Abstract
A multi-component method focussing on thorough sample preparation has been developed for simultaneous analysis of swine manure for three classes of antibiotic—tetracyclines, sulfonamides, and tylosin. Liquid manure was initially freeze-dried and homogenised by pulverization before extraction by pressurised liquid extraction. The extraction was performed at 75°C and 2,500 psig in three steps using two cycles with 0.2 mol L−1 citric acid buffer (pH 4.7) and one cycle with a mixture of 80% methanol with 0.2 mol L−1 citric acid (pH 3). After liquid–liquid extraction with heptane to remove lipids, the pH of the manure was adjusted to 3 with formic acid and the sample was vacuum-filtered through 0.6 μm glass-fibre filters. Finally the samples were pre-concentrated by tandem SPE (SAX-HLB). Recoveries were determined for manure samples spiked at three concentrations (50–5,000 μg kg−1 dry matter); quantification was achieved by matrix-matched calibration. Recoveries were >70% except for oxytetracycline (42–54%), sulfadiazine (59–73%), and tylosin (9–35%) and did not vary with concentration or from day-to-day. Limits of quantification (LOQ) for all compounds, determined as a signal-to-noise ratio of 10, were in the range 10–100 μg kg−1 dry matter. The suitability of the method was assessed by analysis of swine manure samples from six different pig-production sites, e.g. finishing pigs, sows, or mixed production. Residues of antibiotics were detected in all samples. The largest amounts were found for tetracyclines (up to 30 mg kg−1 dry matter for the sum of CTC and ECTC). Sulfonamides were detected at concentrations up to 2 mg kg−1 dry matter (SDZ); tylosin was not detected in any samples.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotic residues from veterinary use can enter the environment when liquid manure is spread on agricultural fields as organic fertilizer [1, 2]. Sulfonamides have been detected in wet swine manure at concentrations up to 20 mg kg−1 in one study [3]; another study showed concentrations to be below 0.5 mg L−1 [4]. Tetracycline concentrations up to 41 mg kg−1 have been determined in liquid swine manure [5] and approximately 1 mg L−1 was found in swine lagoon samples [4]; amounts of macrolides were somewhat lower, e.g. 0.11 mg kg−1 for tylosin [6].
The objective of the work discussed in this paper was to develop a multi-component method for analysis of swine manure for tylosin, sulfonamides, and tetracyclines used in Danish pig farming, i.e. tylosin A (TYL A), sulfadiazine (SDZ), sulfamethazine (SMZ), sulfadoxine (SDX), tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), doxycycline (DOXY), and the epimerised tetracyclines epi-tetracycline (ETC), epi-oxytetracycline (EOTC), and epi-chlortetracycline (ECTC). These compounds have a wide range of physicochemical properties, as is shown in Table 1. Because liquid swine manure is a complex and heterogeneous matrix consisting of faeces and urine from the farm animals, feed residues, straw, and wash waters, several analyte–matrix interactions and possible impurities must be considered during method development. The dry matter content of manure is usually 1–10%; it has been shown to consist largely of organic matter (62–73%) and nutrients (N and P) [7] but may also contain substantial amounts of proteins and lipids (approximately 10 and 2%, respectively) [8]. The organic matter pool of swine manure has been examined in detail and the humic and fulvic acids contents were 0.70–2.47% and 0.50–1.00%, respectively [7]. These substances are characterised by low molecular weight and high content of functional groups such as carboxylic acids and phenols and, hence, contain numerous sites for binding of the tetracyclines, sulfonamides, and tylosin by hydrogen bonding and ion-exchange. Tetracyclines also interact strongly with divalent metals ions by complexation (e.g. up to 2.7% Mg and 6.3% Ca in swine manure [9]), as shown in several studies [10, 11]. The higher molecular-weight organic matter constituents (humus) are more hydrophobic substances, for example straw and feed, and sorption may occur as a result of hydrophobic interactions, i.e. van der Waals forces. The sorption coefficient (K d) for OTC in swine manure was determined to 78–83 L kg−1 [12]; in other studies K d values for TYL were 36–56 L kg−1 [12] and 39–108 L kg−1 [13]. As far as we are aware sorption of sulfonamides by manure has not been investigated, but K d for soil–manure (50:1) mixtures was 0.59–1.18 L kg−1 [14].
