLE), was used for the microwave-assisted extraction (MAE) of priority phenolic compounds from soil samples. A central composite design was applied to optimize the extraction parameters, namely, time and power. Under the optimized conditions, the method was applied to different soil samples in order to analyze the influence of soil characteristics on the phenol extraction. Results demonstrated that most of these compounds can be recovered from the soils investigated in good yields (higher than 80%). The standard deviation is lower than 9% (n = 6) for most analytes. Validation of the method by analyzing a reference soil sample containing eight phenols and a comparison with Soxhlet extraction are also reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds are prevalent in environmental waters and soils due to their widespread use in industrial processes [1]. These compounds are generated in the production of plastic, dyes, drugs, pesticides, antioxidants, and paper, and in the petrochemical industry [2]. Pentachlorophenol (PCP) is used as a wood preservative [3]. Furthermore, common pesticides, such as lindano and hexachlorobenzene, can be metabolized to PCP by plants, animals, and microorganisms. Phenol is generated from lignin degradation in paper production [4] and chlorophenols can be generated from phenols as the result of chlorinating drinking water. Nitrophenols are formed photochemically in the atmosphere from vehicle exhaust [5]. As a result of years of use, these compounds have contaminated soils, surface waters, and groundwaters, and affected fish populations.
Phenolic compounds can cause toxicity, persistence, and bioaccumulation effects in animal and vegetable organisms and may be dangerous for human health. Phenols have been included in the US Environmental Protection Agency (EPA) list of priority pollutants due to their toxicity [6]. Furthermore, the European Union (EU) has classified several phenols as priority contaminants [7].
Determination of phenols from contaminated soil is required to evaluate the extent of pollution and to apply the best soil remediation technology. The extraction step has so far been performed by following the official methods issued by national and international environmental protection agencies, such as the EPA 3500 B and International Organization for Standardization (ISO) TC 190/SC3/WG6 methods. These require the use of relatively large volumes of organic solvents and there is therefore great concern regarding their negative environmental effects and their disposal. Moreover, the procedures involved in the extraction of phenols from soil samples (e.g., by Soxhlet extraction) are usually lengthy, non-selective, and entail a great deal of sample handling, which increases the risk of errors [8, 9].
Recent concerns about the hazardous nature and environmental dangers of organic solvents applied in environmental sample preparations have led to the development of several extraction techniques that are free of organic solvents or only use low volumes of these solvents, such as solid-phase microextraction (SPME), supercritical fluid extraction (SFE), subcritical water extraction (SBWE), and microwave-assisted extraction (MAE) [10–14]. The last of these, MAE, has been successfully applied to the extraction of organic compounds from soil, sediments, plants, and animal tissues [15–18], and needs less organic solvent and a shorter extraction time than traditional extraction methods. In general, the compounds can be extracted more selectively and quicker with similar or better recoveries in comparison with conventional extraction processes [17].
However, there are several disadvantages to using organic solvent for MAE. First, most organic solvents may be dangerous to the operators and may result in environmental pollution due to waste solvent disposal. Second, the organic solvent should generally be capable of absorbing the microwave energy. In some cases, a material must be added to absorb the energy and transfer it to the sample. This could mostly be achieved by adding water to the sample matrix [19]. Moreover, organic solvent at a relatively high temperature and pressure may corrode the equipment [20].
The use of micellar system to substitute the organic solvents as extractant in MAE solves several problems caused by these solvents. Therefore, MAE with micellar medium, MAME, seems to be a viable alternative to other extraction techniques [21]. The main advantages are a shorter extraction time, a reduction in the amount of sample required for the analysis, higher sample throughput, less cost, and great safety, since it does not require the use of hazardous organic solvents.
The aim of this study was to apply the MAME process, with the non-ionic surfactant polyoxyethylene 10 lauryl ether (POLE), to the extraction of phenolic compounds from soil samples. The performance and application of this method in soils is important because of the difficulty in extracting the analytes from such complex matrices. We studied the extraction of phenols from various types of soils with a view to analyzing the influence of soil organic matter and pH on the desorption of phenols. Fifteen phenolic compounds, including the priority, and three different types of soil were studied.
