Abstract
Logarithmically growing batch cultures of Escherichia coli were exposed to sublethal concentrations of pyrethroid and carbamate pesticides of four different technical grades. This induced 17–20 stress proteins, as observed using two-dimensional polyacrylamide gel electrophoresis. An E. coli culture growing exponentially in Luria Bertani medium (cell density ~2.3×109 cells/ml) was exposed to predetermined sublethal doses of individual pesticides. The cells were harvested after 30 minutes of induction and the stress response was developed in fresh LB medium for three hours under the same growth conditions. Cell pellets were obtained and stored in sonication buffer. Two-dimensional polyacrylamide gel electrophoresis was performed to resolve the proteins. Visualization of the protein spots by rapid silver staining showed 17–20 stress proteins which were absent in the standard protein profile of E. coli. On average 29% of these stress proteins were unique to the pollutant, while the remaining stress proteins overlapped with those of other pesticides. The iso-electric points (PIs) and molecular weights of the proteins were determined by comparing with protein markers with known PIs and molecular weights. Furthermore, upon comparing the pesticide-induced proteins within the same class and between the two different classes (pyrethroid and carbamate), it was apparent that the general nature of the stress remained the same throughout, which indirectly proved that the gene or set of genes responsible for stress expression are also the same, irrespective of the chemical nature of the substituents of the pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When organisms or cells are exposed to low levels of certain harmful physical or chemical agents, the organisms acquire an induced tolerance against the adverse effects [1–4]. Studies in microorganisms have provided evidence for increased longevity, cell division rate and survival when exposed to low doses of ionizing radiation, ultraviolet radiation, electromagnetic radiation, and peroxide [5–8]. Pretreatment with a sublethal heat challenge has also been implicated in the acquisition of cross-tolerance to additional forms of insult, including exposure to alcohol, anoxia, glumate toxicity, reactive oxygen species, salinity, nutrient limitation, osmolarity, and endotoxins [9, 10]. The stress proteins in E. coli have been characterized using heat, radiation, heavy metals, oxidizing agents, nutrient starvation, the SOS response, and organic solvents [11–18]. The fact that specific patterns of proteins are expressed for a particular stress has led to the use of stress proteins to monitor environmental samples for the presence of particular pollutants [19, 20]. Organisms other than E. coli have also been subjected to various stresses in order to investigate cellular behavior when the conditions in the medium change [21, 22].
In the present study, the stress proteins induced in response to technical grade samples of four different pesticides were analyzed in the hope that they could be used to qualitatively identify these pesticides in the environment. The model system used to induce the stress proteins was E. coli (BK 2012), and the model pollutants utilized as the stressors were cypermethrin, zeta-cypermethrin, carbofuran and bifenthrin. Technical grade pesticides were used as the experimental pollutants in order to provide a similar environmental situation to that encountered agriculturally. The effects of these pesticides were studied by comparing the 2-D PAGE protein profiles with the protein profile of E. coli without any induction. The induced proteins thus determined may provide a method of quantifying adverse biological effects of exposure to pesticides in the environment when examined in E. coli or other microorganisms from contaminated areas.
Materials and methods
Determining sublethal concentrations of E. coli for different pesticides
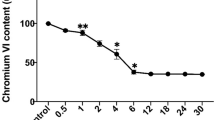
Escherichia coli (BK 2012, CEMB Culture Collection Lab, Lahore, Pakistan) was used as the model system. Technical grade samples of four pesticides—bifenthrin, carbofuran, cypermethrin and zeta-cypermethrin—belonging to two different classes of pesticides (pyrethroid and carbamate) were obtained from FMC (Pvt.) Ltd., Gulberg, Lahore, Pakistan. Different concentrations of these pesticides were solvated in absolute ethanol (ethanol final concentration <1% [vol/vol]). The effects of the chemical pollutants on bacterial growth rates were examined by introducing various concentrations of pesticides to batch cultures of the bacteria. The sublethal concentration of each pesticide for E. coli was determined by examining its effect on the survival rate of the bacteria. For this purpose, an inoculum of E. coli grown overnight was diluted 100 times with LB medium and incubated in a water bath shaker at 250 rpm and 37 °C. At mid-log phase, when the cell density was 2.3×109 cell/ml, the culture was transferred from the flask to five culture tubes. At this stage, different concentrations of the four individual pollutants were introduced into different culture tubes. One of the culture tubes was used as a control. The optical density at 595 nm was then noted every 30 minutes and sublethal doses of the pesticides were determined by constructing growth curves for the bacteria (Table 1 and Fig. 1).