The objective of method development in this study was to obtain homogeneous and representative samples by sample pretreatment and extraction procedures that encompassed the breaking of numerous analyte–matrix interactions and to remove impurities differing strongly in physicochemical properties, for example hydrophilic humic acids and hydrophobic lipids.
Finally, the suitability of the developed method was assessed by analysis of manure samples from six different swine-production farms, varying in production type, i.e. finishing pigs, sows, or mixed production. The antibiotic content was expected to vary according to the general health of the respective farm animals.
Experimental
Materials and chemicals
Oxytetracycline hydrochloride (OTC) (>95%), chlortetracycline hydrochloride (CTC) (79%), tetracycline hydrochloride (TC) (>95%), tylosin tartrate (TYL) (89.8%), oleandomycin triacetate (OLEA), sulfamethazine sodium salt (SMZ) (>99%), sulfadoxin (SDX), and sulfadiazine sodium salt (SDZ) (>99.0%) were all from Sigma-Aldrich, Germany. Doxycycline hydrochloride (DOXY) (>99%) was obtained from Calbiochem (La Jolla, USA) and epi-oxytetracycline (EOTC), epi-chlortetracycline (ECTC), and epi-tetracycline (ETC) from Acros Chemicals. Methanol and n-heptane of HPLC grade was obtained from Lab-Scan (Dublin, Ireland) and formic acid (GR for analysis, 98–100%), citric acid monohydrate, and sodium hydroxide pellets (GR for analysis) from Merck (Darmstadt, Germany). Ottawa sand standard, general-purpose grade (S/0365/63), was purchased from Fisher Scientific (Leicestershire, UK). Water was obtained from a Millipore purification system equipped with an Ultrapure organex cartridge (Billerica, MA, USA).
Solid-phase extraction (SPE) was performed with SAX-cartridges (strong anion exchange, 500 mg sorbent, 6 mL cartridge) purchased from Isolute (IST, Mid Glamorgan, UK) and HLB-cartridges (poly(divinylbenzene-co-N-pyrrolidone), 200 mg sorbent, 6 mL cartridge) purchased from Waters Oasis (MA, USA).
Manure sampling and pretreatment
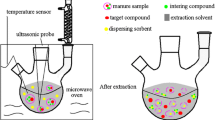
Completely mixed manures were sampled from manure storage tanks from six different swine-producing farms in May 2005, immediately before application of the manure to agricultural fields. The samples were immediately frozen to reduce degradation of the antibiotics during transport. On arrival at the laboratory the samples were freeze-dried to complete dryness in fractions of 100–500 mL and subsequently homogenised by pulverization in a coffee mill. A summary of the procedure used for sample pretreatment and subsequent extraction and clean-up is illustrated in Fig. 1.
Selected physicochemical properties of the liquid manures are listed in Table 2. Total nitrogen and ammonium nitrogen were determined by the Kjeldahl method and ICP was used for determination of P, K, Mg, and Cu, all by a commercial laboratory (Steins Laboratories, Holstebro, Denmark). The authors determined dry matter, organic matter, and pH. Dry matter was determined as the weight-loss during freeze-drying, organic matter was determined by loss-on-ignition at 360°C as described elsewhere [15], and pH was measured directly in the liquid manure.
Pressurised-liquid extraction (PLE)
PLE was performed with an ASE 200 system equipped with a solvent selector, both from Dionex Sunnyvale, California, USA). Approximately 0.750 g freeze-dried manure sample was accurately weighed into 22 mL extraction cells (lined with glass microfibre filters from Whatman, Maidstone, UK) and mixed with 20 g Ottawa sand standard before extraction.
The extraction procedure was optimised with regard to extraction solvent, pressure, temperature and number of cycles. Optimum extraction conditions were found to be 2,500 psig and 75°C and a three-step extraction procedure using two different extraction buffers—0.2 mol L−1 aqueous citric acid buffer with pH adjusted to 4.7 with NaOH (two cycles) then 80% methanol with 20% 0.2 mol L−1 citric acid (pH 3.5) (one cycle). The program for each cycle of the PLE-procedure was: 5 min heat (no pre-heat), 5 min static, 50% flush volume, and 60-s purge.