Experimental
Reagents
Phenolic compounds were obtained from Sigma-Aldrich (Madrid, Spain) and prepared by dissolving appropriate amounts of the commercial products in methanol to obtain a concentration of 200 μg L–1. Working solutions were prepared by further diluting these concentrations. The phenolic compounds are listed in Table 1 (numbers and abbreviations identify the compounds in figures). The certified reference soil with phenolic compounds was obtained from Resource Technology Corporation (provided by LGC Promochem, Barcelona, Spain). The non-ionic surfactant used in this study, polyoxyethylene 10 lauryl ether (POLE), was obtained from Sigma-Aldrich and prepared in deionized water. HPLC-grade methanol was obtained from Panreac Química S.A. (Barcelona, Spain).
All the solvents and analytes were filtered through a 0.45-μm nylon membrane filter, and ultra-high-quality water obtained from a water purification system was used throughout.
Apparatus
The chromatograph system consists of two Waters 510 pumps fitted with a Waters Rheodyne 7725 I injector, with a 20-μL sample loop and a Waters 996 photodiode array detector. The system and the data management were controlled by Millennium software from Waters (Waters Cromatografía S.A. Barcelona, Spain). The stationary-phase column was a Waters Nova-Pack C18, 3.9 × 150 mm, 4-μm particle diameter. The analytical column and the mobile phase reservoir were water-jacketed and thermostated at 25±1°C with a circulating bath.
The microwave oven used in this study was a Multiwave (Anton Paar, Graz, Austria) with a 6 EVAP rotor and 6 MF100 vessels (Anton Paar, Graz, Austria).
Procedures
Soil characteristics
The soil samples were air-dried at room temperature for more than 2 weeks and sifted to a particle size of less than 0.3 mm. To determine the soil pH, 5 g of each soil was mixed with 20 mL of distilled water; the slurry was stirred and then allowed to separate before the surpernatant pH was measured potentiometrically. The organic matter content was determined by the Sauerlandt method (organic matter oxidation by potasic dichromate and sulfuric acid).
In order to study the influence of soil pH and organic matter on the extraction of phenols, three different soil samples were chosen from two gardens and a pine forest. Characteristics of the soils are given in Table 2.
Preparation of spiked soils
Phenols-free soil samples were collected from different locations on Gran Canaria (Canary Islands). After sieving, soil fractions with particle size under 0.3 mm were taken. These samples were air-dried at room temperature. These sediment samples were spiked as follows: to optimize the method, 2 g of each soil was spiked with a volume of a chlorophenol solution to obtain a concentration of each analyte of 2 μg g−1. The same amount of soil was spiked with a solution of fifteen phenolic derivatives to obtain a final concentration of 4 μg g−1 for analytical applications. The samples were then stored in the dark at room temperature for 24 h before analysis.
The samples identified as “aged” samples were spiked with the mixture of phenolic derivatives to obtain a concentration of each analyte of 4 μg g−1 . The samples were stored in a refrigerator at 4°C for 1, 2, 4, and 8 weeks. It was assumed that the phenols were uniformly distributed in the sample and that, as the sediment still retained residual moisture throughout the storage period, any analyte–matrix interactions would have occurred over the weathering period and to a similar extent to those in real contaminated sediments with similar properties.
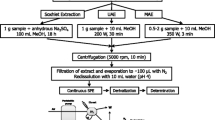
Microwave-assisted micellar extraction
Once the sediment sample was transferred to the vessel, 8 mL of the surfactant solution with a concentration of 5% (v/v) was added and the sediment was subjected to the MAME process. The vessels were then allowed to cool down to room temperature, 10–15 min before being opened. The extracted solution was filtered with a 0.45-μm syringe-driven filter and transferred to a glass tube before injection.