Preparation of protein lysate
An inoculum of E. coli grown overnight was diluted 100 times with LB medium and then incubated in a water bath shaker at 37 °C and moving at 250 rpm. At mid-log phase, the cultures were exposed to predetermined sublethal doses of pesticides, while the control was not exposed to any pesticide. After 30 minutes of exposure, the cells were harvested by spinning the culture in a centrifuge (JA 20, Beckman, Fullerton, CA, USA ) at 7000 rpm for 10 minutes. The cell pellets thus formed were transferred to fresh LB medium, and again incubated in a water bath shaker under the same conditions to develop the stress response. After 180 minutes, the cells were harvested using the Beckman centrifuge. The cell pellets formed were stored in sonication buffer in a refrigerator at –70 °C. The protein lysate was obtained by sonicating the cells and concentrating them into 10 kDa Centricones (Pall, Boston, MA, USA).
Two-dimensional polyacrylamide gel electrophoresis
Protein profiles of E. coli for bifenthrin, carbofuran, zeta-cypermethrin and cypermethrin were prepared by two-dimensional polyacrylamide gel electrophoresis using a Hoefer (San Francisco, CA, USA) ID 125 System. The procedure originally described by O’Farrel [23] was followed. The rapid silver staining technique [24] was used to visualize the proteins. Profile analysis was carried out using Bio 2-D software (V 6.32, Vilber Lourmat, Marne-la-Vallée, France).
Results and discussion
The E. coli batch cultures were exposed to individual pesticides in order to create an environment of stress. Three different concentrations of each pesticide were introduced to logarithmically growing batch cultures and the resultant growth curves of E. coli were compared with the standard (untreated) growth curve. Concentrations of 25 mg/l, 50 mg/l, and 75 mg/l of bifenthrin and carbofuran were found to be sublethal concentrations for E. coli, whereas 50 mg/l, 75 mg/l and 100 mg/l were sublethal concentrations of zetacypermethrin. Similarly, 30 mg/l, 45 mg/l and 60 mg/l of cypermethrin were sublethal doses for E. coli. These concentrations had little effect on the growth rate of E. coli.
The stress proteins induced in response to different individual pesticides (bifenthrin, carbofuran, zetacypermethrin and cypermethrin) were identified by comparing the 2-D PAGE protein profiles of E. coli cultures treated with sublethal concentrations of these pesticides with the standard protein profile. The results obtained are summarized in Table 2. The molecular weights and PIs of the stress proteins were determined using Bio2-D by comparing them with markers with known molecular weights and PIs.
The introduction of the four pesticides to E. coli induced 76 individual stress proteins (Table 2). Some of these proteins overlapped with those induced by other pesticides, while an average of 29% of these were unique to a specific pesticide. Previous research on stress proteins has focused on the use of induced stress proteins as biomarkers [17]. However, that research did not consider the use of stress proteins in the context of soil pollution caused by excessive and improper use of pesticides in developing countries like Pakistan. Therefore, the present study was performed to investigate this aspect of pollution. Any proteins that could potentially be used to qualitatively identify stressors will not occur with the control E. coli and they will not overlap with the heat shock proteins already reported in the literature.
Sublethal concentrations of individual pesticides were determined by introducing at least three different pesticide concentrations to exponentially growing cultures of E. coli and then comparing the resulting curve with the standard curve of E. coli (the control). The results showed that the stress proteins are induced at concentrations that have little or no effect on the growth rate, and so these serve as a more sensitive index than the growth rate. These results suggest that stress protein analysis is a promising alternative (and more sensitive) method for measuring toxic effects on organisms at sublethal levels. There was a clear temporal pattern to the synthesis of pesticide-induced stress proteins, indicating sequential gene expression, as has been observed for starvation [25], heat shock [26], SOS [27] and oxidation stresses [28].
The challenge protocol (the concentration of the pesticide, the duration of exposure and the time allowed for stress response development) was selected in order to optimize the resolution of stress proteins. Exposure of E. coli to the abovementioned individual pesticides induced the production of a large number of stress proteins to combat these harmful effects. Some of the proteins were induced by more than one pesticide. In contrast to the work of Blom et al. [17], where a radioisotope labeling technique was used to show that at least 50% of the stress-induced proteins were unique to a particular pollutant, in the present study a rapid silver staining technique was used to visualize protein spots of 2-D PAGE protein profiles. Therefore, the relative decrease in the number of unique stress proteins may be explained by the detection limit (2–5 ng) of the rapid silver staining technique. The protein profile of E. coli exposed to bifenthrin showed the induction of 20 stress proteins (Fig. 2). The molecular weights of these proteins ranged from 38.33 to 29.20 kDa and the PIs ranged from 8.3 to 8.8 (see Table 3). The protein profile of E. coli exposed to carbofuran showed the induction of 20 stress proteins. The iso-electric points and molecular weights of these stress proteins ranged from 5.0 to 8.8 and 28.69 to 102.18 respectively. Blom et al. [17] prepared protein profiles for E. coli exposed to benzene and hexachlorobenzene (HCB), and visualized the protein spots by autoradiography. They observed 21 and 11 unique stress proteins for benzene and HCB respectively. Therefore, it is evident that the sensitivities of the silver staining technique and autoradiography of radiolabeled proteins are similar. The protein profile of E. coli exposed to zeta-cypermethrin showed 19 stress proteins. The molecular weights of these proteins fell between 72.92 and 28.03 kDa, while their iso-electric points ranged from 5.42 to 9.01. Likewise, exposure to cypermethrin induced 17 stress proteins. The molecular weights of these proteins ranged from 37.22 to 28.00 and the PIs ranged from 6.36 to 9.34.