Sample clean-up and pre-concentration
Liquid–liquid extraction with 10 mL heptane per 40 mL manure extracts (lipid removal) was performed end-over-end for 15 min and the heptane phase was discarded. The extracts were diluted to 500 mL with Millipore water, adjusted to pH 3 by addition of 1.5 mL formic acid, and then vacuum-filtered through GF-3 glass fibre filters (0.6 μm retention; Macherey–Nagel, Germany). Finally the samples were concentrated using SAX-HLB tandem SPE as described elsewhere [16, 17]. In brief, the SAX cartridge was placed on top of the HLB cartridge and both columns were conditioned with 3×1 mL methanol and 3×1 mL 0.04 mol L−1 citric acid buffer (pH 3). The samples were passed through both cartridges at approximately 5 mL min−1, washed with 5 mL Millipore water, and, finally, dried under vacuum for 15 min. The SAX cartridge was then discarded and the antibacterial agents were eluted from the HLB cartridge with 2 mL methanol. Before LC–MS–MS analysis the methanol extracts were diluted 1:1 with Millipore water, and oleandomycin (OLEA) was added, as instrument standard, at a constant concentration of 250 μg L−1 (100 μg L−1 during validation).
LC–ESI-MS–MS analysis
Analysis of the manure extracts was performed by LC–ESI-MS–MS with an Agilent Technologies (Palo Alto, CA, USA) 1100 series HPLC system equipped with a degasser, a cooled autosampler (4°C), and a cooled column oven (15°C) and coupled to a Sciex API 2000 triple quadrupole mass spectrometric detector with an electrospray source (ESI) (Applied Biosystems, Foster City, CA, USA). Collection and data treatment were performed with Analyst 1.4 Software (Applied Biosystems).
HPLC gradient separation of the antibacterial agents was achieved on Xterra MS-C18 guard and analytical columns (10 mm×2.1 mm and 100 mm×2.1 mm, respectively; particle size 3.5 μm). Mobile phases A and B consisted of 95% and 20% methanol, respectively, in Millipore water at pH 3 (adjusted with formic acid). The antibacterial agents were separated by use of a 25 min gradient running from 5 to 75% mobile phase A, then 15 min equilibration.
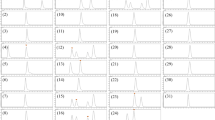
The mass spectrometer was operated in positive-ion mode and the antibacterial agents were detected using MRM in two periods with different settings (Table 3). Sulfonamides and tetracyclines were analysed together in one period (0–22 min) and tylosin and OLEA in a subsequent period (22–40 min). Chromatograms showing the total ion count (TIC) for all the antibiotics and the extracted ion-counts (XIC) for MRM analysis of the individual analytes are presented in Fig. 2.
LC–ESI-MS–MS total-ion count (TIC) chromatogram showing the separation of sulfonamides, tetracyclines, and tylosin included in this method, and corresponding extracted ion counts (XIC) for MRM detection of the individual analytes. The dotted vertical line at 22 min in the TIC chromatogram indicates separation of the detection method into the two periods
Validation of manure extraction procedure (PLE–SPE–LC–ESI-MS–MS)
The entire extraction and clean-up procedure was validated for recovery, limit of detection, and day-to-day variation using manure samples #1 and #2 in Table 2. Recoveries were determined for six replicate samples at three concentrations (50, 500, and 2,500 μg kg−1 dry matter (5,000 μg kg−1 for tetracyclines)). The liquid manure was fortified with the antibiotics before freeze-drying then subjected to the sample preparation procedure described above. Recoveries were calculated using an external calibration plot prepared for the sample matrix (matrix-matched calibration) to take into account matrix effects and background levels of the antibacterial agents. To determine the day-to-day variation this procedure was repeated after one week with three replicate samples at each concentration level. The limit of detection (LOD) and limit of quantification (LOQ) for the entire method were determined as signal-to-noise ratios (S/N) of 3 and 10, respectively. S/N values were calculated by correlation with the sample concentration determined by standard addition, by use of the software tool Analyst 1.4.
Application to farm samples
The applicability of the method to real samples was demonstrated by analysis of manure samples from six different farms (Table 2) representative of different types of swine production (finishing pigs, sows, and mixed production), production size, storage tank capacity, and general health status of the livestock.
Liquid manure (200 mL) from each farm was freeze-dried and pulverised. Triplicate samples of the freeze-dried material were prepared as already described. The amounts of antibiotics were quantified by standard addition. Each sample furnished 2 mL methanol extract; this was divided into six sub-samples of 250 μL and the remaining 500 μL was saved for potential further analysis. Each sub-sample was subsequently diluted 1:1 with aqueous standards to create a standard addition plot with six concentration levels (0–250 μg L−1). The instrument standard (OLEA) was also added with the aqueous standards.