Liquid chromatography analysis with UV detection
Analysis of the extracted samples was carried out by using high-performance liquid chromatography with UV detection. The separation and determination of the compounds under study were performed by injecting 20-μL aliquots obtained into the liquid chromatograph, and the absorbance for each analyte, corresponding to the wavelength maxima, was then measured. The retention time and the wavelength for each compound are listed in Table 1. The eluent used for the separation of the mixture of 15 phenols was water (with 1% acetic acid)/methanol (70:30) for 16 min (isocratic), up to 100% methanol for 24 min. The eluent used for the separation of the eight chlorophenol mixture was methanol/water (40:60) up to 100% methanol for 20 min. In both instances, the flow-rate was 1 mL min−1 . The range of calibration curve concentration was between 100–2,000 and 100–1,200 μg L−1, respectively. These curves were obtained by duplicate injection of the solution containing 2% (v/v) polyoxyethylene 10 lauryl ether, 5% (v/v) methanol, and the corresponding analyte concentration. A linear relationship was obtained between peak areas and the analyte concentrations, with high correlation coefficients (0.995).
Soxhlet extraction
The fresh and aged samples were Soxhlet extracted over 16 h using 70 mL of acetone/hexane (1:1). After the extraction step, the extract was evaporated to dryness using a rotary evaporator, dissolved in methanol, and analyzed by HPLC.
Statistical analysis
All statistical tests (ANOVA, experimental design) were performed by using Statgraphics plus software, Version 4.0 (Manugistic, Rockville, MD, USA).
Results and discussion
Optimization of MAME methodology
Parameters that have a major influence on the MAME process are extractant volume and irradiation time and power. Optimization of MAE conditions has been reported in several applications and many studies have used factorial, central composite, and orthogonal array designs to find the optimal conditions [15, 22–26].
Optimization experiments were performed using soil samples with an organic matter content of 4.8% and a pH of 8.3 (Tafira soil) and a mixture of eight chlorophenols. A concentration of 5% (v/v) of polyoxyethylene 10 lauryl ether was used to achieve the optimization process, as a higher surfactant concentration did not improve the recoveries.
The extractant volume must be sufficient to ensure that the entire sample is immersed; this parameter was optimized previously. We studied the influence of the volume of surfactant solution on the extraction efficiency of phenols by varying the volume of surfactant and keeping the sample mass constant: 4, 8, and 12 mL of surfactant 5% (v/v) and 2 g of sample were used. The results obtained, Table 3, show that 4 mL is not sufficient to wet the sample, but use of 8 mL allows one to wet the samples satisfactorily. When the volume of surfactant is higher, the temperature in the vessels also increased and the data obtained for phenol and 2-chlorophenol, using 12 mL surfactant, show that some solutes seem to degrade at high temperatures. We therefore chose 8 mL for the MAME optimization.
Microwave irradiation conditions
The time and power of irradiation are parameters that are interrelated, so their influence on the extraction efficiency was investigated by applying a statistical approach using a factorial design. This reduces the development time and provides less ambiguous data. We used a central composite design, 22 + star with three central points. The experimental design, involving 11 runs, was used as an approach to the response surface of the microwave extraction process. The experimental design parameters and the response values in the screening design are shown in Table 4. Other variables involved in the extraction process were kept constant, namely, surfactant concentration (5% v/v), surfactant volume (8 mL), and soil sample amount (2 g). The concentration of chlorophenols spiked was also kept constant at 2 μg g−1 .
The data analysis of the results given in Table 4 was performed using a regression analysis and the response surface (Y) was taken as a function of the considered variables (xI) using polynomials. The general polynomial function is
where Y is the recovery, x1 and x2 the variables considered in the optimization process, and βI are the parameters to be calculated.
Figure 1a shows the response surface for 4-CMC. The amount of analyte extracted increases with the microwave power at short time intervals. This behavior is similar for the other target compounds, except for PH (Fig. 1b). The recoveries for this analyte increase with the power and time. However, these conditions increase the temperature inside the vessels and degradation of more substituted analytes can occur, increasing the amount of phenol obtained. Therefore, a power level of 700 W and a time interval of 3 min were chosen for subsequence studies.
Influence of other parameters
Once the microwave irradiation parameters were optimized, we studied the effect of the solution pH on the recovery using the optimal time and power. When working with soil samples, the extraction process might also be influenced by the pH of the extracting solution as it can alter the ionic form of the analytes under study. To investigate the effect of this parameter on the process, samples containing 2 g of soil in an acid and basic medium, achieved by adding HCl or NaOH, were subjected to MAME using a surfactant concentration of 5% (v/v) and the aforementioned power and time. The results obtained showed that there is no significant variation on the recoveries for most analytes under study. However, the more polar compounds, such as PH and 2-CP, show a slight increase in the recoveries when the pH was increased. This may be due to a basification of the extract producing phenolate ions, which are non-volatile [27, 28] and losses of the most volatile compounds are thus avoided. Despite this, we decided not to modify the pH solution as no significant changes were obtained.