The analysis of stress proteins will aid the development of more sensitive techniques for pollutant analysis. The unique stress proteins could be purified and antibodies could be raised to enable quick and sensitive detection of xenobiotics in the environment.
The above work shows that unique stress protein synthesis is a sensitive tool for environmental stress diagnosis, and that the stress proteins could be used as biomarkers for environmental pollutant identification. Although modern analytical techniques can help us to find pollutants in the environment, they are as yet unable to predict the responses of cells to adverse changes in the environment. Therefore, stress proteins could provide a way to measure the effects of toxins on living organisms.
Our study of the stress proteins produced in response to two different classes of pesticides (pyrethroids and carbamates) showed that the same stress patterns were obtained for different substituent chemical groups within the same class and for the two different classes, indicating that the gene or set of genes responsible for stress expression were the same irrespective of the class or nature of the substituents on the pesticides.
References
Hartl F (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132
Kabakov AE, Gabai VL (1995) Heat shock-induced accumulation of 70-kD stress protein (HSP70) can protect ATP-depleted tumor cells from necrosis. Exp Cell Res 217:15–21
Flahaut S, Hartke A, Giard JC, Auffray Y (1996) Alkaline stress response in Enterococcus faecalis: adaptation, cross-protection, and changes in protein synthesis. Appl Environ Microbiol 62:2416–2420
Luckey TD (1987) Ionizing radiation promotes protozoan reproduction. Radiat Res 108:215–221
Smith SJ, Barbee AS (1998) Exercise stress response as an adaptive tolerance strategy. Environ Health Persp 106(Suppl 1):325–330
Dihel L, Sonneborn JS (1985) Effects of low frequency electromagnetic field on cell division and the plasma membrane. Bioelectromagnetics 6:61–71
Smith SJ, Stansbury M, Thomas J (1996) Induced hydrogen peroxide tolerance in paramecium. Eur J Protistol 32(1):153–157
Rordorf G, Koroshetz WJ, Bonventre JV (1991) Heat shock protects cultured neurons from glutamate toxicity. Neurons 7(6):1043–1051
Maulik N, Watanabe M, Engelman D, Engelman RM, Kagan VE, Kishin E, Tyurin V, Cordis GA, Das DK (1995) Myocardial adaptation to ischemia by oxidative stress induced by endotixin. Am J Physiol 269:907–916
Van BR, Kelley PM, Neidhardt FC (1987) Differential induction of heat shock, SOS and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. Bacteriol 169:26–32
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Lindquist S (1986) The heat shock response. Annu Rev Genet 22:631–677
Matin A (1994) Starvation promoters of Escherichia coli: their function, regulation, and use in bioprocessing and bioremediation. Ann NY Acad Sci 721:277–291
Weiner L, Brissette JL, Model P (1994) Stress-induced expression of the Escherichia coli phage-shock protein operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev 5:1912–1923
Walker G (1984) Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev 48:60–93
Blom A, Harder W, Matin A (1992) Unique and overlapping pollutant stress proteins of Escherichia coli. Appl Environ Microbiol 58:331–334
Kobayashi H, Yamamoto M, Aono R (1998) Appearance of a stress-response protein, phage-shock protein A in Escherichia coli exposed to hydrophic organic solvents. Microbiology 144:353–359
Belkin S, Smulski DR, Vollmer AC, Van Dyk TK, LaRossa RA (1996) Oxidative stress detection with Escherichia coli harboring a KatG’::lux fusion. Appl Environ Microbiol 62:2252–2256
Sanders BM, Martin LS (1993) Stress proteins as biomarkers of contaminant exposure in archived environmental samples. Sci Total Environ 139/140:459–470
Goulhen F, Grenier D, Mayrand D (2003) Stress response in Actinobacillus actinomycetemcomitans: induction of general and specific stress proteins. Res Microbiol 154(1):43–48
Roccheri MC, Agnello M, Bonaventura R, Matranga V (2004) Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem Biophys Res Commun 321(1):80–87
O’Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Blom H, Beier H, Gross HS (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Groat R, Schultz J, Zychlinsky E, Bockman A, Matin A (1986) Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol 168:486–493
VanBogelen R, Acton M, Neidhardt F (1987) Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev 1:525–531
Nystrom T, Neidhardt FC (1992) Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol 6:3187–3198
Morgan B, Christman M, Jacobson F, Storz G, Ames A (1986) Hydrogen peroxide-inducible proteins in Salmonella typhumurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA 83:8059–8063
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asghar, M.N., Ashfaq, M., Ahmad, Z. et al. 2-D PAGE analysis of pesticide-induced stress proteins of E. coli . Anal Bioanal Chem 384, 946–950 (2006). https://doi.org/10.1007/s00216-005-0233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0233-0