Data treatment
In the following sections recoveries are expressed as mean values from analysis of three replicate samples unless otherwise indicated, and standard deviations are shown in parentheses as ±values. Throughout the manuscript significant differences between treatments are determined as P<0.05 calculated by one-way ANOVA at the 95% confidence level using GraphPad Prism, version 4.01.
Results and discussion
Sample pretreatment (stability and freeze-drying procedures)
Freeze-drying of large volumes of liquid manure samples and subsequent pulverisation resulted in homogeneous samples including the contents of liquid and solid phases of the manure. The procedure also facilitated sample handling, including the possibility for using PLE. Numerous other research groups have analysed antibiotics in manure, but the analysis was usually performed using smaller volumes (1–15 g wet weight) of liquid manure samples [3, 5, 18, 19], which may hamper the homogeneity of the samples. For comparison 200–500 mL liquid manure was freeze-dried per run in this study, resulting in approximately 10–20 g dried manure. Reproducibility of liquid samples may be improved by sample homogenisation, e.g. by blending, but handling is still complicated. Different approaches for sample pretreatment include filtration or centrifugation of liquid manure slurries and subsequent handling of the liquid phase only, i.e. by SPE clean-up [4, 20–22]; substantial amounts of antibiotics may, however, be removed with the solid phase.
Instability of the antibiotics was observed in the liquid manure samples before freeze-drying. Tylosin was particularly rapidly dissipated in the manure samples; it has been reported in several publications that this is because of degradation and strong sorption [12, 23–25]. Conservation of the liquid manure samples with three levels of sodium azide (0.5, 1.0 and 2.5% w/v) was therefore investigated. Addition of 2.5% NaAz significantly improved recoveries (P<0.05); mean recoveries were still as low as 11.5% for TYL, however (data not shown) so use of this strategy was discontinued. Inhibition of antibiotic degradation by immediate freezing was also investigated. Liquid manure samples were fortified with OTC, SDZ, and TYL and either frozen at −18°C immediately after fortification or left for 5, 20, 40, 90 or 210 min before freezing (data not shown). No significant degradation of OTC and SDZ was observed within the first 90 min after spiking the manure but recoveries of TYL declined from 58(±7.7)% for samples frozen immediately after spiking to 29(±6.2)% when samples were left for just 5 min before freezing. After 90 min only 5.2(±1.1)% was recovered. Subsequently, therefore, samples were frozen immediately after sampling.
Extraction solvent and procedure
The procedure for extraction of the antibiotics from the freeze-dried manure was optimised for SDZ, OTC, and TYL, unless otherwise indicated. A wide range of organic solvents were investigated for extraction, for example methanol, methanol–ammonium buffer, acetonitrile, dimethyl sulfoxide (DMSO), acetone, dichloromethane, ethyl acetate (EtAc), tetrahydrofuran (THF), iso-propanol, methyl t-butyl ether (MTBE), and hexane (data not shown). The efficiency of the solvents was usually in accordance with the solubility of the antibiotics [26] and only the more polar solvents (methanol, acetone, DMSO) proved promising as extraction solvents. This was somewhat surprising, because numerous methods successfully employ EtAc for extraction despite the low solubility of the antibiotics in this solvent [26]. For example, extraction of sulfonamides from manure has been performed using EtAc and sodium chloride and/or McIlvaine buffer [3, 18] and EtAc, EDTA, urea, and phosphate buffer have been used for macrolide extraction [19]. Hamscher et al. [5] isolated sulfonamides and tetracyclines from liquid manure by extraction with citric acid and EtAc. Because this method seemed promising, with recoveries near unity, we repeated the procedure in our laboratory; we achieved recoveries of only 38–61% for tetracyclines (CTC and OTC) and 70–79% for sulfonamides (SDZ and SMZ), however, for both concentrations tested (0.2 and 1.0 mg kg−1). We also observed heavy precipitation in the final manure extracts, possibly because of precipitation of lipids, so use of EtAc was not pursued further. Use of chelating agents is widespread in these methods and the approach has also traditionally been used for analysis of antibiotics residues in food products [27]. Extraction with aqueous buffers was therefore investigated, and citric acid, EDTA, phosphoric acid, and ammonium acetate buffers all resulted in satisfactory extraction. The optimum extraction solvent was found to be 0.2 mol L−1 aqueous citric acid at pH 4.7 (referred to below as “buffer A”). Increasing the molarity or reducing the pH did not affect the extraction efficiency (data not shown). We also included methanol as an extraction solvent, because the solubility of the antibiotics in this solvent was higher than in water—6.9 mg mL−1 water compared with 16.4 mg mL−1 methanol for OTC [28]. Inclusion of a second extraction-step using methanol was also expected to increase extraction efficiency for a variety of manure matrices. The extraction efficiency for methanol was significantly improved by addition of 20% 1.0 mol L−1 citric acid compared with that of pure methanol (P<0.05); no additional effect was observed when the citric acid content was increased to 50% (data not shown). Hence, 80% methanol with 0.2 mol L−1 citric acid and pH 3.5 was for extraction (referred to below as “buffer B”). Hence the extraction solvent finally chosen is comparable with those used previously [6, 29]. Use of McIlvaine buffer, EDTA, and methanol. EDTA was also investigated in this study, but precipitation was observed within few hours when mixed with methanol, potentially causing blockage of the PLE system. Therefore, only citric acid was used as chelating agent.