In order to determine the influence of analyte concentration on the extraction process, samples spiked with the mixture of chlorophenols in the concentration range of 2–8 μg g−1 were extracted using MAME. The values obtained demonstrate that this parameter has no influence on the extraction process for this range of concentrations.
Analytical applications
Once the MAME conditions were optimized, the same soil used in the optimization procedure (Tafira soil) was spiked with a mixture of 15 phenolic derivatives to a final concentration of 4 μg g−1. This new mixture contained the chlorophenols and 11 phenolic derivatives included in the EPA list of priority pollutants. These compounds behave differently to the chlorophenols, and therefore different interactions between matrix and analytes could occur. The values obtained show that the previously established conditions are adequate for most of the analytes studied, except for methylphenols, for which the recoveries are lower (Table 5). Figure 2 shows the chromatogram obtained for an extract of 15 phenolic derivatives after MAME procedure.
Chromatogram of an extract of a spiked sample soil after MAME procedure. Chromatographic conditions as described in the text. Numbers correspond to those listed in Table 1
To determine the method accuracy and precision, six soil samples containing the 15 phenolic compounds were extracted simultaneously for 3 min and 700 W (optimal conditions), 24 h after being spiked. The average recoveries and RSD for the 15 phenolic derivatives are shown in Table 5. As can be seen, the recovery values for most of the analytes extracted are higher than 80%. The extraction yields for alkylphenols, PC, 2,4-DMP, and 2,4,6-TMP, were very low, probably due to their interacting more strongly with the soil than is the case with the other phenols [29]. The other compounds present in the mixture with methyl groups behave more similarly to chlorophenols than to alkylphenols. The behavior of nitrophenols is similar to that of chlorophenols. The corresponding values of RSD are under 9% for 13 of the 15 compounds studied.
Comparison of MAME with Soxhlet extraction
Soxhlet extractions were performed for comparison purposes. Figure 3 compares the recovery values achieved with MAME and those obtained with Soxhlet extraction for Tafira soil. The results obtained with MAME are in line with the values obtained using Soxhlet extraction, except for 2,4,6-TMP and 2,4-DCP. The compounds eluted in the first 20 min cannot be quantified due to the high amount of interferences in this part of the chromatogram.
Influence of matrix nature
In order to study the influence of soil characteristics on the extraction, we applied the MAME procedure to soils with different levels of organic matter, pH, and texture. The effect of organic matter on phenols sorption was studied by using soil with the same pH and different content of organic matter (Tafira, 4.8%; and San Roque, 12.5%). Table 6 shows the recoveries obtained for these soil samples tested after 24 h of conditioning. The results obtained with San Roque soil are better than those achieved using Tafira soil. The main difference is the good recoveries of alkylphenols in the soil with a higher content of organic matter. However, the mononitrophenols have recovery values slightly lower than 80% in this type of soil.
The pH of soil can affect the sorption of phenols because the organic matter (in particular, the humic acids) behaves rather differently depending on this parameter, altering its sorbent capacity. The influence of soil pH on phenol extraction was studied using an acid soil (pine forest, pH 5.9) and an alkaline one (Tafira, pH 8.3). These soils have a similar organic matter content of 4–5%. The results obtained (Table 6) indicate that the recoveries of 13 phenols are higher than 80% in the acid soil, whereas the values obtained in the alkaline soil are slightly lower. Furthermore, the alkylphenols are extracted satisfactorily, but their recoveries are lower than those obtained in the samples that are high in organic matter.
The texture of a soil is extremely important in the sorption process. If a soil is mostly made up of clay and organic matter, a significant amount of sorption will take place. Clay, above all when intermixed with organic particles, is by far the most adsorbent of the three main soil textures (clay, silt, and sand) due to its small particle size, high surface area, and high surface charge. Although the soils tested in this study are sandy ones, the Tafira soil has a higher amount of particles with a smaller size. Alkylphenols interacted more strongly with this kind of soil, which is probably due to this fact. It could explain the poor recoveries of these types of compounds in this soil. The results obtained indicate that for these soil types, with a high sand content, the soil texture is the parameter that has greater influence on the sorption process than the soil pH or organic matter content.