PLE was chosen as the extraction technique because of possible optimisation of pressure and temperature to achieve vigorous extraction of the strongly adsorbed antibiotics. Combination of cycles was also investigated and significantly better recoveries were achieved when two cycles of buffer A and one cycle of buffer B were used (P<0.05) (Fig. 3).
Results obtained from optimisation of pressurised liquid extraction (PLE). The procedure was optimised for OTC, SDZ, and TYL as representatives of the three classes of antibiotic. The method was optimised for cycles (number and combination), pressure, and temperature. The temperature setting “OFF” corresponds to room temperature. Each bar represent mean values from replicate extraction of three samples; the standard deviation is shown as error bars
Extraction was performed at 500, 1,500, and 2,500 psig and, although no statistically significant differences were observed (P>0.05), mean values for recoveries where higher at 2,500 psig (Fig. 3). The extracts did not contain additional impurities when pressure was increased, so 2,500 psig was chosen as the extraction pressure. Increasing the temperature to above 75°C caused recovery of OTC to decrease significantly (P<0.05), because of the heat sensitivity of this class of antibiotic [28], whereas extraction at room temperature (OFF), 50, or 75°C did not significantly affect recovery (Fig. 3). Although no significant effects (P<0.05) of temperature on recoveries of SDZ and TYL were observed, higher mean values for extraction efficiency were obtained at 75°C than at lower temperatures; this temperature was therefore chosen for further extractions.
Clean-up and pre-concentration procedure
An array of clean-up procedures was applied for purification and pre-concentration of the manure samples, as illustrated in Fig. 1.
Lipid removal was efficiently achieved by liquid–liquid extraction of the manure extract with nonpolar organic solvents such as pentane, hexane, cyclohexane, heptane, toluene, and 1,1,1-trichlorethane. Heptane was selected for lipid removal, because this solvent phase-separated well from the aqueous manure extract and is less toxic than hexane, which has previously been employed successfully [18].
After the heptane-wash the manure extract were diluted with Millipore water, which forced hydrophobic constituents to precipitate while the hydrophilic antibiotics remained dissolved in the aqueous phase. The subsequent filtration step completely removed the turbidity from the sample, without any detectable removal of the antibiotics. Dilution of the sample reduced the methanol content to <5% so antibiotics were not eluted from the HLB cartridges during subsequent tandem SPE (SAX-HLB). Formic acid was added to achieve pH 3 in the sample, so all the antibiotics were either positively charged or neutral (zwitterions) (pK a values are given in Table 1), and so were not retained on the SAX cartridge, whereas humic acids and other negatively charged species were removed. Although HLB cartridges have been widely used for clean-up of manure extracts [21, 22, 29], a disadvantage of the broad adsorption affinity of the HLB material is co-sorption of impurities; this may be avoided by use of more specific SPE materials. Hydrophobic materials such as LiChrolute EN and C18 materials have been used for clean-up of manure extracts of different classes of antibiotic, including sulfonamides, tetracyclines, and macrolides, but recoveries were not reported [4]. In a previous study of aqueous samples, however, the method worked well for the hydrophobic macrolides but was not applicable to tetracyclines and recoveries for sulfonamides were poor (15–75%) [30]. The hydrophilicity of the antibiotics has also been exploited in SPE clean-up. Schlüsener used diol-material for clean-up of macrolides, ionophores and tiamulin from manure extracts and obtained recoveries of 75–94% except for TYL, for which recoveries were not reproducible [19]. Pfeifer et al. used aminopropyl SPE for sulfonamides and trimethoprim, achieving recoveries of 77–91% [18]. The analytes were retained by hydrogen-bonding to the NH2-groups, a sorption mechanism that is applicable to all the antibiotics studied in this work. The hydrophilic interactions also enable removal of lipids from the cartridge by washing with hexane, without eluting the analytes [18]. Hence, the aminopropyl material may be an excellent alternative to the HLB cartridge; this was not investigated in this study.
LC–ESI-MS–MS analysis
Separation of fourteen compounds, including eight antibiotics, three epimerised tetracyclines, and three tylosin degradation products, was achieved by use of a 25-min gradient LC–ESI-MS–MS method with 15 min equilibration time between samples (40-min method). The chromatograms in Fig. 2 show the total ion count (TIC) for all the analytes with the extracted ion counts (XIC) for the individual antibiotics. Baseline separation was achieved for the tetracyclines and the corresponding epimers, and the other compounds were easily separated by mass using MRM detection. The tetracyclines and sulfonamides were analysed in one period (0–22 min) using the same mass spectrometry conditions; a second period was included for analysis of TYL and degradation products (22–40 min) to obtain satisfactory sensitivity.
Oleandomycin (OLEA) was included as an instrument standard to monitor instrumental variation during runs. The response was very stable when the matrix was not changed; this is apparent from Fig. 4a, the OLEA signal during the three-week period of validation when all samples were run using the same manure (see below). The signal was affected by changing the matrix, however; Fig. 4b shows the OLEA signal during analysis of samples from six different farms. The MS signal was constant within runs for each farm (approximately 50 injections), but varied dramatically between runs. This effect confirmed the need to use standard additions as the method of quantification; this compensates for matrix effects of the sample being quantified and has been shown to be the most reliable method of quantification in ESI-MS analysis of highly matrix-loaded samples [31].
Response for the instrument standard (OLEA) during LC–ESI-MS–MS analysis; each point represent injection of a sample. (a) Constant response during the validation of the method when all samples were run using the same manure matrix (100 μg L−1 OLEA). (b) Changes in response during analysis of manures from six different farms (250 μg L−1 OLEA), illustrating substantial matrix variation
Method validation
Validation of the method was performed using a mixture of manures #1 and #2 and was achieved using standard plots obtained for this background matrix, which simultaneously takes into matrix effects and the background amounts of the antibiotics. All the manures used in this study contained DOXY at concentrations >500 μg kg−1, however, and recoveries for this compound at low concentrations were inconsistent. Recoveries determined for sulfonamides and tetracyclines were above 70%, except for SDZ and OTC. These recoveries are satisfactory for a multi-component method and are usually in the same range as for other published methods, i.e. 77–91% [18], 47–89% [3] and 72–108% [5, 32] for sulfonamides and 82–127% for tetracyclines [5, 32]. Recoveries of TYL were poor; this is probably because of degradation in the liquid manure, as previously discussed, and more work should be performed to improve sample preservation. Other authors also experienced difficulties analysing TYL in manure samples, either because of interfering peaks in the UV chromatogram [29] or because of inconsistent recoveries [19]. In this study, however, we wished to include TYL despite the low recoveries, because this compound is widely used in Danish pig farming.
Recoveries were determined at three concentrations—from 50 μg kg−1 dry matter, the lowest concentration, near the LOQ, to 5 mg kg−1, regarded the maximum amount likely to be found in manure samples. Recoveries at the 50-μg kg−1 level may be affected by background concentration (particularly for CTC, ECTC, TC, and ETC) and higher standard deviations were observed at this level. Otherwise no tendency toward concentration-dependence was observed, despite the wide concentration-range. Repeating the recovery determination after one week also established that the day-to-day variation of the method at the 50-μg kg−1-concentration level was below 25%, except for EOTC and ECTC.