On the other hand, taking into account that the surfactants have a high capacity to extract humic and fulvic acids and that the phenols can be adsorbed into organic matter, this fact may explain the high recoveries of the compounds extracted from San Roque soil, with a higher organic matter content.
Influence of aging time
Decreasing recoveries resulting from aging of matrices is a well-known phenomenon [30]. The analytes present in recent soils samples are more easily extracted than those that have had a longer contact time. This can be explained according to whether the analytes are incorporated by adsorption (short periods) or by sequestration (longer periods) [31]. The former phenomenon occurs at the early stages of sorption, where hydrogen bonding and Van der Waals forces prevail. On the other hand, sequestration involves sorption at remote microsites within the soil matrix [32].
In order to study the aging effects, we applied the MAME procedure to the three soils for different time periods after conditioning. The soil samples were spiked with the phenol mixture and stored at 4°C in the dark before their extraction. Figure 4a–c shows the recoveries obtained for the different groups of phenolic compounds in San Roque soil with the time aging. It can be seen that the recoveries decrease slightly during the two first weeks for the nitro and chlorophenols (Fig. 4a, b), but the amount of analyte extracted remains practically constant for these last compounds after this time. In the same figure, it can be observed that the recoveries of 2-nitrophenol, 4-nitrophenol, and 2-chlorophenol increase slightly with time. This can be attribute to the fact that the more substituted analytes suffer microbial degradation and chemical transformation, such as losses of atoms of chloro or nitro groups, so they are converted into more simple compounds.
In Fig. 4c, it can be observed that the behavior of alkylphenols is quite different. In general, the recovery values decrease sharply with aging time up to 1 month. From then onwards, para-cresol increases its recovery as the aging time progresses, but we suspect that, as is the case of monochloro and mononitrophenols, this may be due to the degradation of other compounds with a greater number of substituents. As can be seen in the same figure, 4-C-3,5-DMP behaves in a more similar way to chlorophenols than to alkylphenols. The behavior of the analytes over time in the others soils tested is similar to that obtained for San Roque soil.
Comparison of MAME with Soxhlet extraction
To compare the extraction of aged samples, Soxhlet extractions were performed using 2 g of Tafira soil with 8 weeks of conditioning. Figure 5 shows the recoveries obtained with both the MAME and Soxhlet methods. It can be observed that, in general, the values obtained are quite similar, except for 2,4-DMP. Consequently, the MAME procedure is a viable alternative for the extraction of phenols from both fresh and aged soil samples.
Validation with a certified soil
Recoveries obtained with spiked compounds may not be representative of those obtained with native compounds. Spiked analytes are generally lightly coated on the surface of the matrix, whereas native ones can be strongly adsorbed inside the porous matrix. This can be explained by the diffusional and the kinetic limitations of the sorption process, and the several interactions, which may have been simultaneously established between native analyte and the matrix [33–35]. This is the reason why it is necessary to validate the extraction procedure with certified reference matrices. For this purpose, we used a certified reference material with phenolic derivatives. The soil, sandy loam with pH 6.96, was contaminated with phenols from a wood-treating site in the Rocky Mountain region of the United States. The proposed extraction procedure was applied to 2 g of this soil using the optimal conditions. To check the presence of interfering species, the standard addition method was adopted to analyze the extracts. Table 7 reports the phenols present in the certified soil and gives the certificate values, the confidence intervals, and the amounts of each analyte that we obtained using the MAME procedure. The recoveries obtained, falling within the certified range for all compounds analyzed, indicate that the proposed extraction procedure is suitable.
Conclusions
Nowadays, efforts are being directed towards the development of analytical techniques which rapidly achieve an accurate measurement of organic pollutants in environmental samples. According to the results obtained, the applicability of the proposed method provides a viable alternative to other extraction techniques. The main advantages of MAME are shorter extraction times, higher sample throughput, and organic-free solvents, which result in reduced costs and environmental toxicity.