The limits of detection and quantification were determined as signal-to-noise ratios of 3 and 10, respectively, for a sample in which concentrations of the antibiotics were near the LOQ. The LOQ measured were between 10 and 100 μg kg−1 dry matter, corresponding to approximately 0.5–10 μg L−1 liquid manure, assuming 5–10% dry-matter content. This sensitivity is sufficient for quantification of typical levels of the antibiotics in manure samples and also enables evaluation of the manures in accordance with environmental risk assessment criteria (cut-off value 100 μg kg−1) stipulated by the European agency for evaluation of medicinal products [33]. Furthermore, LOQ of other published methods are usually at the low μg kg−1-level, e.g. 20–50 μg kg−1 [5, 32], <100 μg kg−1 [3], and 5 μg kg−1 [18] for sulfonamides, 50 μg kg−1 [5, 32], 0.5 μg L−1 [4] and 140 μg L−1 [29] for tetracyclines, and 10 μg L−1 manure for tylosin [6]. This level is, therefore, generally regarded as the requirement for quantification of antibiotics in manure. The validation data ontained in this work are listed in Table 4.
Application to farm samples
The freeze-dried manure from the six different farms varied widely in appearance, i.e. colour and density, but all samples passed easily through the clean-up procedure and the final extracts all seemed similar and homogenous. Three replicate samples were analysed for each farm and the content was quantified by using linear standard-addition plots for each sample. The mean values and corresponding 95% confidence intervals are listed in Table 5.
Manures #1 and #2 were collected from swine-producing farms with extremely low consumption of antibiotics because of the very high health standards of the farm animals. This is reflected in the results shown in Table 5; for manure #1 the amounts of several of the antibiotics were below the LOQ. Manures #3–#6 were collected from conventional farms, and substantially larger amounts of the antibiotics were observed, i.e. concentrations up to 30 mg kg−1 for the sum of CTC and ECTC. These high concentrations are comparable with previously published results, i.e. tetracycline concentrations up to 41 mg kg−1 [5] and 1 mg L−1 in liquid manure [4]. Sulfonamide concentrations in this study did not exceed 10 mg kg−1; previous studies have found 0.5 mg L−1 liquid manure [4] and 20 mg kg−1 [3].
TYL was not detected in any samples and may already have been degraded in the manure tank, during mixing of the manure, or after sampling. In a study by De Liguoro and co-workers a TYL concentration of 0.11 mg kg−1 was found in liquid manure [6]. Despite low recoveries, such levels of TYL should be detectable using the method presented here.
Conclusions
A multi-component method has been developed for analysis of tetracyclines, sulfonamides, and tylosin in swine manure samples. The focus was on development of a thorough sample-preparation procedure furnishing homogeneous and reproducible samples. Homogeneous samples were achieved by freeze-drying of a large volume of liquid manure and subsequent pulverisation. Extraction of the antibiotics was performed by PLE, using a combination of citric acid and methanol as extraction solvents. Sample clean-up consisted of lipid removal by liquid–liquid extraction with heptane and subsequent vacuum-filtration. Remaining humic materials were removed by SPE with SAX and HLB cartridges in tandem. The antibiotics were analysed by LC–ESI-MS–MS and quantification was performed by standard addition to compensate for matrix effects.
Recoveries for the entire method were >70%, except for SDZ, OTC and TYL, and did not vary with concentration or from day-to-day. Limits of quantification were in the range 10–100 μg kg−1.
The suitability of the method was assessed by analysis of manure samples from six different farms. Antibiotics were detected in all the samples, with the highest amounts found for tetracyclines (up to 30 mg kg−1 for the sum of CTC and ECTC). Sulfonamides were detected at concentrations up to 2 mg kg−1 (SDZ) and tylosin was not detected in any samples. Antibiotics were detected even in samples from farms with very low consumption of antibiotics, indicating that the method is suitable for analysis of swine manure containing only trace levels of antibiotics.
References
Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lutzhoft HCH, Jørgensen SE (1998) Chemosphere 36:357–393
Halling-Sørensen B, Jacobsen AM, Jensen J, Sengeløv G, Vaclavik E, Ingerslev F (2005) Environ Toxicol Chem 24:802–810
Haller MY, Müller SR, McArdell CS, Alder AC, Suter MJF (2002) J Chromatogr A 952:111–120
Campagnolo ER, Johnson KR, Karpati A, Rubin CS, Kolpin DW, Meyer MT, Esteban JE, Currier RW, Smith K, Thu KM, McGeehin M (2002) Sci Total Environ 299:89–95
Hamscher G, Pawelzick HT, Höper H, Nau H (2005) Environ Toxicol Chem 24:861–868
De Liguoro M, Cibin V, Capolongo F, Halling-Sørensen B, Montesissa C (2003) Chemosphere 52:203–212
Moral R, Moreno-Caselles J, Perez-Murcia MD, Perez-Espinosa A, Rufete B, Paredes C (2005) Biosource Technol 96:153–158
Hansen KH, Angelidaki I, Ahring BK (1998) Water Res 32:5–12
Choudhary M, Bailey LD, Grant CA (1996) Waste Manag Res 14:581–595
Wessels JM, Ford WE, Szymczak W, Schneider S (1998) J Phys Chem B 102:9323–9331
Lunestad BT, Goksøyr J (1990) Dis Aquat Org 9:67–72
Loke ML, Tjørnelund J, Halling-Sørensen B (2002) Chemosphere 48:351–361
Kolz AC, Ong SK, Moorman TB (2005) Chemosphere 60:284–289
Thiele-Bruhn S, Seibicke T, Schullten H-R, Leinweber P (2004) J Environ Qual 33:1331–1342
Schulte EE, Hopkins BG (1996) Soil organic matter: analysis and interpretation. SSSA, Madison, WI
Jacobsen AM, Halling-Sorensen B, Ingerslev F, Hansen SH (2004) J Chromatogr A 1038:157–170
Blackwell PA, Lützhoft HCH, Ma HP, Halling-Sørensen B, Boxall ABA, Kay P (2004) J Chromatogr A 1045:111–117
Pfeifer T, Tuerk J, Bester K, Spiteller M (2002) Rapid Commun Mass Spectrom 16:663–669
Schlüsener MP, Bester K, Spiteller M (2003) Anal Bioanal Chem 375:942–947
Christian T, Schneider RJ, Färber HA, Skutlarek D, Meyer MT, Goldbach HE (2003) Acta Hydrochim Hydrobiol 31:36–44
Aga DS, Goldfish R, Kulshrestha P (2003) Analyst 128:658–662
Eichhorn P, Aga DS (2004) Anal Chem 76:6002–6011
Kolz AC, Moorman TB, Ong SK, Scoggin KD, Douglass EA (2005) Water Environ Res 77:49–56
Teeter JS, Meyerhoff RD (2003) Environ Res 93:45–51
Loke ML, Ingerslev F, Halling-Sørensen B, Tjørnelund J (2000) Chemosphere 40:759–765
Salvatore MJ, Katz SE (1993) J AOAC Int 76:952–956
Fedeniuk RW, Shand PJ (1998) J Chromatogr A 812:3–15
Mitscher LA (1978) The chemistry of the tetracycline antibiotics. Marcel Dekker Inc., New York, NY, USA
Blackwell PA, Lützhoft HCH, Ma HP, Halling-Sørensen B, Boxall ABA, Kay P (2004) Talanta 64:1058–1064
Hirsch R, Ternes T, Haberer K, Mehlich A, Ballwanz F, Kratz K-L (1998) J Chromatogr A 815:213–223
Stüber M, Reemtsma T (2004) Anal Bioanal Chem 378:910–916
Hamscher G, Sczesny S, Höper H, Nau H (2002) Anal Chem 74:1509–1518
EMEA. (1998) Note for guidance: Environmental risk assessment for veterinary medicinal products other than GMO-containing and immunological products. EMEA/CVMP/0055/96. Committee for veterinary medicinal products. 1998. London, UK, The European agency for the evaluation of medicinal products
Qiang Z, Adams C (2004) Water Res 38:2874–2890
Morishita T, Yamazaki M, Yata N, Kamada A (1973) Chem Pharm Bull 21:2309–2322
Schumacher GE, Linn EE (1978) J Pharm Sci 67:1717–1720
Combined Chemical Dictionary (2005) http://www.chemnetbase.com/scripts/ccdweb.exe. Accessed 15 Nov 2005
McFarland JW, Berger CM, Froshauer SA, Hayashi SF, Hecker SJ, Jaynes BH, Jefson MR, Kamicker BJ, Lipinski CA, Lundy KM, Reese CP, Vu CB (1997) J Med Chem 40:1340–1346
Acknowledgements
Mette and Anders Lundsgaard, Niels Sørensen and Niels Jacobsen are all gratefully acknowledged for providing manure samples. This study was partly funded by grants from the European Union (ERApharm, project no. 511135) and from the Danish Directorate for Food, Fisheries and Agri Business (project no. 3401-65-03-45).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobsen, A.M., Halling-Sørensen, B. Multi-component analysis of tetracyclines, sulfonamides and tylosin in swine manure by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 384, 1164–1174 (2006). https://doi.org/10.1007/s00216-005-0261-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0261-9