References
Moore JW, Ramamoorthy S (1984) Phenols in organic chemicals in nature waters. Applied monitoring and impact assessment. Springer, Berlin Heidelberg New York
Martínez D, Pocurull E, Marcé RM, Borrull F, Calull M (1996) J Chromatogr A 734:367–373
Kontsas H, Rosenberg C, Pfäffli P, Jäppinen P (1995) Analyst 120:1745–1749
Mckague AB (1981) J Chromatogr A 208:287–293
Tremp J, Mattrel P, Fingler S (1993) Water Air Soil Pollut 68:113–123
Environmental Protection Agency (1984) EPA method 604 phenols in Federal Register, Part VIII, 40 cFR Part 136, p 58
Drinking Water Directive 80/778/EEC (1980) Commission of the European Communities, Brussels
DiCorcia (1973) J Chromatogr 80:69–76
Hoshika Y (1977) J Chromatogr 144:181–187
Prosen H, Zupancic-Kralj L (1999) Trends Anal Chem 18:272–282
Kawata K, Ibaraki T, Tanabe A, Yagoh H, Shinoda A, Suzuki H, Yasuhara A (2001) J Chromatogr A 911:75–83
Li B, Yang Y, Eaton CD, He P, Jones AD (2000) J Chromatogr A 873:175–184
Buchhloz KD, Pawliszyn J (1994) Anal Chem 66:160–167
Lopez-Avila V, Young R (1994) Anal Chem 66:1097–1106
Llompart MP, Lorenzo RA, Cela R, Jocelyn Paré JR (1997) Analyst 122:133–137
Lee MR, Yeh YC, Hsiang WS, Hwang BH (1998) J Chromatogr A 806:317–324
Zlotorzynski A (1995) Rev Anal Chem 25:43–76
Ho WH, Hsieh SJ (2001) Anal Chim Acta 428:111–120
Jocelyn Paré JR, Belanger MR, Stafford SS (1994) Trends Anal Chem 13:176–184
Xiong GH, Liang JM, Zou SC, Zhang ZX (1998) Anal Chim Acta 371:97–103
Bianco Prevot A, Gulmini M, Zelano V, Pramauro E (2001) Anal Chem 73:3790–3795
Eguizabal A Zuloaga O, Etxevarría N, Fernández LA, Madariaga JM (1998) Analyst 123:1679–1684
Pino V, Ayala JH, Afonso AM, González V (2001) Int J Environ Anal Chem 81:281–294
Pino V, Ayala JH, Afonso AM, González V (2000) J Chromatogr A 869:515–522
Padrón Sanz C, Eiguren Fernández A, Sosa Ferrera Z, Santana Rodríguez JJ (2002) J AOAC Int 85:44–49
Mahugo Santana C, Sosa Ferrera Z, Santana Rodríguez JJ (2004) Anal Chim Acta 524:133–139
Galcerán MT, Jáuregui O (1995) Anal Chim Acta 304:75–84
DiCorcia A, Marchese S, Samperi R (1994) J AOAC Int 77:446–453
Crespín MA, Gallego M, Varcárcel M (2000) J Chromatogr A 897:279–293
Hacothorne SB, Björklund E, Bøwadt S, Mathiasson L (1999) Environ Sci Technol 33:3152–3159
Dec J, Bollag J (1997) Soil Sci 162:858–874
Kopinke F, Pörschmann J, Stottmeister U (1995) Environ Sci Technol 29:941–950
Burford MD, Hawthorne SB, Miller DJ (1993) Anal Chem 65:1497–1505
Richter BE, Jones BA, Ezzel JL, Porte NL, Avdanovic N, Pohl C (1996) Anal Chem 68:1033–1039
Dupeyron S, Dudermel PM, Counturier D (1997) Analusis 25:286–292
Acknowledgements
This study was funded by the Spanish Ministry of Science and Technology Project No. PPQ2002/04683.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahugo Santana, C., Sosa Ferrera, Z. & Santana Rodríguez, J.J. An environmentally friendly method for the extraction and determination of priority phenols in soils using microwave-assisted micellar extraction. Anal Bioanal Chem 382, 125–133 (2005). https://doi.org/10.1007/s00216-005-3167-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3167-7